Abstract
Cis-diammineedichloroplatinum (cisplatin) is a chemotherapeutic agent that is widely used in the treatment of many cancers. Nephrotoxicity, ototoxicity and neurotoxicity are dose-limiting adverse effects for cisplatin. The cellular and molecular mechanisms underlying cisplatin-induced ototoxicity aren’t fully understood. It has been proposed that cisplatin primarily cause damage at the cochlea, outer hair cells in particular, leading to excessive production of free oxygen radicals in the organ of Corti, stria vascularis, spiral ligament, and spiral ganglionic cells. The cytotoxicity is associated with the generation of reactive oxygen species (ROS); thus, there is an increasing interest on antioxidants with an effort to discover the established protection against cisplatin-induced ototoxicity over time. Misoprostol (MP) has gained considerable interest as a reactive oxygen species scavenger in recent years. To best of our knowledge, there is no study about protective effect of MP, a prostaglandin E1 (PGE1) analogue, on cisplatin-induced ototoxicity. In our study, we show that protective effects of misoprostol on cisplatin-induced ototoxcity on rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cis-diammineedichloroplatinum (cisplatin) is a chemotherapeutic agent that is widely used in the treatment of many cancers. Nephrotoxicity, ototoxicity and neurotoxicity are dose-limiting adverse effects for cisplatin [1, 2]. Nephrotoxicity can be prevented by increased hydration and forced diuresis; however, no cure or preventive treatment is currently available for ototoxicity. In previous studies, it was reported that hearing thresholds can be elevated in up to 75–100 % of patients. The rate of this adverse effect is even higher in children [2, 3]. The cellular and molecular mechanisms underlying cisplatin-induced ototoxicity are not fully understood. It has been proposed that cisplatin primarily cause damage at the cochlea, outer hair cells in particular, leading to excessive production of free oxygen radicals in the organ of corti, stria vascularis, spiral ligament, and spiral ganglionic cells [4, 5]. It is believed that primary cytotoxic effects of cisplatin are mediated by the monohydrated cisplatin complex, which reacts with nuclear DNA to form platinum–DNA adducts [6]. The cytotoxicity is associated with the generation of reactive oxygen species (ROS); thus, there is an increasing interest on antioxidants with an effort to discover the established protection against cisplatin-induced ototoxicity over time [7]. To date, there is no FDA-approved product that is proven to be effective in preventing or reducing cisplatin ototoxicity [8]. Misoprostol (MP) has gained considerable interest as a reactive oxygen species scavenger in recent years [9]. In addition to antioxidant properties such as anti-apoptotic or cytoprotective effects, it also has additional properties [10, 11].
To best of our knowledge, there is no study about protective effect of MP, a prostaglandin E1 (PGE1) analogue, on cisplatin-induced ototoxicity. Therefore, we designed this study in rats to investigate the effects of MP on CP-induced histopathological changes and auditory parameters including auditory brainstem response (ABR) and distortion product otoacoustic emission (DPAOE). This is the first study demonstrating attenuating effects of misoprostol against cisplatin ototoxicity in a rat model as indicated by DPOAEs.
Objective Ototoxicity is one of the most important adverse effects that limit use aminoglycoside antibiotics. In this study, it was aimed to investigate the potential protective role of misoprostol against cisplatin by electrophysiological tests (auditory brainstem response [ABR] and distortion product otoacoustic emission [DPOAE]) and histopathology.
Material and method
This experimental study was approved by Ethics Committee on Animal Studies of Erciyes University, Medicine School (date: 12.03.2014; #14/039). It is conducted at Hakan Çetinsaya Experimental and Clinical Research Center of Erciyes University, Medicine School.
