Abstract
The main objective of the meta-analysis was to investigate whether intratympanic steroid injections in combination with systemic steroids would provide an additional advantage over systemic steroid therapy (SST) alone in patients with idiopathic sudden sensorineural hearing loss (ISSNHL). The results will provide a meaningful suggestion in clinical therapy of ISSNHL. The electronic database search was based on the database in OVID Medline, Embase and PubMed up to December 15, 2015 with the goal of identifying all available observational studies examining the effects of combination therapy and SST in ISSNHL patients. Observational studies that compared the pure tone average (PTA) improvement and recovery rate between combination therapy and SST group in ISSNHL patients were selected. Finally we have identified eight eligible studies that focused on comparing the combination therapy and SST in ISSNHL from designated researches. In the PTA improvement group, seven studies have been analyzed to compare the pooled mean differences between two therapy modalities and subgroups based on initial hearing loss and treatment delay. In the recovery rate group, six studies were calculated for pooled risk ratios and subgroup analysis was also conducted. Through our meta-analysis, we have reached the conclusion that combination therapy exhibited better outcomes in PTA improvement than SST alone, especially in severe-profound initial hearing loss cases. Combination therapy also showed advantages in recovery rate. Whether time of treatment delay would influence the PTA improvement and recovery rate requires further researches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic sudden sensorineural hearing loss (ISSNHL) is widely identified as a hearing loss of greater than 30 dB in at least three contiguous frequencies that occur within 3 days [1, 2]. In general, it has an estimated incidence of 5–20 cases per 100,000 per year and the peak incidence occurs in patients in their 50–60s [3]. The etiologies and pathogenesis of the disease remain controversial over a long period of research. Many untreated patients who recovered hearing early did not seek further medical treatment. This might partly explain the spontaneous recovery rate ranging from 32 to 65 % [4]. In addition, the prognosis of the disease has also been associated with various factors. Especially, profound hearing loss [5], vestibular symptoms, prolonged time from onset to treatment [6], and down-sloping audiogram [7] are considered as negative prognostic variables.
Since early treatment is recommended, suitable therapeutic approaches were introduced including steroids, antiviral drugs, anticoagulants, antioxidants, vasoactive agents and hyperbaric oxygen [7, 8]. For now, most of appropriate therapeutic modalities for ISSNHL remains controversial. This has led to a great number of researches and clinical trials initiated for identifying the reason, and yet no standard protocols have been universally determined. In 1980, Wilson et al. first introduced systemic steroids as the most effective and commonly accepted treatment for ISSNHL [9]. Steroids can be administrated orally, intravenously or locally such as intratympanic injection into middle ear in combination with, or without other agents. However, there were still 30–50 % patients who responded poorly to steroid therapy [10, 11]. Besides, it was also identified that high-dose administration of systemic steroids might raise the risk of unpleasant side effects, such as endocrine problems, hypertension, avascular necrosis of the femur and osteoporosis [12, 13].
Taking contraindication into consideration, in certain pathological situations, an ameliorated treatment, intratympanic steroid treatment (ITS), was developed and introduced by some otolaryngologists [14–16]. It delivers steroid to the cochlea via the round window membrane and thus provides a higher concentration of steroid in the cochlea. Comparing with systemic steroids, it had advantages in reducing systemic steroid toxicity during long-term application. In addition, pharmacokinetic animal experiments also observed a much higher perilymphatic concentrations of corticosteroids with intratympanic application compared with oral or intravenous administration [15]. Due to these two key advantages of intratympanic steroids, their popularity has increased. Besides, intratympanic steroids were also used as salvage therapy in refractory cases of ISSNHL. The promising results have made some authors to promote their use as first-line therapeutic option in patients with contraindication to systemic steroids [17, 18]. Additionally, intratympanic steroid administration has been applied as an adjunctive treatment given concomitantly with systemic steroids. However, the efficacy of combination therapy for ISSNHL remains controversial in reports. Some researchers showed that combination therapy had the potential to promote pure tone average (PTA) improvement and recovery rate compared with systemic steroids alone [4, 19, 20], while others did not [21]. The difference of results needs to be analyzed and summarized to provide more convincing outcomes for the effects of combination therapy.
