Abstract
Classical understanding of the function of the pharyngeal plexus in humans is that it relies on both motor branches for innervation of the majority of pharyngeal muscles and sensory branches for the pharyngeal wall sensation. To date there has been no reported data on the role of the pharyngeal plexus in vocal cord innervation. The aim of this study is to evaluate whether or not the plexus pharyngeus contributes to the innervation of the vocal cords. One hundred twenty-five sides from 79 patients (59 female, 20 male) undergoing thyroid surgery with intraoperative neuromonitoring were prospectively evaluated. While vocal cord function was evaluated with endotracheal tube surface electrodes, cricothyroid and cricopharyngeal muscle electromyographic recordings were obtained with a pair of needle electrodes. The ipsilateral pharyngeal plexus, external branch of the superior laryngeal nerve, and recurrent laryngeal nerve were stimulated with a monopolar probe at 1 mA. With stimulation of the plexus pharyngeus on 125 operated sides, positive electromyographic waveforms were detected from five ipsilateral vocal cords (accounting for 3.2% of all vocal cords monitored and 6.3% of patients). The mean EMG amplitude of the vocal cords with stimulation of the plexus pharyngeus was 147 ± 35.5 μV (range 110–203). In one case, the long latency time of 19.8 ms correlated with innervation by the glottic closure reflex pathway. The short latencies seen in the other four cases [3.9 ± 1.1 ms (range 3.2–5.5)] correlated with direct innervation. In some cases, the plexus pharyngeus may contribute to vocal cord innervation by reflex or direct innervation patterns in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The physiologic functions of the larynx involve respiration, phonation, and the protective glottic closure reflex with swallowing. All of these functions are heavily influenced by vocal cord motion. Traditional laryngeal neuroanatomic descriptions have stated that the recurrent laryngeal nerves (RLNs) innervate all of the intrinsic laryngeal adductor and abductor muscles except for the cricothyroid muscles, which are innervated by the external branch of the superior laryngeal nerves (EBSLNs). The cricothyroid (CT) muscle changes vocal cord tension by elongating the cord. The internal branch of the superior laryngeal nerve (IBSLN) supplies sensory innervation to the larynx [1]. In recent years, several studies have shown that the laryngeal nerve supply is more complex and variable than was established in classic anatomical descriptions. Connections between the IBSLN and RLN, the IBSLN and EBSLN, and EBSLN and RLN have been defined [2–4].
The glottic closure reflex (GCR), also called the laryngeal adductor reflex, has a crucial role in laryngeal protection, preventing material from inappropriately entering the upper airway. The general consensus is that this reflex is a polysynaptic brain response in which the afferent component consists of the IBSLN innervating the mechanoreceptors and chemoreceptors in the laryngeal mucosa. Sensory information is then transduced through the central nervous system via the nucleus tractus solitarius to the ipsilateral nucleus ambiguus in the medulla of the brainstem. The motor neurons within the nucleus ambiguus then project to the RLN (the efferent component). In response to a stimulus, this reflex results in bilateral vocal cord adduction [5]. While the neuronal mechanics responsible for this critical reflex are quite complex, electrical stimulation of various sensory nerves has elicited reflex laryngeal adduction in some animal models [6]. Interestingly, in other animal models it has been observed that electrical and mechanical stimulation of the pharyngeal plexus (PP) leads to glottic closure [7, 8]. Additionally, two studies performed on porcine models in the same center have identified a specific neural pathway termed the glottic closure reflex-like (GCR-like) pathway, in which the afferent component is the PP. In these porcine models, the PP also demonstrated a variable direct innervation pattern to the thyroarytenoid (TA) muscle, the main adductor of the vocal cords [9, 10].