Animal preparation and experimental procedure
All rats underwent otoscopic examination (Zeiss S1, Germany). Emission and normal hearing were assessed by DPOAE and ABR measurements. The study included 80 ears of 40 rats with normal hearing threshold and DPOAE value. The rats (n = 40) were randomized into 4 groups as follows:
Group I (n = 10) Cisplatin (14 mg/kg; Cisplatin-Teva, 50 mg vial, MED ILAC, Turkey) was given via intraperitoneal route,
Group II (n = 10) Cisplatin (14 mg/kg) plus misoprostol (100 mcg/kg; Sigma Aldrich Chemical Co, St. Lousi, Mo, USA) was given via intraperitoneal route,
Group III (n = 10) Ethanol 20 % (1 cc) was given via intraperitoneal route,
Group IV (n = 10) Misoprostol (100 mcg/kg; Sigma Aldrich Chemical Co, St. Lousi, Mo, USA was given via intraperitoneal route,
All drugs used in the study were given once daily for 14 days.
In all animals, sedation was achieved by using combination of ketamine hydrochloride (80 mg/kg, i.p., Ketalar, Eczacibaşı, Turkey) and xylazine (10 mg/kg, i.p.; Rompun, Bayer, Germany). After anesthesia, external auditory canal and tympanic membrane was evaluated in 40 rats by using an otoscope (Riester 2101, Germany) with an appropriate speculum in place. Cerumen was removed from external auditory canal. No rat with abnormal auditory canal or middle ear was detected. In all rats, DPOAE and ABR measurements were performed in both ears. Then, animals were assigned into four groups. After 7-days drug exposure, otoscopic examination was repeated under general anesthesia and three rats with pathology were excluded. Again, three rats died during study period. Thus, DPOAE and ABR measurements were repeated in 34 rats (68 ears). Baseline DPOAE and ABR values were compared to those obtained after drug exposure and cochlear toxicity was evaluated in electrophysiological manner.
DPOAE test procedure
DPOAE measurements were performed by using Otodynamic ILO-288 Echoport device (Otodynamics Ltd., London, UK). Measurements were performed in a silent room. An appropriate plastic tube adaptor (1 cm in size) attached with plastic tympanometer probe was inserted to external auditory canal. Primary stimulus levels were equalized at 80 dB (L1/L2) were chosen for DP-gram measurements. Two distinct frequencies (f 1 and f 2) were set as f 1/f 2 ratio of 1.22 to obtain maximum responses. DP gram measurements were performed at frequencies of 1001, 1501, 2002, 3003, 4004, 6006, 7996 Hz.
ABR test procedure
ABR test was performed in both ears under anesthesia. Measurements were performed by using Interacoustics (Interacoustics, Denmark). ABR responses were recorded by using silver subdermal needle electrodes (Technomed Europe, The Netherlands). In the study, ipsilateral recording was performed via three electrodes by using one channel. Electrode positioning was as follows: active electrode (+) at vertex, grounding electrode over contralateral mastoid bone and reference electrode (−) over ipsilateral mastoid bone. Click stimulus was used as auditory stimuli. The band-pass filter 100–3000 Hz for click stimuli, and repetition rate of 21 s were set as filtering. The threshold was determined beginning from 70 dB by 20 dB decrements. Normal hearing is defined as detection of normal ABR configuration at 10 dBHL. Behavioral reproducibility was tested by two repetitions at threshold level and proof is demonstrated. ABR threshold is defined as lowest level where wave V of ABR was observed. ABR measurements were repeated 14 days after drug initiation and results were compared with baseline ABR measurements.
Histopathological examination
The cochlea was fixed in 10 % formalin. Then, tissue samples were embedded into paraffin blocks to avoid cell destruction by autolysis or bacteria, and to preserve tissue morphology and composition. To enable histopathological examination, the specimens were decalcified in a solution consisting of formic acid and sodium citrate. Then, cochlea was bisected to obtain a transverse section. One paraffin-embedded block tissue was selected from each case and 5 µm sections were cut, which were then stained with hematoxylin and eosin. The sections were evaluated under light microscopy (Nikon Eclipse Ni) and digital images were obtained by digital camera (Nikon DS2-Fi2) attached to light microscope. Tissue sections were deparaffinized with xylene and washed with ethanol.