Therefore, the main objective of the meta-analysis was to investigate whether ITS in combination with systemic steroids would provide an additional advantage over systemic steroids alone in patients with ISSNHL. Moreover, this analysis could ultimately provide some meaningful suggestions in clinical therapy of ISSNHL.
Methods
Literature search strategy
The electronic database search was based on the database in OVID Medline, Embase and PubMed up to December 15, 2015, to identify all available observational studies focusing on comparing the effects of combination therapy and systemic steroid therapy (SST) in ISSNHL. Literature and studies were searched using a random combination of the following keywords: ‘sudden deafness’ or ‘sudden sensorineural hearing loss’ or ‘sudden sensorineural deafness’ or ‘idiopathic sudden sensorineural hearing loss’, ‘systemic steroid’, ‘intratympanic steroid’ and ‘combined intratympanic and systemic steroid’. The search used clinical trials as publication filter and was limited to human being and the English language. All articles were de-identified (blinded title, authors, journal and year of publication) before selection. The titles and abstracts of potential references were manually examined to exclude irrelevant publications. The bibliographies were also examined for relevant articles to identify more eligible studies. Two reviewers (Y.G. and D.L.) conducted the literature search process independently.
Eligibility criteria
The studies included in this meta-analysis were all observational studies (including prospective and retrospective studies) that compared PTA improvement and recovery rate between combination therapy and SST groups. Eligible studies should meet the following criteria: (a) valid proven diagnosis of ISSNHL; (b) combination group represented that combined ITS and SST in the same time without other adjuvant therapy; (c) studies that included ISSNHL patients who had undergone combined therapy or SST from the beginning; (d) PTA improvement based on the average PTA measured by the researchers; (e) full recovery referred to those patients with complete recovery or good recovery.
Articles were excluded with the following criteria: (1) review articles or letters; (2) patients with chronic otitis media, trauma, previous radiotherapy or chemotherapy, recent use of ototoxic drugs, liver or renal dysfunction, retro-cochlear lesion; (3) studies did not evaluate effects of combination therapy or SST as primary treatment for ISSNHL; (4) insufficient data for presenting PTA improvement or recovery rate.
Data extraction
All the eligible articles, including the titles and abstracts were read by two reviewers (Y.G. and D.L) independently to exclude irrelevant publications. Then, the full texts of the extracted articles were carefully examined for comprehensive evaluation. Moreover, when multiple studies contained overlapping data, the one with the largest data set or newest data was chosen. In addition, the references of extracted articles were also manually searched to avoid missing relevant studies. If the full text was unavailable, we contacted the authors for the data required for the meta-analysis. Finally, we have introduced two main groups by the aims of the study. One group clarified the hearing outcomes (PTA improvement) before and after combination therapy or SST in ISSNHL patients while the other group clarified the recovery rate. All data from eligible studies are extracted to Table 1, including number of patients, ISSNHL definition and details, combination therapy and systemic therapy protocols and study design. Patient demographics and initial audiological results of eligible studies are extracted to Table 2 in the meantime.
Assessment of risk of bias and quality ranking
Two reviewers (Y.G. and D.L.) rated the quality of retrieved studies with Cochrane’s collaboration tool independently. Based on the Cochrane Handbook 5.1 [32], we have assessed the risk of bias from included studies for the following domains: random sequence generation (selection bias), allocation concealment (selection bias), binding of participants and personnel (performance bias), binding of outcome assessors (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other bias. The risk of bias was graphically demonstrated and summarized via methodological quality graph and quality summary. For quality ranking, two reviewers rated included studies separately by Jadad score (Table 1) and a score ≥3 was regarded as a high-quality study [22].