Galen’s anastomosis was described as the direct connection between the dorsal branch of the IBSLN and the dorsal branch of the RLN. Galen’s anastomosis has been believed to be primarily an anastomosis of sensory fibers [4], but the new evidence suggests that it may have some motor function. This is an intralaryngeal anastomotic connection that is often a single trunk of variable diameter formed by the two branches of the vagus nerve (95%) [4]. However, PP was described to be a composition of pharyngeal branches mainly from the glossopharyngeal and vagus nerves, with lesser contributions from the EBSLN and sympathetic nerve fibers derived from the superior ganglion [11]. The PP distributes throughout the pharyngeal wall and consists of numerous branches categorized into motor, sensory, and mixed fibers. The motor branches of the PP innervate the majority of the muscles in the pharynx, whereas the sensory branches act as sensors in the pharyngeal walls [9, 12].
In recent years, intraoperative neural monitoring (IONM) during thyroid surgery using endotracheal tube surface electrodes for EMG analysis has gained widespread acceptance as an adjunct to the gold standard of visual nerve identification for identifying and evaluating RLN and EBSLN function [13]. With this technique, the amplitude of the EMG waveform is correlated with the adductor function of the vocal cords [14]. Multichannel IONM systems can be used to simultaneously evaluate the function of other laryngeal muscles such as the cricopharyngeus (CP) and CT muscles, which are present in the dissection field during thyroidectomy. Recently, it was showed in a study of intraoperative EMG performed after thyroidectomy that both of the RLN and EBSLN contributed to the motor innervation of the CP muscle [15].
By general consensus, there is currently a lack of data regarding the connection between the PP and complex neural networks associated with vocal cord adduction. To our knowledge, there is no study in the literature that evaluates the contribution of the PP to vocal cord function in humans. We hypothesized that if the laryngeal nerves contribute to the innervation of the pharyngeal muscles in humans and PP contributes to the direct innervation of the vocal cords in porcines, PP may also contribute to the innervation of the vocal cords in humans considering the complex anastomotic pattern of the nerves within the laryngo-pharyngeal region. This study was undertaken to test this hypothesis, electrophysiologically.
Materials and methods
We studied a consecutive series of 79 patients (59 female, 20 male) with a mean age of 47.2 years (range 20–75 years) undergoing thyroid surgery (lobectomy or total thyroidectomy, with or without central neck dissection) with IONM for various diseases from February to October of 2015. The study was approved by the Institutional Review Board, and written informed consent was obtained from all patients. There were no financial or professional affiliations between the authors and the commercial company whose nerve monitoring product was used. Each side of the neck operated on was considered a separate entity, with a total of 125 sides evaluated in our study. The demographic profile of the patients and neuromonitoring data of the PP, RLN, and EBSLN were documented at the end of each case and recorded in a prospective database.
Exclusion criteria included preoperative RLN palsy, thyroid cancer with massive extrathyroidal extension, intentional nerve transection because of cancer invasion, failure to identify the PP intraoperatively, loss of signal of the RLN during surgery, and failure to assess nerve function because of technical difficulties with the IONM equipment.
All patients underwent routine direct laryngoscopy by an independent laryngologist preoperatively and within two days postoperatively.
Neuromonitoring setup
The four-channel NIM 3.0 Nerve Monitoring System (Medtronic Xomed, Jacksonville, FL, USA) was used to test each nerve intraoperatively. All monitoring setup, applications, and data interpretation were in compliance with the International Neural Monitoring Study Group Guidelines [14]. All operations were performed under general anesthesia including the same anesthetic protocol. Induction of anesthesia was initiated by consecutive intravenous injection of midazolam (2 mg), propofol (2 mg/kg), fentanyl (1 μg/kg) and a single low-dose of rocuronium (0.3 mg/kg). Once the neuromuscular inhibition was achieved, the patient was intubated with a size 6.0–8.0 endotracheal tube with surface electrodes (NIM Standard Reinforced EMG endotracheal tube; Medtronic Xomed, Jacksonville, FL, USA). After the endotracheal intubation, all the patients were put on mechanical ventilation. The anesthesia depth was maintained using the mixture of 2% sevoflurane and nitrous oxide-oxygen (1:1) providing a minimal alveolar concentration (MAC) of 1, without any use of additional neuromuscular blocking agents. If the depth of anesthesia is not sufficient to suppress spontaneous activity of the vocal cords, a bolus low-dose of propofol (0.5 mg/kg) was given to increase the anesthesia depth and prevent further patient movement. The patients were monitored with electrocardiogram, noninvasive blood pressure and pulse oximetry.
