Abstract
Surface electromyography (sEMG) is a well-established procedure for recording swallowing-related muscle activities. Because the use of a large number of sEMG channels is time consuming and technically sophisticated, the aim of this study was to identify the most significant electrode positions associated with oropharyngeal swallowing activities. Healthy subjects (N = 16) were tested with a total of 42 channels placed in M. masseter, M. orbicularis oris, submental and paralaryngeal regions. Each test subject swallowed 10 ml of water five times. After having identified 16 optimal electrode positions, that is, positions with the strongest signals quantified by the highest integral values, differences to 26 other ones were determined by a Mann–Whitney U test. Kruskal–Wallis H test was utilized for the analysis of differences between single subjects, subject subgroups, and single electrode positions. Factors associated with sEMG signals were examined in a linear regression. Sixteen electrode positions were chosen by a simple ranking of integral values. These positions delivered significantly higher signals than the other 26 positions. Differences between single electrode positions and between test subjects were also significant. Sixteen most significant positions were identified which represent swallowing-related muscle potentials in healthy subjects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surface electromyography (sEMG) provides real-time spatiotemporal information about muscle activities related to the oropharyngeal swallowing. Nowadays, due to its non-invasive character, sEMG is often used in clinical routine and research.
The idea to obtain swallowing-related information by EMG recording is not a new one. In 1956, Doty and Bosma published among the first an electromyographic analysis of reflex deglutition in an animal trial [1]. Whereas the first EMG studies on swallowing were predominantly conducted even in humans with needle and hook-wired electrodes [2, 3], the development of sEMG increased the attractiveness of this method for diagnostic and rehabilitation purposes.
Although the swallowing-related muscles are often small in size and overlap in fibers, sEMG turned out to deliver constant results in respect to frequency and amplitude values [4], even if sEMG, in contrast to EMG recordings using needle electrodes, is not able to record activities of single swallowing muscles. Also, sEMG was reliable in repetitive recordings over multiple days in comparison with simultaneous intramuscular recordings, at least in masticatory muscles such as masseter [5, 6]. Moreover, muscle activity of the M. orbicularis oris, M. masseter and the submental muscle group correlated reliably with videofluoroscopy results [7]. Biomechanical correlates could be found between sEMG and separate swallowing functions such as hyoid elevation, constriction of pharyngeal muscles, and opening of the upper oesophageal sphincter [4].
Due to this link between sEMG signals and muscle activities, sEMG can provide information on the physiology of swallowing [8–11], which can be utilized for biofeedback interventions in therapeutic settings [12–16]. The placement of sEMG electrodes is one of the most important factors associated with the measurement accuracy and minimization of possible misinterpretations of electromyography results [17–20]. However, electrode placement is still more based on practice of diverse study groups and laboratories than on results of empirical studies [19, 21].
Some studies focus on isolated regions such as masticatory muscles [6, 22, 23], submental [2, 16, 24, 25] and hyolaryngeal regions [26, 27] or a combination of those [7, 11, 28] in healthy test subjects [7, 29] and patients [30–32]. Topics of interest for sEMG research included, apart from swallowing, other issues such as voice [32, 33] or playing trumpet [34–36]. However, despite the existing abundance of sEMG research, it is hard to compare study results in respect to benefit, application or even recording recommendations for both clinicians and investigators because of different study designs used, including different electrode placement sites.
Given the paucity of empirically based recommendations regarding sEMG conduction, the aim of the present study was the identification of optimal electrode placement sites for sEMG recording of swallowing-related activities by means of a systematic analysis of a large number of available placement positions. Because common commercial recording systems are predominantly equipped with no more than 16 sEMG channels and also because the use of large sets of sEMG channels is both time consuming and technically sophisticated, the purpose of the study was to find 16 most significant positions out of a total of 42, which were located as densely as possible in the four swallowing-related regions of (1) M. masseter, (2) M. orbicularis oris, (3) submental and (4) paralaryngeal regions. Due to the limitations of sEMG in recording pharyngeal muscle activity, this region was not in the focus of the present study.