Statistical methods of meta-analysis
Meta-analysis was then conducted regarding the PTA improvements in combined therapy group and SST group, and the recovery rate in two groups (complete recovery). The mean differences (MDs) and 95 % confidential intervals (CIs) were estimated for PTA improvement, and pooled risk ratios (RRs) and 95 % CI were estimated for recovery rate in the two design groups. Cochrane’s I 2 index was calculated to assess heterogeneity. If the data were not significant, the MDs and RRs were pooled via the fixed-effects model. Otherwise, the random-effects model was used.
We used the software RevMan 5.3 for data analysis and graph making in this meta-analysis. The statistical process was performed according to the guidelines proposed by the Meta-Analysis of Observational Studies in Epidemiology groups. Forest plots were used to estimate the MDs and 95 % CI of hearing outcomes and RRs and 95 % CI of recovering rate in designed groups. Subgroup analysis was also designed for estimating variation of study design, severity of initial hearing loss and treatment delay. Begg’s funnel plots and Egger’s tests were carried out to control the potential publication bias. P values <0.05 were considered statistically significant. All P values above are two tailed.
Results
Search results
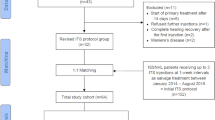
After initial review of the titles, abstracts and duplicates removed, we included 336 articles related to steroid therapy in ISSNHL patients by electronic and manual searching. Through a further detailed review of full articles and data, 244 studies were excluded for not meeting the criteria and then 84 full-text articles were excluded, including 65 studies on salvage treatment or no initial combination therapy, 12 insufficient data studies and 7 non-clinical studies. Finally, we identified eight eligible studies (including seven prospective [4, 19–21, 23–25] and one retrospective study [26]) focusing on the comparison of combination therapy and SST in ISSNHL. Based on the objectives of evaluating the outcomes of different therapy modalities in ISSNHL, they were mainly divided into two groups: [4, 19–21, 23–25] have been designed to focus on PTA improvement while [4, 19–21, 23, 26] have been clustered to address the difference in recovery rates. All eligible studies scored highly in Jadad scale tests (Table 1). The flowchart of the article selection is shown in Fig. 1.
Characteristics of eligible studies
The characteristics of eligible studies are extracted to Tables 1 and 2 separately. Table 1 mainly represents the criteria for diagnosis, protocols in combined therapy and steroid therapy alone. Table 2 is mainly listed with the personalized clinical characteristic in each study, in which severity of initial disease and duration from onset to treatment were also included.
Hearing outcomes in combination therapy and SST groups
There were seven clinical trials that compared the hearing outcomes of combination therapy and SST from date of therapy to end point. Namely, PTA improvement was calculated based on the PTA assessment before and after treatment as displayed in Table 3. Pooled MDs and 95 % CIs are depicted using forest plots in Fig. 2a. Since there was no significant heterogeneity identified during the meta-analysis according to I 2 statistics described above, the fixed-effects model was used (I 2 = 45 %, P = 0.09). Overall, the total model showed that combined therapy indeed provided a better outcome than SST (MD 12.47, 95 % CI 9.25–15.70, P < 0.00001).
Base on the hypothesis that severity of initial hearing loss and duration of onset to treatment would probably influence the therapeutic effects, we have also designed the subgroup analysis based on the two factors. As shown in Fig. 3a, there were five eligible studies in the mild-moderate subgroup and three in the severe-profound subgroup (the criteria of initial hearing loss severity is based on whether the initial PTA <70 dB). These results illustrated that both groups showed no significant heterogeneity (I 2 = 0 %, P = 0.97 and I 2 = 0 %, P = 0.88) while the main difference between subgroups came from the PTA improvements under chosen therapy modalities (mild–moderate MD 7.99, 95 % CI 3.91–12.07; severe–profound MD 22.34, 95 % CI 15.93–28.76; P = 0.0002). On the other hand, no significant heterogeneity was discovered in time delay subgroups either (Fig. 3b). The two subgroups did not show obvious difference in the outcomes (time interval ≤7 days MD 13.14, 95 % CI 9.16–17.11; time interval >7 days MD 10.87, 95 % CI 4.28–17.47; P = 0.56). Therefore, we have initially concluded that in PTA improvement, combination therapy could elevate hearing outcomes better than SST alone to a certain extent. It may serve as a more effective modality for primary ISSNHL treatment, especially in severe–profound initial hearing loss cases.