A sterile single-use pulse-generated monopolar stimulator probe (Medtronic Xomed, Jacksonville, FL, USA) was used for nerve stimulation in the surgical field. The endotracheal tube electrodes (left vocal cord to channel 1, right vocal cord to channel 2), probe and grounding electrodes were plugged into the interface-connector box. Standard IONM for the RLN was performed as a 4-step procedure (V1, R1, R2, V2), and the EBSLN was monitored during upper pole dissection in compliance with the International Neural Monitoring Study Group Guidelines [14, 16].
Neuromonitoring of the vocal cord, CP and CT muscles
After the completion of the thyroidectomy or lobectomy, intraoperative EMG data was obtained. EMG recordings were accomplished with a pair of needle electrodes inserted at the end of surgery into the CT muscle and also the midportion of the CP muscle on the lateral side of the RLN that is located 1–1.5 cm far from the nerve’s entry point to the larynx. The needle electrode was inserted into the CP muscle in a supero-inferior direction, perpendicular to the muscle fibers, with a depth of 1 cm to prevent the insertion through the other intralaryngeal muscles. These needle electrodes were plugged into the third and fourth channels of the interface-connector box of the NIM 3.0 Nerve Monitoring System, respectively.
The EBSLN, RLN, and PP were stimulated with a monopolar stimulator probe at 1 mA. The stimulation duration was set at 100 µs, and the current was set at a frequency of 4 Hz. During the stimulation of these nerves, the EMG recordings of the left and right vocal cords, CT and CP muscles were obtained via the first, second, third and fourth channels of the NIM 3.0 Nerve Monitoring System, respectively.
We evaluated the innervation pattern of the CT and CP muscles in our other studies and found that the EBSLN and PP are the main suppliers of the CT and CP muscles, respectively. The EMG findings of these muscles were used to confirm the EBSLN and PP in our current study, respectively.
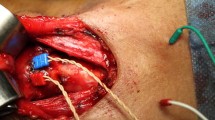
The constant points of neural stimulations were standardized. EBSLN was stimulated at a point that is located 2 cm proximally to the nerve’s entry point to the CT muscle. While rotating the larynx manually to the medial side, PP was found with the stimulator probe upon the surface of the dorso-lateral fibers of the inferior pharyngeal constrictor muscle nearby the posterior border of the thyroid cartilage lamina. PP was stimulated at the same level of EBSLN’s stimulation point. RLN was stimulated at a distance of 2 cm proximally, before it enters the larynx.
Positive stimulation was defined by both the audible alarm from the NIM system and achievement of a recognizable vocal cord EMG waveform ≥100 µV with nerve stimulation. There is no uniform consensus in the literature regarding the definition of latency. We used the definition by Sritharan et al. [13], which describes latency as the time from the stimulation spike to the first evoked waveform peak.
Analysis
All amplitude and latency data are presented as mean data with standard deviation (min–max). Positive EMG response values are expressed as percentages. Statistical analyses were performed using the Mann–Whitney U and Wilcoxon test to compare continuous variables.
Results
The study included 79 patients (59 female and 20 male) whose mean age was 47.2 years (range 20–75 years). Forty-six patients underwent bilateral and 33 underwent unilateral operations, for a total of 125 operated sides and 125 monitored vocal cords. With stimulation of the PP on 125 operated sides, positive EMG waveforms were detected from five ipsilateral vocal cords (accounting for 3.2% of all vocal cords monitored and 6.3% of patients) (Figs. 1, 2). Of these five positive waveforms, two were seen in unilaterally explored patients and three in bilaterally explored patients. The vocal cord EMG amplitudes and latencies obtained with stimulation of the PP, RLN, and EBSLN are given in Table 1. Evoked potentials were obtained from the vocal cords in four out of five cases by stimulating the EBSLN. Stimulation of the PP, EBSLN and RLN produced the following EMG amplitudes and latencies: 147 ± 35.5, 320 ± 248, 1582.4 ± 345.6 μV and 3.9 ± 1.1, 3.4 ± 0.7, 2.9 + 0.4 ms, respectively. The long latency time of 19.8 ms seen in case 4 (Fig. 2) was excluded as an outlier in calculating the mean latency value for the PP. The EMG amplitudes and latency times achieved from the CP muscle with stimulation of the PP were 3239.6 ± 1349.3 μV (range 1858–5103 μV) and 3.7 ± 1.1 ms (range 2.5–5.3 ms), respectively.