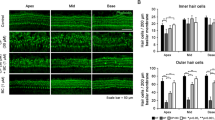
Histologically, tissue samples obtained from experimental animals revealed normal micro-architecture of the organ of Corti (Fig. 1). Histopathologically, the presence of stria vascularis, edema, leukocyte infiltration, neovascularization and fibroblast proliferation were rated subjectively as −, +, ++ or +++.
Results
This is the first study investigating the protective effects of misoprostol against cisplatin-induced ototoxicity. Our results suggest that DPOAE responses and histopathological structure were preserved in the cisplatin plus misoprostol group when compared to the group received cisplatin alone. In light of these findings, we concluded that cisplatin-induced ototoxicity may be prevented by use of misoprostol the rats. However, further studies with comprehensive electrophysiological and histopathological evaluations are needed to investigate the protective effect of the misoprostol on cisplatin-induced ototoxicity.
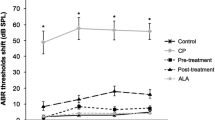
ABR tests
When ABR threshold values were compared at baseline, there was no significant difference in ABR threshold values of left and right ear between groups (p > 0.05).
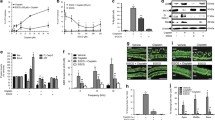
DPOAE tests
DPOAE responses were measured at frequencies of 1000, 1500, 2000, 3000, 4000, 6000 and 8000 Hz at baseline and after 14-days drug exposure. When DPOAE responses were analyzed, it was seen that there was significant difference in DPOAE response at frequency of 1001 Hz across groups (p < 0.05). However, no significant difference was detected between groups regarding DPOAE responses at frequencies of 2002, 3003, 4004 and 6006 obtained at baseline (p < 0.01), but there was significant difference in DPOAE response at 7996 at significance level of <0.05.
No significant difference was found in DPOAE response at frequencies 1001 and 1501 Hz obtained after 14-days drug exposure among groups (p > 0.05).
In misoprostol group, there was significant difference between DPOAE response at frequency of 6006 Hz obtained at baseline and after drug exposure at significance level of <0.05 (p < 0.05).
In cisplatin group, there was significant difference between DPOAE responses at frequencies of 4004 and 7996 Hz obtained at baseline and after drug exposure at significance level of <0.01 (p < 0.01) (Table 1).
In cisplatin misoprostol group, there was significant difference between DPOAE responses at frequencies of 4004 and 6006 Hz obtained at baseline and after drug exposure at significance level of <0.01 (p < 0.0) while in DPOAE response at frequency of 7996 Hz at significance level of <0.05 (Table 2).
Histopathological results
There were significant differences in measurements of epithelial vacuolization in strial epithelium and spiral limbus among groups (p > 0.05) (Table 3).
There were significant differences in measurements of epithelial vacuolization in strial epithelium and spiral limbus among groups (Figs. 1, 2, 3). In the histopathological examination, there was vacoulization in fibroblasts of stria vascularis at cochlea in rats given cisplatin (Fig. 4).
Discussion
Cisplatin is commonly used as an anti-neoplastic agent in the treatment in a wide spectrum of neoplastic diseases including ovarian, testicular, bladder, lung, head and neck [12]. Its anti-neoplastic action is associated to inhibition of the deoxyribonucleic acid (DNA) synthesis [13]. However, nephrotoxicity, neurotoxicity and ototoxicity observed during cisplatin therapy are dose-limiting adverse events [14]. Ototoxicity may occur within hours to days after cisplatin exposure. Cisplatin-induced ototoxicity is usually bilateral with progressive and irreversible nature. Sensorineural hearing loss is initially observed at high frequencies; however, it may progress to involve all frequencies at high cumulative doses of cisplatin [12].The exact mechanism of cisplatin-induced ototoxicity hasn’t been fully understood. The most popular mechanism proposed is excessive production of free oxygen radicals in the cochlear tissues [12]. Cisplatin also decreases antioxidant enzymes in the cochlea [4]. In experimental animal studies, cisplatin-induced ototoxicity was demonstrated and there are many reports on the protective effects of various antioxidant agents such as resveratrol, melatonin, dexamethasone, Vitamin E, d-methionine, N-acetylcysteine, gingko biloba extract, allopurinol and ebselen, amiphostine, sodium salicylate, Salvia miltiorrhiza and pomegranate extract [3, 4, 15–23].