Recovery rate between combination therapy and SST groups
We analyzed six studies focused on the recovery rate under combination therapy or SST in primary ISSNHL treatment. According to Siegel’s criteria, the recovery outcomes have been divided into four types: complete recovery (CR), partial recovery (PR), slight improvement (SI) and no recovery (NR). Due to the fact that in most studies, we only had access to CR and NR data collection and the criteria for PR and SI varied between studies, we initially determined the recovery rate calculated in our analysis would be based on CR and NR for the moment. The details for recovery rate in eligible studies are displayed in Table 4.
There was also no obvious heterogeneity discovered and the fixed-effects model was chosen (I 2 = 22 % P = 0.27). Total pooled RRs and 95 % CIs are exhibited in Fig. 2b (RR 1.49, 95 % CI 1.19–1.87, P = 0.0006). To address more valid outcomes, subgroup analysis with respect to design variables, initial severity of hearing loss and treatment delay time was performed and is exhibited in Fig. 4a–c. The results demonstrated that in both prospective group (RR 1.50, 95 % CI 1.20–1.89, P = 0.0005) and retrospective group (RR 1.17, 95 % CI 0.26–5.26), combination therapy provided a better prognosis with better recovery rate than SST in ISSNHL patients. Insignificant difference between the two subgroups demonstrated the design method did not affect the analysis results to the best of our knowledge (P = 0.75). Indeed, both in mild–moderate and severe–profound groups, combined therapy presented a better recovery rate than SST alone (RR 1.40, 95 % CI 1.12–1.76, P = 0.003 and RR 3.47, 95 % CI 1.30–9.28, P = 0.01, respectively) (Fig. 4b). Similar results were observed in the treatment time delay subgroup, with time interval ≤7 days (RR 1.53, 95 % CI 1.11–2.12, P = 0.009) and time interval >7 days (RR 1.41, 95 % CI 1.03–1.92, P = 0.03) (Fig. 4c). However, neither of these inter-subgroup differences were significant (P = 0.08 and 0.70, respectively). Taken all into consideration, we had the confidence to address that combination therapy had advantages beyond SST alone, including in subgroup analysis based on initial hearing loss severity and treatment delay time. Heterogeneity was insignificant in all subgroups.
Risk of bias in included studies
The risk of bias of the included studies has been graphically demonstrated and summarized (Fig. 5a, b). All of the included studies have kept to random sequence generation except Battaglia et al. [4] and Günel et al. [26] which did not display whether the study followed the randomization rules. There were two studies [20, 21] which described the allocation concealment clearly while another six studies did not provide details. For participant and personnel binding, three studies [4, 19, 20] followed the rules and described their binding clearly, while other four studies [21, 23, 24, 26] did not provide detailed descriptions. There was one study [25] which did not follow the binding. In outcome assessment binding, apart from one study [25], there were two studies [4, 21] that performed blind assessment while other five studies [19, 20, 23, 24, 26] did not report their binding. Additionally, there were no incomplete outcome assessment bias, selective reporting bias and other bias existed in all of our included studies.
Publication bias
Overall, to address the potential publication bias that might be introduced during the meta-analysis, Egger’s tests and Begg’s funnel plots were utilized as control. Publication bias was not identified by either Egger’s tests (Table 5) or Begg’s funnel plots (Fig. 6a, b) including the subgroup analysis (Fig. 6c–g), which indicated the absence of publication bias in our meta-analysis results.