Materials and methods
Sixteen healthy subjects without swallowing dysfunctions, five males and 11 females aged 21;1–62;5 years (median 27;2 years), were included in the study. Neurological diseases, anomalies of oropharyngolaryngeal structures as well as factors that might affect swallowing such as cold, tooth and throat pain or other medical and medication problems were negated by all participants. There was no attempt to constitute a homogeneous group, as far as gender, age, race or other demographical factors are concerned. Informed written consent was obtained from all participants prior to initiating data collection.
Ethics approval was granted by the health ethics review board of University Hospital of Frankfurt/Main, Germany (# 393/13).
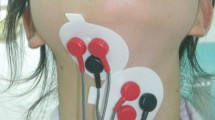
sEMG was recorded with 42 surface electrodes placed as densely as possible in the (1) M. masseter, (2) M. orbicularis oris, (3) submental and (4) paralaryngeal regions using a bipolar technique with the reference electrode at mid-forehead (see Fig. 1). The surface electrode pairs (NeoLead; Neotech Products, Valencia, USA) were placed on a cleaned, dry skin with an interelectrode center-to-center distance of 4 mm, parallel orientated to muscle fiber direction to obtain the maximum of spatial potential gradient [37]. Neolead electrodes are pre-wired, made of latex and phthalate/DEHP and have very compact dimensions (1″ × 3/8″) allowing high placement density. The sEMG signals were recorded with a 16-channel amplifier (Buck Elektromedizin, Bad Rappenau, Germany; sampling rate: 500 Hz; sample quantification: 8 bit for a voltage range of between −500 and +500 μV, amplification 10,000; high-pass filter about 80 Hz, low-pass filter about 300 Hz), preamplified and band-pass filtered (3–250 Hz) with sufficiently high signal-to-noise ratio (about 34 dB at the level of 50 Hz).
The 16-channel amplifier was connected to the notebook Compaq 160 (Hewlett-Packard Development Company, L.P.; Palo Alto, USA) running the sEMG software “B-F-EAT” (Buck Elektromedizin, Bad Rappenau, Germany).
For the measurements, subjects were instructed to sit upright, with head in a neutral position, without moving and using mimics during the check of the electrode impedances and the swallowing study. Each subject completed five swallows of 10 ml water (cf. [38]), self-delivered by a graduated syringe, each time after observing the signal in rest for at least 3 s. To avoid fatigue effects, recovery breaks of about 1 min were made between subsequent swallows.
The obtained sEMG signals were high-pass filtered, rectified, and smoothed with a moving-average filter of 80 ms in length, prior to analysis.
To compare the sEMG signals of all 42 channels and to define the 16 most important swallowing-related electrode positions in the four examined regions, the integral of the rectified sEMG signals with respect to time was chosen as an indicator of the strength of the corresponding muscles.
A baseline of at least 0.5 s before the swallow, without any artifacts, was used to calculate a line indicating activities surpassing the limit of two standard deviations from the baseline. By means of this line, onset and offset of the sEMG signal were identified. Rectified and smoothed sEMG signals V i (t) (i indicates the channel) with removed baseline drift were processed by an experienced clinician who set marks at the onset and offset time (t on, t off: intersection point of sEMG signal and the “two-standard-deviations-line”) and highest peak in each channel and each recording (see Fig. 2).
The integral (area under the curve) was calculated automatically by the software with the following formula:
The integral was calculated not on the basis of five sEMG records per person, but on the basis of one record averaged over five synchronized swallows.
In order to examine the preciseness of the integral calculation method, the intra-judge (ρ = .992, p < .001, N = 320) and inter-judge reliability (ρ = .930, p < .001, N = 320) were analyzed for a sample of integral values of two clinicians.
Statistical data were exported from the sEMG recording program and analyzed in SPSS 20. As the data demonstrated no normal distribution according to Kolmogorov–Smirnov test, only non-parametric tests were used where possible.