The present study showed that misoprostol treatment may play a protective role against cisplatin-induced ototoxicity in rats. To best of our knowledge, there is no study on this issue in the literature. In our study, it was seen that there was significant difference in DPOAE response at frequency of 1001 Hz across groups (p < 0.05). However, no significant difference was detected between groups regarding DPOAE responses at frequencies of 2002, 3003, 4004 and 6006 obtained at baseline (p < 0.01), but there was significant difference in DPOAE response at 7996 at significance level. In misoprostol group, there was significant difference between DPOAE response at frequency of 6006 Hz obtained at baseline and after drug exposure at significance level of <0.05 (p < 0.05).
Our results showed that DPOAE responses were preserved in the cisplatin plus misoprostol treatment group when compared with cisplatin alone group. In our study, there were significant differences in measurements of epithelial vacuolization in strial epithelium and spiral limbus among groups, while there were significant differences in measurements of epithelial vacuolization in strial epithelium and spiral limbus among groups. The histopathological findings observed also supported protective effect of misoprostol in cisplatin-induced ototoxicity.
In drug-related ototoxicity, pathogenesis may be linked with the accumulation of drugs within cells. Laboratory animals as well as in vitro studies have shown that cisplatin leads to hearing loss by influencing on several cochlear regions. Outer cell degeneration is the most commonly reported histopathological manifestation of ototoxicity. At the beginning, outer hair cells stereocilia tip links of outer hair cells are injured; followed by loss of outer hair cells [4].
Cisplatin ototoxicity causes progressive damage in cochlear outer hair cells starting from the base and extending to the apex. It also includes collapse of Reissner’s membrane, atrophy of the stria vascularis and supporting cells the organ of Corti [24]. In our study, DPOAE amplitudes decreased in the higher frequencies but the lower frequencies were spared in the cisplatin group. This is an apparent sign of progressive damage starting from base of cochlea to the apex which is induced by cisplatin. Moreover, the ototoxic effect may be profound as cisplatin accumulates in inner ear.
The precise cellular and molecular mechanisms underlying cisplatin-induced ototoxicity haven’t been fully understood; however, in recent studies it has been proposed that oxidative stress may be one of the underlying mechanisms in the pathogenesis of ototoxicity. In the literature, there are studies that suggest a linkage between cisplatin-induced ototoxicity and excessive production of free oxygen radicals in the cochlea, outer hair cells, spiral ligament, stria vascularis and spiral ganglionic cells [1]. In addition, cisplatin also decreases antioxidant enzymes involved in removal and neutralization of increased super oxidase [12]. Cisplatin accumulates in the cochlear tissues and integrates into the DNA, resulting in synthesis of dysfunctional proteins and enzymes. This will lead excessive free oxygen radical generation in association with decreased antioxidant enzyme system. A wide spectrum of enzymatic and non-enzymatic mechanisms involved in control of biologic effects of free oxygen radicals in vivo. Extreme increases in the production of free oxygen radicals or decrease in the antioxidant system results in potentially cytotoxic oxidative stress [25]. Once the stability between free oxygen radicals production and the anti-oxidative defiance mechanisms is impaired, oxidative stress can occur, which can result in cochlear cell injury or death.
Endogenous or exogenous antioxidant agents may protect against cisplatin-induced ototoxicity. In experimental animal studies, exogenous antioxidant agents have been used to reduce cisplatin ototoxicity, presumably by scavenging free oxygen radicals. These agents include resveratrol, melatonin, dexamethasone, Vitamine E,d-methionine, N-acetylcysteine, gingko biloba extract, ebselen, amiphostine, sodium salicylate, Salvia miltiorrhiza, pomegranate extract, thymoquinone, bilberry extract, lycopene, chrysin,sertralin, molsidomine and beta glukan [3, 4, 15–33]. There is no FDA-approved agent that has shown efficacy in preventing cisplatin ototoxicity so far [4].