Funnel plots of the meta-analysis of PTA improvement and recovery rate. a PTA improvement; b complete recovery rate; c severity of hearing loss subgroup in PTA improvement; d treatment delay time subgroup in PTA improvement; e study design subgroup in recovery rate; f severity of initial hearing loss subgroup in recovery rate; g treatment delay time subgroup in recovery rate
Discussion
Recently, the choice of ISSNHL therapy method remains under-challenge in clinical settings. In general, most of sensorineural hearing loss is considered to be idiopathic. Among the introduced therapy modalities, systemic therapy developed by Wilson et al. is still considered to be one of the most effective therapy methods in ISSNHL treatment [9]. However, considering its side effects of suppression of immune response, improvement of decreased microvascular circulation, mineral corticoid effects, or decrease in endolymphatic pressure, ITS therapy has been gradually administered to such non-responding patients or severe side effects patients as primary or salvage treatment. After it was first introduced by Silverstein et al. to employ treatment for ISSNHL patients [27], ITS therapy has shown its efficiency with direct delivery of a high concentration of steroid to the inner ear. For now, to seek for its better performance, it has always been administered with combination of SST. In randomized controlled studies performed by Gundogan et al., it was proven that combination therapy exhibited greater efficiency than SST alone, in hearing improvement [25] and similar to Gundogan’s findings, Battaglia et al. and Koltsidopoulos et al. also demonstrated that combination therapy showed higher hearing improvement in PTA and a greater likelihood in hearing recovery compared with SST alone [4, 20]. However, some other researchers illustrated that combination therapy did not show significant improvements, such as Lim et al. [21].
As known to all, based on the PTA level during pre-treatment, patients could be divided into four groups with various severities of initial hearing loss: mild, moderate, severe and profound [28]. In this aspect, Koltsidopoulos et al. have demonstrated that combination therapy could be a beneficial therapeutic option for patients with mild–severe SSNHL [20]. On the other hand, in 2014 Kim et al. proved that combination therapy has potentially greater efficiency than SST in profound ISSNHL patients [29]. So subgroup analysis based on severity of initial hearing loss was considered to be necessary in this analysis. In addition, previous researches have also indicated that the date from disease onset to treatment has great impact on the PTA outcomes of ISSNHL treatment as well as the prognosis of disease. For instance, Battaglia et al. illustrated the effects of treatment delay on hearing outcomes [23]. To address this hypothesis, we also conducted subgroup meta-analysis focusing on the potential effects of treatment delay time on PTA improvement outcome and recovery rate in ISSNHL patients.
Overall, the main aim of our meta-analysis was to pool the effects of comparing combination therapy and SST in hearing outcomes of PTA and recovery rate. Moreover, due to the controversial opinions on the severity of hearing loss and the delay of treatment’s effects in combination therapy versus systemic therapy, we have also conducted subgroup analysis based on two objectives above. In addition, since we have included seven prospective studies and one retrospective study, the subgroup analysis was also necessary to clarify whether design variables would affect the outcomes.
The results of our meta-analysis revealed that with a combination of ITS therapy, ISSNHL patients could benefit more with steroid administration. Consistent with the achievements of Battaglia et al. and Kim et al. [23, 29], our meta-analysis also suggested that combination therapy would benefit severe–profound ISSNHL patients more after pooled eligible studies. In this case, we have the confidence to justify that combination therapy in severe–profound patients is more effective than it in mild–moderate patients for hearing outcomes, but not recovery rate (Figs. 3a, 4b). Therefore, in consideration of the well-known side effects of systemic therapy, combination with ITS therapy might serve as an alternative modality for seeking better outcomes.
In the subgroup meta-analysis of time delay effects, no matter whether the delay of treatment was within or outside of 7 days, the difference was insignificant according to our analysis results (P = 0.56 for PTA improvement and P = 0.70 for recovery rate). Therefore, this result indicated that combination therapy might be a potentially more beneficial treatment modality for ISSNHL patients, regardless of the time delay effects. Even though several previous results [23], demonstrated that combination therapy benefited more than SST alone within 7 days, our analysis results did not exhibit this tendency. This result might be due to the small amount of studies involved in the analysis. Though no significant inter-subgroup difference was indicated in time interval ≤7 and >7 days subgroups, we still have the confidence to further confirm that combination therapy behaves better than SST alone.