The electromyography (EMG) waveform (black line) from the right vocal cord (thyroarytenoid (TA) muscle) with stimulation of the right PP was obtained via the second channel. (Channel 1 left vocal cord, Channel 2 right vocal cord, Channel 3 cricothyroid (CT) muscle, Channel 4 cricopharyngeal (CP) muscle) in case 3. Additionally, the second EMG waveforms (gray line) are obtained by left continuous vagus nerve stimulation
The EMG waveform (black line) with a long latency time, obtained from the right vocal cord with right PP stimulation (Channel 2) in case 4. (Channel 1 left vocal cord, Channel 2 right vocal cord, Channel 3 inferior pharyngeal constrictor muscle, Channel 4 cricopharyngeal (CP) muscle). Additionally, the second EMG waveforms (gray line) are obtained by right continuous vagus nerve stimulation
The EMG amplitude of the vocal cords was significantly lower and latency significantly longer with PP stimulation compared to RLN stimulation (p = 0.046 and p = 0.009, respectively). No significant difference was detected between vocal cord EMG amplitudes (p = 0.327) and latencies (p = 1) when comparing EBSLN and PP stimulation. Finally, the latency of the vocal cord EMG waveform with stimulation of the PP was similar to that of the CP muscle (p = 0.7).
Discussion
Although the afferent pathway of the classical GCR is the IBSLN, this protective reflex has also been demonstrated with electrical stimulation of other sensory nerves in an animal model [6]. Vocal cord adduction is observed with stimulation of the posterior region of the pharynx by a bolus of water in cats. The glossopharyngeal nerve has been demonstrated as the afferent pathway of this reflex [8]. Kitagawa et al. [17] found in a rat model that electrical stimulation of the glossopharyngeal nerve’s pharyngeal branch elicited reflexive swallowing, with a similar relationship between stimulus frequency and latency of swallowing as that seen with stimulation of the SLN. However, when both the SLN and pharyngeal branch of the glossopharyngeal nerve are electrically stimulated simultaneously, the latency of reflexive swallowing becomes shorter than that seen with independent stimulation of the nerves [7]. With EMG of the TA muscle, Matsuzaki et al. [9] showed that electrical stimulation of the PP evokes vocal cord adduction, with variable latencies obtained with TA muscle contraction. Three distinct neural pathways were identified in another study reported from the same center [10]. First, an evoked long latency GCR-like pathway was identified, with sensory fibers of the PP acting as the afferent route projecting to the brainstem via the nucleus tractus solitarius and nucleus ambiguous, and the RLN acting as the efferent route. Second, they identified a short-latency connection between the PP and the EBSLN leading to direct TA muscle contraction. Finally, they noted a likely communicating branch from the PP directly to the RLN and ultimately to TA muscle contraction. These pathways all influence glottic closure [10].
This study is the first one demonstrating the contribution of PP stimulation to vocal cord innervation in humans. The PP latency responses of this study also support that there may be at least three distinct neural pathways playing a role in conduction. It is essential to separate the short and long latency responses to understand the innervation patterns. Latency is the amount of time it takes the neural impulse to travel to its end target after the nerve is stimulated [10]. The latency of the ipsilateral TA muscle contraction in our fourth case is 19.8 ms, leading us to consider that this electrical stimulation is conducted via a GCR-like pathway. The branch of the PP that is thought responsible for this pathway should be considered a mixed neural branch as it is also thought to contribute to motor function of the CP muscle.