Misoprostol is a synthetic methyl prostaglandin E1 (PGE1) analogue that is used in the treatment of peptic ulcer as it increases secretion of mucus lining gastrointestinal tract and mucosal blood flow [34]. In recent years, it became apparent that misoprostol has effects beyond protecting the gastroduodenum from NSAIDs and promoting uterotonic activity. In preclinical and clinical studies, it was indicated that the drug may have roles in the treatment of organ systems such as the heart, lungs, kidneys, brain, pancreas, and liver. The mucosal edema and increased mucus layer may be important components in the drug’s cytoprotective mechanism. This can lead dilution in the concentration of potentially harmful molecules such as NSAIDs and increase the distance that such molecules have to penetrate before reaching susceptible cells [35]. It is also likely that misoprostol acts as an inhibitor of leukocyte adherence and/or directly modulates expression of specific adhesion molecules throughout the body in various disease states [36, 37, 38]. Many novel effects of the agent have been discovered in a variety of diseases and organ systems, which are generally associated to cell protective properties of the drug. For example, in one double-blind controlled trial, it was indicated that misoprostol was associated with significant improvement in renal function as assessed by serum creatinine and creatinine clearance after kidney transplantation [39]. In some clinical trials, it was shown that misoprostol is associated with significantly improved NSAID-induced tinnitus [40, 41]. Misoprostol (MP) has gained considerable interest as a scavenger of reactive oxygen species [9]. It also has other properties in addition to antioxidant such as anti-apoptotic or cytoprotective effects [10, 11]. Misoprostol, a prostaglandin E1 analogue, is widely used in preventing NSAID-induced gastric ulcers; prolongs the survival in heart and kidney transplantation; and protects against cyclosporine-induced renal damage. Misoprostol has interaction with broad range of physiologic and pharmacologic activities in diverse disease states and organ systems. Its cell protective effects extend its proven ability to block NSAID-induced gastroduodenal damage. Additional protective effects may lead to more successful management of malignancy and hepatic, renal, lung, and cardiac dysfunction. Immunomodulatory effects may be helpful in chronic arthropathies, allergies, and cartilage deterioration [10, 42]. The effect of misoprostol on cisplatin-induced changes in lipid peroxidation products, and the activity of antioxidant enzymes in the rat kidney have also been investigated to determine the extent of tissue damage due to oxidative stress [43].
To best of our knowledge, there is no study about protective effect of MP, a prostaglandin E1 (PGE1) analogue, on cisplatin-induced ototoxicity. Therefore, we designed this study in rats to investigate the effects of MP on CP-induced histopathological changes and auditory parameters including auditory brainstem response (ABR) and DPAOE (distortion product otoacoustic emission).
Conclusion
Our results revealed statistically significant differences between study groups, suggesting that misoprostol had protective effect against cisplatin ototoxicity. Our results also showed that misoprostol treatment achieves significant protection to the cochlea from cisplatin toxicity; thus, intraperitoneal dose of misoprostol used in this study may have a protective effect against cisplatin ototoxicity in rats.