From previous studies’ conclusion, salvage treatment with ITS after systemic therapy failed to respond demonstrated significant intratympanic steroid effects [30]. However, in this case, there were not enough detailed designed comparison studies resolving the argument of combination therapy in salvage ITS therapies. Therefore, no valid analysis could be established so far. More importantly, our arguments provided a proper evidence to suggest that combination therapy benefits more than SST alone in ISSNHL patients, filling the gap of evidence with other agent combination, including antivirals, thrombolytics, antioxidants, or vasodilators from the recommendation of the 2012 AAO-HNS clinical practice guidelines [31].
Although several researchers have previously conducted analysis on ISSHNL therapy topics, ours is still the first meta-analysis for comparison of combination therapy and SST, including subgroup analysis performed for severity of initial hearing loss and delay of treatment effects. The conclusion from our meta-analysis is shown as follows: first, to the extent of our study, combination therapy produced better hearing performance in PTA improvements than SST in ISSNHL patients. Second, in the aspect of hearing recovery, combination therapy also acted better than SST with a significant higher recovery rate, regardless of the initial hearing loss severity and treatment delay time. Third, severe to profound hearing loss reflected better response in combination therapy than SST compared with mild to moderate types. Lastly, although delay to treatment variation was not showing a significant difference in PTA improvement and recovery rate, we still believe that earlier treatment with combination therapy would benefit the most. We will continue to collect related data for delay of treatment with two treatment modalities in the future for comparison.
There were still some limitations underlying our meta-analysis. First, the eligible studies in our study were limited due to combination therapy not widely being introduced, thus limited studies were performed comparing the superiority of treatment modalities. Secondly, we did not only take randomized controlled trials into our meta-analysis. It is known to all that introduction of non-randomized controlled trials would possibly introduce publication bias. However, to the best of our knowledge, due to limited amount of clinical trials comparing the combination therapy and SST, these were all the eligible studies we could obtain so far. Besides, according to our results, no significant publication bias was shown. Even after excluding the non-randomized clinical trials, our meta-analysis results were not affected. This further revealed that our results were trustworthy. Third, for the lack of complication of some trials, it was beyond our capacity to conduct an analysis based on it to provide meaningful advice for patients with variable complications, such as diabetes. However, we have the faith that the meta-analysis on ISSNHL therapy with different complications could be accomplished as soon as enough trials and data could be obtained in the future.
Conclusions
Through our meta-analysis on comparing combination therapy and SST alone in treatment of ISSNHL patients, we came up with the conclusion that combination therapy exhibited better outcomes in PTA improvement than SST alone, especially in severe–profound initial hearing loss cases. Combination therapy also showed advantages in recovery rate. Whether time of treatment delay would influence the PTA improvement and recovery rate still requires further research.
References
Xenellis J et al (2006) Idiopathic sudden sensorineural hearing loss: prognostic factors. J Laryngol Otol 120(9):718–724
Chau JK et al (2010) Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope 120(5):1011–1021
Korpinar S et al (2011) Factors influencing the outcome of idiopathic sudden sensorineural hearing loss treated with hyperbaric oxygen therapy. Eur Arch Otorhinolaryngol 268(1):41–47
Battaglia A, Burchette R, Cueva R (2008) Combination therapy (intratympanic dexamethasone + high-dose prednisone taper) for the treatment of idiopathic sudden sensorineural hearing loss. Otol Neurotol 29(4):453–460
Edizer DT et al (2015) Recovery of idiopathic sudden sensorineural hearing loss. J Int Adv Otol 11(2):122–126
Giannoni B, Pagnini P, Vannucchi P (1998) Delayed endolymphatic hydrops. Acta Otorhinolaryngol Ital 18(4 Suppl 59):66–70