Differences in the distribution of the integral between electrode placement sites and between test subjects were determined by a Kruskal–Wallis H test. Differences between swallowing patterns were also assessed by Kruskal–Wallis H tests.
Most significant electrode positions were chosen by a simple ranking of integral values, numerically balanced for four anatomical regions. To represent all anatomical regions, at least two electrode positions were chosen in each one. The difference between (a) the chosen electrode positions and (b) the other positions was demonstrated by the Mann–Whitney U test for integral to examine whether the chosen 16 electrode placement sites deliver significantly stronger sEMG signals than the other 26 electrode placement sites.
Additionally, a linear regression was calculated with the integral as dependent variable and several independent factors which potentially influence the distribution of sEMG signals: (1) “42 electrode placement sites”; (2) “16 test subjects”; (3) “sex”; (4) “age”; (5) “placement of electrodes on the left, right side or centrally”; (6) “4 swallowing regions”; (7) “16 chosen electrode positions vs the 26 other positions”; (8) “3 subgroups of test subjects”. The factor (7) quantifies the influence of the division of all electrode positions into more and less important ones. The factor (8) refers to the subdivision of test subjects into those who predominantly use the muscles (a) in the orbicularis oris region, (b) in the masseter region, and (c) in the submental region during swallowing. All of the chosen factors were shown to be associated with the distribution of sEMG signals in some of the previous studies, although often inconsistently (see “Discussion”). Standardized beta coefficients (β) and mean values (M) are given for statistically significant results. Adjusted R 2 was used to determine the explained variance.
Results
The differences between 42 electrode positions were highly significant for the integral: χ 2(41) = 129.41, p < .001, N = 42. Also, highly significant differences were revealed between test subjects: χ 2(15) = 138.37, p < .001, N = 16.
A further analysis of the sEMG signals of test subjects identified three subgroups regarding swallowing patterns: those with the highest activity (1) in the orbicularis oris region (N = 5), (2) in the masseter region (N = 4), and (3) in the submental region (N = 7). In three Kruskal–Wallis H tests, the integral values of those test subjects “preferring” a certain region were significantly higher on electrode positions from this region compared with the other two groups: (1) M. orbicularis: χ 2(2) = 20.78, p < .001, (2) M. masseter: χ 2(2) = 14.67, p = .001, (3) submental region: χ 2(2) = 9.98, p = .007.
For the choice of the most significant sEMG electrode positions, all positions were subdivided into two groups for each of the four regions (M. masseter, M. orbicularis oris, submental and paralaryngeal regions) according to the integral values: 16 most significant electrode positions with the highest values in the ranking vs 26 positions with lower values (see Fig. 3). The integral value was significantly higher for the 16 most significant electrode positions than for the 26 remaining positions: U = 40,551, Z = −6.42, p < .001, N = 42.
According to a linear regression (F (8,663) = 6.74, p < .001, adjusted R 2 = .064, that is, 6 % of explained variance), the influence of factors “42 electrode placement sites”, “16 test subjects”, “3 subgroups of test subjects”, “age”, and “4 swallowing regions” did not reach statistical significance. The influence of the factors “16 chosen electrode positions vs the 26 other positions” (β = −.190, p < .001) and “sex” (β = −4.04, p < .001) were highly significant. Integral calculated for the chosen 16 electrode positions (M = 3.0) was more than twice as high as the integral of the other 26 positions (M = 1.2). Mean values demonstrated that men’s results (M = 3.0) were on average higher than those of women (M = 1.4). The influence of the factor “placement of electrodes on the left, right side or centrally” was also significant (β = 2.09, p = .037). Central electrode positions (M = 2.9) delivered higher values than those on the right and left sides (both Ms = 1.7). In regard to the low percentage of the explained variance it should be noted that zero integral values made out 63 % in the dependent variable in the regression, which means that on 63 % of channels sEMG activities, measured by integral, did not surpass the line indicating two standard deviations from the baseline recorded in rest.