Three categories of protective laryngeal responses have been observed after stimulation of the IBSLN in the classical laryngeal adductor reflex. First, an early response involves adduction of the ipsilateral vocal cord with a latency of approximately 10–18 ms, as seen in anesthetized cats and dogs. This short-latency evoked response, termed R1, has also been consistently noted with a latency of approximately 16–17 ms in anesthetized humans [18, 19]. A second category of short-latency evoked R1 response involves simultaneous contralateral adduction, also known as the crossed adductor reflex. Although this response has consistently been found in anesthetized cats, it is less consistently found in dogs and is rarely observed in anesthetized human subjects [18]. Additionally, deepening anesthesia alters the central facilitation and abolishes the crossed adductor reflex in human subjects [19]. The third category of adductor response involves a longer latency reflex and has been termed R2. This R2 has been observed to produce bilateral vocal cord responses and is most readily noted in awake human subjects, with a latency of 50–80 ms [20].
The mean latency time of TA muscle contraction with stimulation of the PP in four out of five of our cases (excepting the fourth case) was 3.9 ms (range 3.2–5.5 ms), which was longer than that of the RLN and similar to the EBSLN. This means latency recorded with PP stimulation is similar to the mean latencies reported in the literature with stimulation of the RLN and EBSLN (3.96 and 3.56 ms, respectively) [13]. These results lead us to believe that vocal cord innervation can be elicited via connections between the PP and RLN or PP and EBSLN, as identified by Paskhover et al. [10]. Additionally, an amplitude with PP stimulation of 10% that with stimulation of the RLN, the main supplier of the vocal cords, supports this direct pattern via connections between thin branches. The stimulated PP branch may be motor or mixed functioning in these patients. In our third case, EBSLN stimulation did not cause vocal cord adduction, suggesting an elicited response by the connection between the RLN and PP. Vocal cord adduction might occur via the connection between the RLN and EBSLN in the other three cases. The latency obtained with stimulation of the PP was shorter than that for the EBSLN in cases 2 and 5, so this stimulation might be via a connection with the RLN. Although less likely, it must also be considered that stimulation might be via a connection between the EBSLN and PP, which is located distal to our fixed stimulation points for the EBSLN and PP. Latency with PP stimulation in the first case was longer than those of the EBSLN and RLN, suggesting stimulation via the connection with RLN or EBSLN.
The technical limitations such as stimulation of RLN or EBSLN instead of PP, which might affect these results, must be considered. The PP was described to spread its branches over the dorso-lateral surface of the superior and middle constrictors, and lower branches descend onto the inferior pharyngeal constrictor muscle [21]. The PP can be found with the stimulator probe upon the surface of the dorso-lateral fibers of the inferior pharyngeal constrictor muscle nearby the posterior border of the thyroid cartilage lamina, with the help of the rotation maneuver of the larynx medially. This area is not close to the EBSLN’s course and the RLN’s entry point to the larynx, therefore the inadvertent stimulation of the RLN or EBSLN instead of the PP branches is not possible. Besides, the direct stimulation of the intralaryngeal branches of the RLN is not possible, because the electrical transmission distance to adjacent tissues is less than 1 mm with 1 mA intensity [22].
Some anatomical studies observed communications between the PP and RLN [23], in addition to the electrophysiologic findings of Paskhover et al. [10]. Communications of the PP with the external and internal branches and main trunk of the SLN were also sometimes found [24]. In addition, the SLN may actually arise from two roots, with one coming from the glossopharyngeal nerve [25].
A recent IONM study with an endotracheal tube with surface electrodes revealed the activation of the vocal cord in 70–80% of the patients, when EBSLN was electrically stimulated [26, 27]. This response is present due to the existence of the human communicating nerve between the superior laryngeal nerve and RLN [26, 27], which was described in up to 83% of anatomical dissection studies [28]. Additionally, it is found that the TA muscle is directly cross-innervated by an EBSLN in the porcine model [29].