Filipo R et al (2014) Oral versus short-term intratympanic prednisolone therapy for idiopathic sudden hearing loss. Audiol Neurootol 19(4):225–233
Castro Junior NP, Almeida CI, Campos CA (2007) Sudden sensorineural hearing loss and vertigo associated with arterial occlusive disease: three case reports and literature review. Sao Paulo Med J 125(3):191–195
Wilson WR, Byl FM, Laird N (1980) The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch Otolaryngol 106(12):772–776
Gianoli GJ, Li JC (2001) Transtympanic steroids for treatment of sudden hearing loss. Otolaryngol Head Neck Surg 125(3):142–146
Hunchaisri N, Chantapant S, Srinangyam N (2010) Intratympanic dexamethasone for refractory sudden sensorineural hearing loss. J Med Assoc Thail 93(12):1406–1414
Vidal CA et al (2006) Avascular necrosis of both hips and knees in a patient with ulcerative colitis treated for a long term with high-dose corticosteroids. Nutr Hosp 21(1):109–112
Alexander TH et al (2009) Safety of high-dose corticosteroids for the treatment of autoimmune inner ear disease. Otol Neurotol 30(4):443–448
Plontke SK et al (2009) Randomized, double blind, placebo controlled trial on the safety and efficacy of continuous intratympanic dexamethasone delivered via a round window catheter for severe to profound sudden idiopathic sensorineural hearing loss after failure of systemic therapy. Laryngoscope 119(2):359–369
Fu Y et al (2011) Intratympanic dexamethasone as initial therapy for idiopathic sudden sensorineural hearing loss: clinical evaluation and laboratory investigation. Auris Nasus Larynx 38(2):165–171
Lavigne P, Lavigne F, Saliba I (2015) Intratympanic corticosteroids injections: a systematic review of literature. Eur Arch Otorhinolaryngol 1–8. doi: 10.1007/s00405-015-3689-3
Seggas I et al (2011) Intratympanic steroid therapy for sudden hearing loss: a review of the literature. Otol Neurotol 32(1):29–35
Erdur O, Kayhan FT, Cirik AA (2014) Effectiveness of intratympanic dexamethasone for refractory sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol 271(6):1431–1436
Arastou S, Tajedini A, Borghei P (2013) Combined intratympanic and systemic steroid therapy for poor-prognosis sudden sensorineural hearing loss. Iran J Otorhinolaryngol 25(70):23–28
Koltsidopoulos P et al (2013) Intratympanic and systemic steroids for sudden hearing loss. Otol Neurotol 34(4):771–776
Lim HJ et al (2013) Efficacy of 3 different steroid treatments for sudden sensorineural hearing loss: a prospective, randomized trial. Otolaryngol Head Neck Surg 148(1):121–127
Jadad AR et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12
Battaglia A et al (2014) A prospective, multi-centered study of the treatment of idiopathic sudden sensorineural hearing loss with combination therapy versus high-dose prednisone alone: a 139 patient follow-up. Otol Neurotol 35(6):1091–1098
Arslan N et al (2011) Combined intratympanic and systemic use of steroids for idiopathic sudden sensorineural hearing loss. Otol Neurotol 32(3):393–397
Gundogan O et al (2013) Therapeutic efficacy of the combination of intratympanic methylprednisolone and oral steroid for idiopathic sudden deafness. Otolaryngol Head Neck Surg 149(5):753–758
Gunel C et al (2015) Efficacy of low-dose intratympanic dexamethasone for sudden hearing loss. Auris Nasus Larynx 42(4):284–287
Silverstein H et al (1996) Intratympanic steroid treatment of inner ear disease and tinnitus (preliminary report). Ear Nose Throat J 75(8):468–71, 474, 476 passim
Siegel LG (1975) The treatment of idiopathic sudden sensorineural hearing loss. Otolaryngol Clin North Am 8(2):467–473
Kim SH et al (2015) Comparison of steroid administration methods in patients with idiopathic sudden sensorineural hearing loss: a retrospective observational study. Clin Otolaryngol 40(3):183–190
Garavello W et al (2012) Intratympanic steroid treatment for sudden deafness: a meta-analysis of randomized controlled trials. Otol Neurotol 33(5):724–729
Stachler RJ et al (2012) Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg 146(3 Suppl):S1–S35
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Gao, Y., Liu, D. Combined intratympanic and systemic use of steroids for idiopathic sudden sensorineural hearing loss: a meta-analysis. Eur Arch Otorhinolaryngol 273, 3699–3711 (2016). https://doi.org/10.1007/s00405-016-4041-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-016-4041-2