Discussion
In the present experimental study, 16 most significant sEMG electrode placements were determined out of 42 potential positions located all over the oropharyngeal swallowing-related regions. At least two electrode positions were selected in each of the four defined regions that were (1) M. masseter, (2) M. orbicularis oris, (3) submental and (4) paralaryngeal regions. The identification of the most significant positions was based primarily on a simple ranking according to the integral of the rectified and smoothed sEMG signal with respect to time. Since force of a muscle is roughly proportional to the sEMG voltage [39], the integral expresses kind of a force–time product and hence the “effort” of the muscle for the particular swallowing task. The difference between these two groups of electrode positions was also revealed by a linear regression in which the negative beta value means that the 16 most significant electrode positions delivered significantly higher integral values than all other electrode positions.
As was shown in Fig. 3, the chosen 16 electrode positions were located predominantly centrally, which means that the central regions delivered the strongest sEMG signals. These signals were produced by the most superficially located muscle groups, as has already been demonstrated in comparison with other regions in previous research [4, 40, 41].
Significant differences in integral values of sEMG signals were identified between 16 test subjects. This result is in line with some previous studies which revealed considerable interpersonal variance in muscle activity patterns [2, 42–45] and in the corresponding biomechanical movements [46] in healthy subjects. The highly complex adaptive motor activity as well as the considerable intersubject variability in the performance of higher-level control mechanisms was mentioned by authors of previous studies as potential explanations for this finding.
Indeed, as shown in previously published research, there is a certain variation in the normal deglutition processes, whichever parameters are utilized for the analysis [10]. Also, in much larger sEMG datasets no specific consistent swallowing pattern could be detected [28]. In fact, even individual swallowing patterns may vary within one consistency or volume [47].
Motivated by the study of Dodds et al. [48], who outlined that there are at least two different types of oral swallowing, the data collected here were analyzed in regard to different swallowing patterns. Three subgroups of test subjects were identified: those with the highest activities (1) in the orbicularis oris region, (2) in the masseter region, and (3) in the submental region. The existence of the fourth subgroup, those with the strongest sEMG signals in the paralaryngeal region, cannot be excluded and should be verified in a larger sample.
However, although the differences between subgroups were proven to be significant in a series of Kruskal–Wallis H tests, the influence of the factor “subgroups of test subjects” did not reach a significance level in a linear regression, which does not surprise if the very low sample size in each subgroup (Ns < 10) is taken into account.
Age of the test subjects did not influence the integral values significantly according to the linear regression. Obviously, the distribution of sEMG values does not vary much in different age groups except higher senior age, as has already been shown in the study of Vaiman et al. (2004), who could not detect a statistically significant difference between 4- and 12-year-old children (N = 100) and 18- and 30-year-old adults (N = 40) in the sEMG-recorded amplitude (range) of M. orbicularis oris, M. masseter, submental, and infrahyoid activities [49]. With the median age of 27 years, test subjects in the present study could have been too young for senior age-related swallowing dysfunctions identified in other studies. For instance, Wang et al. [50] demonstrated a delayed onset latency in healthy subjects aged 51–70 years compared to the age groups of 20–30 and 31–50 years. Also, concededly, the reason for the missing significant result on age could be traced back to a low sample size.
In contrast, the sex of the test subjects influenced significantly the results, with stronger sEMG signals in the male than in the female subgroup. However, again, because of small sample sizes in both subgroups (male, female), the result cannot be generalized. Findings of the previous studies regarding differences between healthy men and women in their sEMG activities are contradictory. Whereas most authors could not identify such differences irrespective of the study design [7, 51, 52], Moreno et al. [53] did find that men achieved a higher masseter activity at maximum effort than women and women achieved higher values for the digastric muscles in deglutition.
The low percentage of explained variance in the linear regression (6 %) demonstrated that some essential factors influencing the distribution of sEMG values were not included and are still to be identified. A high number of zero integral values also contributed to this low percentage.