The amount of contribution of the PP to vocal cord innervation might be underestimated in this study. Only a single branch of the PP may be stimulated with use of a standardized fixed point for PP stimulation. Other branches of the PP may also contribute to vocal cord innervation via a reflex or direct innervation pattern.
Muscular twitch may not be visualized, because the positive EMG response of 150 microvolt from the thyroarytenoid muscle with the PP stimulation is 10% of that from the vocal cords with stimulation of the RLN. Distant uptake by hook–wire electrode from the TA muscle is possible source of error, but this is unlikely because the uptake of electrical activity was limited to the area just around the needles [30]. The depth of electrode that was inserted into the CP muscle might also be considered as another factor for the technical failure. The needle electrode was inserted into the CP muscle at a point, on the postero-lateral side of the RLN that is at least 1–1.5 cm far from the nerve’s entry point to the larynx to prevent the RLN’s contact to the electrode. However, the needle electrode was inserted into the CP muscle in a supero-inferior direction perpendicularly to the muscle fibers with a depth of 1 cm, remaining within the borders of the muscle, to prevent the potential innervations from the other intralaryngeal muscles.
However, the contribution of the PP to vocal cord innervation may be related in variable degrees to postoperative voice and swallowing disorders after RLN injury. The clinical results regarding the PP’s contribution to the vocal cord innervation after RLN injury, is not known yet. However, the reinnervation of the vocal cords may be maintained via PP in variable degrees. The reinnervation of the vocal cords via PP may diminish the rate of denervation atrophy. Additionally, these pathways in the human larynx could offer surgical targets for rehabilitation, particularly in patients with chronic aspiration due to incomplete or weakened glottic closure, while avoiding direct manipulation of the RLN itself [10].
The depth of anesthesia and use of neuromuscular blocking agents may influence the results of these kinds of EMG studies. The use of intravenous or inhalational anesthetics can be potential factor that would interfere with the reading of neuromonitoring signals [31]. Therefore, thyroidectomy with IONM and intraoperative EMG has exercised additional caution on the administration of general anesthesia. The neuromonitoring of anesthesia depth with bispectral index (BIS) monitoring or the extent of neuromuscular blockade with a train-of-four twitches (TOF) device can be still recommended for the further studies. Although we do not use BIS monitoring and TOF device routinely, we believe that it is not possible for our anesthesia protocol, including low-doses of propofol, sevoflurane and rocuronium, to have an effect upon the results of our study. Propofol, the most commonly used intravenous anesthetic, is used for the induction and maintenance of general anesthesia. Chang et al. [31] found that a single small dose of propofol (0.5 mg/kg) did not alter evoked potential from the RLN during thyroid surgery. Additionally, propofol has no effect on brain stem auditory-evoked potentials under regular maintenance dose [32]. Sevoflurane has little effect on neuromuscular function [33]. However, Sasaki et al. [19] confirmed that ipsilateral R1-evoked responses were achieved at all depths of anesthesia, and contralateral glottic closure reflex could be abolished over anesthesia levels of >1 MAC. The duration of neuromuscular blockade is of great importance in IONM. Rocuronium which is a nondepolarizing shortacting neuromuscular agent has a complete EMG signal recovery can be obtained within 30 min with a single dose of rocuronium (0.3 mg/kg) [34]. It was not possible to have the effect of the neuromuscular blocking agent during the intraoperative EMG, because the EMG was performed at the end of the surgical procedure (at least 60 and 90 min after lobectomy and total thyroidectomy, respectively) in our study.
In conclusion, PP, having variable and complex neural connections in the laryngopharyngeal area, may sometimes contribute to vocal cord innervation via a reflex or direct innervation pattern in humans.