All test subjects included in the study had no swallowing-related disorders or dysfunctions. Therefore, the study did not aim at the description of the sEMG patterns which might be characteristic of swallowing disorders.
Despite the interpersonal variance, the presented study demonstrated a systematically determined pattern of 16 electrode positions that ensures an accurate and reliable recording of swallowing-related sEMG signals. It should be viewed as an initial step in the development of empirically based recommendations on a comprehensive sEMG recording of the oropharyngeal swallowing, although a larger sample size balanced for age and sex distribution would be recommendable for further studies. In addition, further examination of intrapersonal variability of sEMG signals would be of interest [38]. Also, further work is required to link the recorded sEMG signals to the crucial biomechanical functions of oropharyngeal swallowing by simultaneously recorded videofluoroscopy study.
References
Doty RW, Bosma JF (1956) An electromyographic analysis of reflex deglutition. J Neurophysiol 19(1):44–60
Palmer PM, Luschei ES, Jaffe D, McCulloch TM (1999) Contributions of individual muscles to the submental surface electromyogram during swallowing. J Speech Lang Hear Res 42(6):1378–1391
Palmer JB, Rudin NJ, Lara G, Crompton AW (1992) Coordination of mastication and swallowing. Dysphagia 7(4):187–200
Crary MA, Carnaby Mann GD, Groher ME (2006) Biomechanical correlates of surface electromyography signals obtained during swallowing by healthy adults. J Speech Lang Hear Res 49(1):186–193
Koole P, de Jongh HJ, Boering G (1991) A comparative study of electromyograms of the masseter, temporalis, and anterior digastric muscles obtained by surface and intramuscular electrodes: raw-EMG. Cranio 9(3):228–240
Suvinen TI, Malmberg J, Forster C, Kemppainen P (2009) Postural and dynamic masseter and anterior temporalis muscle EMG repeatability in serial assessments. J Oral Rehabil 36(11):814–820
Vaiman M, Eviatar E, Segal S (2004) Evaluation of normal deglutition with the help of rectified surface electromyography records. Dysphagia 19(2):125–132
Vaiman M, Eviatar E, Segal S (2004) Surface electromyographic studies of swallowing in normal subjects: a review of 440 adults. Report 1. Quantitative data: timing measures. Otolaryngol Head Neck Surg 131(4):548–555
Vaiman M, Eviatar E, Segal S (2004) Surface electromyographic studies of swallowing in normal subjects: a review of 440 adults. Report 2. Quantitative data: amplitude measures. Otolaryngol Head Neck Surg 131(5):773–780
Vaiman M, Eviatar E, Segal S (2004) Surface electromyographic studies of swallowing in normal subjects: a review of 440 adults. Report 3. Qualitative data. Otolaryngol Head Neck Surg 131(6):977–985
McKeown MJ, Torpey DC, Gehm WC (2002) Non-invasive monitoring of functionally distinct muscle activations during swallowing. Clin Neurophysiol 113(3):354–366
Steele CM, Bennett JW, Chapman-Jay S, Cliffe Polacco R, Molfenter SM, Oshalla M (2011) Electromyography as a biofeedback tool for rehabilitating swallowing muscle function. In: Steele CM (ed) Applications of EMG in clinical and sports medicine. InTech, Rijeka, pp 311–328
Huckabee ML, Steele CM (2006) An analysis of lingual contribution to submental surface electromyographic measures and pharyngeal pressure during effortful swallow. Arch Phys Med Rehabil 87(8):1067–1072
Steele CM, Huckabee ML (2007) The influence of orolingual pressure on the timing of pharyngeal pressure events. Dysphagia 22(1):30–36
Huckabee ML, Butler SG, Barclay M, Jit S (2005) Submental surface electromyographic measurement and pharyngeal pressures during normal and effortful swallowing. Arch Phys Med Rehabil 86(11):2144–2149
Wheeler-Hegland KM, Rosenbek JC, Sapienza CM (2008) Submental sEMG and hyoid movement during Mendelsohn maneuver, effortful swallow, and expiratory muscle strength training. J Speech Lang Hear Res 51(5):1072–1087
Stepp CE (2012) Surface electromyography for speech and swallowing systems: measurement, analysis, and interpretation. J Speech Lang Hear Res 55(4):1232–1246
De Luca CJ (1997) The use of surface electromyography in biomechanics. J of Appl Biomech 13:135–163
Hermens HJ, Freriks B, Merletti R, Hägg GG, Stegeman D, Blok J, Rau G, Disselhorst-Klug C, Hägg G (1999) European recommendations for surface electromyography. Results of the SENIAM project. Roessingh Research and Development, Enschede. CD-ROM
Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G (2000) Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10(5):361–374
De Luca CJ, Gilmore LD, Kuznetsov M, Roy SH (2010) Filtering the surface EMG signal: movement artifact and baseline noise contamination. J Biomech 43(8):1573–1579
Hugger S, Schindler HJ, Kordass B, Hugger A (2012) Clinical relevance of surface EMG of the masticatory muscles. (Part 1): resting activity, maximal and submaximal voluntary contraction, symmetry of EMG activity. Int J Comput Dent 15(4):297–314
Ko EW, Teng TT, Huang CS, Chen YR (2015) The effect of early physiotherapy on the recovery of mandibular function after orthognathic surgery for class III correction. Part II: electromyographic activity of masticatory muscles. J Craniomaxillofac Surg 43(1):138–143
van den Engel-Hoek L, de Groot IJ, Esser E, Gorissen B, Hendriks JC, de Swart BJ, Geurts AC (2012) Biomechanical events of swallowing are determined more by bolus consistency than by age or gender. Physiol Behav 106(2):285–290
Reyes A, Cruickshank T, Thompson J, Ziman M, Nosaka K (2014) Surface electromyograph activity of submental muscles during swallowing and expiratory muscle training tasks in Huntington’s disease patients. J Electromyogr Kinesiol 24(1):153–158
Watts CR (2013) Measurement of hyolaryngeal muscle activation using surface electromyography for comparison of two rehabilitative dysphagia exercises. Arch Phys Med Rehabil 94(12):2542–2548
Crary MA (1995) A direct intervention program for chronic neurogenic dysphagia secondary to brainstem stroke. Dysphagia 10(1):6–18
Monaco A, Cattaneo R, Spadaro A, Giannoni M (2008) Surface electromyography pattern of human swallowing. BMC Oral Health 26(8):6
Nagy A, Steele CM, Pelletier CA (2014) Barium versus nonbarium stimuli: differences in taste intensity, chemesthesis, and swallowing behavior in healthy adult women. J Speech Lang Hear Res 57(3):758–767
Vaiman M, Nahlieli O (2009) Oral vs. pharyngeal dysphagia: surface electromyography randomized study. BMC Ear Nose Throat Disord 9:3
Maria das Coriolano WS, Belo LR, Carneiro D, Asano AG, Al Oliveira PJ, da Silva DM, Lins OG (2012) Swallowing in patients with Parkinson’s disease: a surface electromyography study. Dysphagia 27(4):550–555
Crary MA, Carnaby Mann GD, Groher ME, Helseth E (2004) Functional benefits of dysphagia therapy using adjunctive sEMG biofeedback. Dysphagia 19(3):160–164
Van Houtte E, Claeys S, D’haeseleer E, Wuyts F, Van Lierde K (2013) An examination of surface EMG for the assessment of muscle tension dysphonia. J Voice 27(2):177–186
Lapatki BG, Van Dijk JP, Jonas IE, Zwarts MJ, Stegeman DF (2004) A thin, flexible multielectrode grid for high-density surface EMG. J Appl Physiol 96(1):327–336