References
Rubin AD, Sataloff RT (2008) Vocal fold paresis and paralysis: what the thyroid surgeon should know. Surg Oncol Clin N Am 17:175–196. doi:10.1016/j.soc.2007.10.007
Maranillo E, Leon X, Orus C, Quer M, Sanudo JR (2005) Variability in nerve patterns of the adductor muscle group supplied by the recurrent laryngeal nerve. Laryngoscope 115:358–362. doi:10.1097/01.mlg.0000154745.78808.02
Martin-Oviedo C, Maranillo E, Lowy-Benoliel A et al (2011) Functional role of human laryngeal nerve connections. Laryngoscope 121:2338–2343. doi:10.1002/lary.22340
Sanudo JR, Maranillo E, Leon X, Mirapeix RM, Orus C, Quer M (1999) An anatomical study of anastomoses between the laryngeal nerves. Laryngoscope 109:983–987
Domer AS, Kuhn MA, Belafsky PC (2013) Neurophysiology and clinical implications of the laryngeal adductor reflex. Curr Otorhinolaryngol Rep 1:178–182. doi:10.1007/s40136-013-0018-5
Suzuki M, Sasaki CT (1977) Effect of various sensory stimuli on reflex laryngeal adduction. Ann Otol Rhinol Laryngol 86:30–36
Kitagawa J, Nakagawa K, Hasegawa M et al (2009) Facilitation of reflex swallowing from the pharynx and larynx. J Oral Sci 51:167–171
Shaker R, Medda BK, Ren J, Jaradeh S, Xie P, Lang IM (1998) Pharyngoglottal closure reflex: identification and characterization in a feline model. Am J Physiol 275:G521–G525
Matsuzaki H, Paskhover B, Sasaki CT (2014) Contribution of the pharyngeal plexus to vocal cord adduction. Laryngoscope 124:516–521. doi:10.1002/lary.24345
Paskhover B, Wadie M, Sasaki CT (2014) The pharyngeal plexus-mediated glottic closure response and associated neural connections of the plexus. JAMA Otolaryngol Head Neck Surg 140:1056–1060. doi:10.1001/jamaoto.2014.2440
Mu L (2007) Sanders I (2007) Neuromuscular specializations within human pharyngeal constrictor muscles. Ann Otol Rhinol Laryngol 116:604–617
Mu L, Sanders I (2000) Sensory nerve supply of the human oro- and laryngopharynx: preliminary study. Anat Rec 258:406–420
Sritharan N, Chase M, Kamani D, Randolph M, Randolph GW (2015) The vagus nerve, recurrent laryngeal nerve, and external branch of the superior laryngeal nerve have unique latencies allowing for intraoperative documentation of intact neural function during thyroid surgery. Laryngoscope 125:E84–E89. doi:10.1002/lary.24781
Randolph GW, Dralle H, Abdullah H et al (2011) Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 121(Suppl 1):S1–S16. doi:10.1002/lary.21119
Uludag M, Aygun N, Isgor A (2016) Innervation of the human cricopharyngeal muscle by the recurrent laryngeal nerve and external branch of the superior laryngeal nerve. Langenbecks Arch Surg. doi:10.1007/s00423-016-1376-5
Barczyński M, Randolph GW, Cernea CR et al (2013) External branch of the superior laryngeal nerve monitoring during thyroid and parathyroid surgery: international Neural Monitoring Study Group standards guideline statement. Laryngoscope 123(Suppl 4):S1–S14. doi:10.1002/lary.24301
Kitagawa J, Shingai T, Takahashi Y, Yamada Y (2002) Pharyngeal branch of the glossopharyngeal nerve plays a major role in reflex swallowing from the pharynx. Am J Physiol Regul Integr Comp Physiol 282:R1342–R1347. doi:10.1152/ajpregu.00556.2001
Sasaki CT, Suzuki M (1976) Laryngeal reflexes in cat, dog, and man. Arch Otolaryngol 102:400–402
Sasaki CT, Jassin B, Kim YH, Hundal J, Rosenblatt W, Ross DA (2003) Central facilitation of the glottis closure reflex in humans. Ann Otol Rhinol Laryngol 112:293–297
Ludlow CL, Van Pelt F, Koda J (1992) Characteristics of late responses to superior laryngeal nerve stimulation in humans. Ann Otol Rhinol Laryngol 101(2 Pt 1):127–134
Sakamoto Y (2009) Classification of pharyngeal muscles based on innervations from glossopharyngeal and vagus nerves in human. Surg Radiol Anat 31:755–761. doi:10.1007/s00276-009-0516-9
Uludag M, Aygun N, Isgor A (2016) Motor function of the recurrent laryngeal nerve: sometimes motor fibers are also located in the posterior branch. Surgery 160:153–160. doi:10.1016/j.surg.2016.02.003
Mu L, Sanders I (1998) Neuromuscular organization of the human upper esophageal sphincter. Ann Otol Rhinol Laryngol 107:370–377
Sakamoto Y (2013) Interrelationships between the innervations from the laryngeal nerves and the pharyngeal plexus to the inferior pharyngeal constrictor. Surg Radiol Anat 35:721–728. doi:10.1007/s00276-013-1102-8
Bergman RA, Thompson SA, Afifi AK, Saadeh FA (1988) Compendium of human anatomic variation: text, atlas, and world literature. Urban & Schwarzenberg, Baltimore
Potenza AS, Phelan EA, Cernea CR et al (2013) Normative intra-operative electrophysiologic waveform analysis of superior laryngeal nerve external branch and recurrent laryngeal nerve in patients undergoing thyroid surgery. World J Surg 37:2336–2342. doi:10.1007/s00268-013-2148-9
Barczyński M, Konturek A, Stopa M, Honowska A, Nowak W (2012) Randomized controlled trial of visualization versus neuromonitoring of the external branch of the superior laryngeal nerve during thyroidectomy. World J Surg 36:1340–1347. doi:10.1007/s00268-012-1547-7
Morton RP, Whitfield P, Al-Ali S (2006) Anatomical and surgical considerations of the external branch of the superior laryngeal nerve: a systematic review. Clin Otolaryngol 31:368–374. doi:10.1111/j.1749-4486.2006.01266.x
Paskhover B, Wadie M, Sasaki CT (2015) Thyroarytenoid cross-innervation by the external branch of the superior laryngeal nerve in the porcine model. Laryngoscope 125:177–179. doi:10.1002/lary.24888
Hydman J (2008) Mattsson P (2008) Collateral reinnervation by the superior laryngeal nerve after recurrent laryngeal nerve injury. Muscle Nerve 38:1280–1289. doi:10.1002/mus.21124
Chang PY, Wu CW, Chen HY et al (2014) Influence of intravenous anesthetics on neuromonitoring of the recurrent laryngeal nerve during thyroid surgery. Kaohsiung J Med Sci 30:499–503. doi:10.1016/j.kjms.2014.05.009
Savoia G, Esposito C, Belfiore F, Amantea B, Cuocolo R (1988) Propofol infusion and auditory evoked potentials. Anaesthesia 43(Suppl):46–49
Han YD, Liang F, Chen P (2015) Dosage effect of rocuronium on intraoperative neuromonitoring in patients undergoing thyroid surgery. Cell Biochem Biophys 71:143–146. doi:10.1007/s12013-014-0176-1
Lu IC, Chang PY, Hsu HT et al (2013) A comparison between succinylcholine and rocuronium on the recovery profile of the laryngeal muscles during intraoperative neuromonitoring of the recurrent laryngeal nerve: a prospective porcine model. Kaohsiung J Med Sci 29:484–487. doi:10.1016/j.kjms.2013.01.002
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All authors have agreed to the manuscript’s content. All authors warrant that the submitted article is original, and has not been submitted to another journal for publication, has not been published elsewhere, or if published in whole or in part, all permissions were granted for publication in Langenbeck’s Archives of Surgery.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. This prospective study was approved by the Institutional Review Board of Sisli Hamidiye Etfal Training and Research Hospital.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Uludag, M., Aygun, N. & Isgor, A. The functional role of the pharyngeal plexus in vocal cord innervation in humans. Eur Arch Otorhinolaryngol 274, 1121–1128 (2017). https://doi.org/10.1007/s00405-016-4369-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-016-4369-7