Lapatki BG, Oostenveld R, Van Dijk JP, Jonas IE, Zwarts MJ, Stegeman DF (2006) Topographical characteristics of motor units of the lower facial musculature revealed by means of high-density surface EMG. J Neurophysiol 95(1):342–354
Lapatki BG, Oostenveld R, Van Dijk JP, Jonas IE, Zwarts MJ, Stegeman DF (2010) Optimal placement of bipolar surface EMG electrodes in the face based on single motor unit analysis. Psychophysiology 47(2):299–314
Loeb GE, Gans C (1986) Electromyography for experimentalists, 1st edn. The University of Chicago Press, Chicago
Macrae PR, Myall DJ, Jones RD, Huckabee ML (2011) Pharyngeal pressures during swallowing within and across three sessions: within-subject variance and order effects. Dysphagia 26(4):385–391
Hof AL (1984) EMG and muscle force: an introduction. Hum Mov Sci 3(1):119–153
Ertekin C, Pehlivan M, Aydoğdu I, Ertaş M, Uludağ B, Celebi G, Colakoğlu Z, Sağduyu A, Yüceyar N (1995) An electrophysiological investigation of deglutition in man. Muscle Nerve 18(10):1177–1186
Ding R, Larson CR, Logemann JA, Rademaker AW (2002) Surface electromyographic and electroglottographic studies in normal subjects under two swallow conditions: normal and during the Mendelsohn maneuver. Dysphagia 17(1):1–12
Tallgren A, Tryde G (1992) Swallowing activity of lip muscles in patients with a complete upper and a partial lower denture. J Oral Rehabil 19(4):329–341
Gay T, Rendell JK, Spiro J (1994) Oral and laryngeal muscle coordination during swallowing. Laryngoscope 104(3 Pt 1):341–349
Spiro J, Rendell JK, Gay T (1994) Activation and coordination patterns of the suprahyoid muscles during swallowing. Laryngoscope 104(11 Pt 1):1376–1382
Perlman AL, Palmer PM, McCulloch TM, Vandaele DJ (1999) Electromyographic activity from human laryngeal, pharyngeal, and submental muscles during swallowing. J Appl Physiol 86(5):1663–1669
Kendall K (2002) Oropharyngeal swallowing variability. Laryngoscope 112(3):547–551
Molfenter SM, Leigh C, Steele CM (2014) Event sequence variability in healthy swallowing: building on previous findings. Dysphagia 29(2):234–242
Dodds WJ, Taylor AJ, Stewart ET, Kern MK, Logemann J, Cook IJ (1989) Tipper and dipper types of oral swallows. AJR Am J Roentgenol 153(6):1197–1199
Vaiman M, Segal S, Eviatar E (2004) Surface electromyographic studies of swallowing in normal children, age 4–12 years. Int J Pediatr Otorhinolaryngol 68(1):65–73
Wang CM, Chen JY, Chuang CC, Tseng WC, Wong AM, Pei YC (2015) Aging-related changes in swallowing, and in the coordination of swallowing and respiration determined by novel non-invasive measurement techniques. Geriatr Gerontol Int 15(6):736–744
Sella O, Jones RD, Huckabee ML (2014) Age and gender effects on submental motor-evoked potentials. Age (Dordr) 36(6):9735
Vaiman M, Gabriel C, Eviatar E, Segal S (2005) Surface electromyography of continuous drinking in healthy adults. Laryngoscope 115(1):68–73
Moreno I, Sánchez T, Ardizone I, Aneiros F, Celemin A (2008) Electromyographic comparisons between clenching, swallowing and chewing in jaw muscles with varying occlusal parameters. Med Oral Patol Oral Cir Bucal 13(3):E207–E213
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial disclosure information
None.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Zaretsky, E., Pluschinski, P., Sader, R. et al. Identification of the most significant electrode positions in electromyographic evaluation of swallowing-related movements in humans. Eur Arch Otorhinolaryngol 274, 989–995 (2017). https://doi.org/10.1007/s00405-016-4288-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-016-4288-7