References
Li Y, Womer RB, Silber JH (2004) Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Eur J Cancer 40:2445–2451
Sluyter S, Klis SF, de Groot JC, Smoorenburg GF (2003) Alterations in the stria vascularis in relation to cisplatin ototoxicity and recovery. Hear Res 185:49–56
Erdem T, Bayindir T, Filiz A, Iraz M, Selimoglu E (2012) The effect of resveratrol on the prevention of cisplatin ototoxicity. Eur Arch Otorhinolaryngol 269(10):2185–2188
Yumusakhuylu AC, Yazici M, Sari M, Binnetoglu A, Kosemihal E, Akdas F, Sirvanci S, Yuksel M, Uneri C, Tutkun A (2012) Protective role of resveratrol against cisplatin induced ototoxicity in guinea pigs. Int J Pediatr Otorhinolaryngol 76:404–408
Laurell G, Bagger-Sjoback D (1991) Dose-dependent inner ear changes after I.V. administration of cisplatin. J Otolaryngol 20:158–167
Siddik ZH (2003) Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 22:7265–7279
Kim HJ, Lee JH, Kim SJ, Oh GS, Moon HD, Kwon KB, Park C, Park BH, Lee HK, Chung SY, Park R, So HS (2010) Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and ototoxicity. J Neurosci 30:3933–3946
Lynch ED, Gu R, Pierce C, Kil J (2005) Reduction of acute cisplatin ototoxicity and nephrotoxicity in rats by oral administration of allopurinol and ebselen. Hear Res 201:81–89
Salam OM, Saleem AA, Omara EA, Hassan NS (2009) Hepatoprotective effects of MP and silymarin on carbon tetrachloride-induced hepatic damage in rats. Fundam Clin Pharmacol 23(2):179–188
Yang H, Majno P, Morel P et al (2002) Prostaglandin E(1) protects human liver sinusoidal endothelial cell from apoptosis induced by hypoxia reoxygenation. Microvasc Res 64:94–103
Sostres C, Gargallo CJ, Arroyo MT, Lanas A (2010) Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract Res Clin Gastroenterol 24(2):121–132. doi:10.1016/j.bpg.2009.11.005
Rybak LP, Mukherjea D, Jajoo S, Ramkumar V (2009) Cisplatin ototoxicity and protection: clinical and experimental studies. Tohoku J Exp Med 219:177–186
Williams CJ, Whitehouse JM (1979) Cis-platinum: a new anticancer agent. Br Med J 1:1689–1691
Hartmann JT, Lipp HP (2003) Toxicity of platinum compounds. Expert Opin Pharmacother 4:889–901
Lopez-Gonzalez MA, Guerrero JM, Rojas F, Delgado F (2000) Ototoxicity caused by cisplatin is ameliorated by melatonin and other antioxidants. J Pineal Res 28:73–80
Daldal A, Odabasi O, Serbetcioglu B (2007) The protective effect of intratympanic dexamethasone on cisplatin-induced ototoxicity in guinea pigs. Otolaryngol Head Neck Surg 137:747–752
Kalkanis JG, Whitworth C, Rybak LP (2004) Vitamin E reduces cisplatin ototoxicity. Laryngoscope 114:538–542
Campbell KC, Meech RP, Rybak LP, Hughes LF (1999) D-Methionine protects against cisplatin damage to the stria vascularis. Hear Res 138:13–28
Feghali JG, Liu W, Van De Water TR (2001) l-n-acetyl-cysteine protection against cisplatin-induced auditory neuronal and hair cell toxicity. Laryngoscope 111:1147–1155
Huang X, Whitworth CA, Rybak LP (2007) Ginkgo bilobaextract (EGb 761) protects against cisplatin-induced ototoxicity in rats. Otol Neurotol 28:828–833
Lynch ED, Gu R, Pierce C, Kil J (2005) Reduction of acute cisplatin ototoxicity and nephrotoxicity in rats by oral administration of allopurinol and ebselen. Hear Res 201:81–89
Church MW, Blakley BW, Burgio DL, Gupta AK (2004) WR-2721 (Amifostine) ameliorates cisplatin-induced hearing loss but causes neurotoxicity in hamsters: dose-dependent effects. J Assoc Res Otolaryngol 5:227–237
Hyppolito MA, de Oliveira JA, Rossato M (2006) Cisplatin ototoxicity and otoprotection with sodium salicylate. Eur Arch Otorhinolaryngol 263:798–803
Yazici ZM, Meric A, Midi A, Arınc YV, Kahya V, Hafız G (2012) Reduction of cisplatin ototoxicity in rats by oral administration of pomegranate extract. Eur Arch Otorhinolaryngol 269:45–52
Halliwell B (1994) Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet 344:721–724
Xu O, Liu Y, Li X, Yang Y, Zhang Z, Wang N, Zhang Y, Lu H (2011) Protective effects of Salvia miltiorrhiza against cisplatininduced ototoxicity in guinea pigs. Am J Otolaryngol 32:228–234
Sagit M, Korkmaz F, Akcadag A, Somdas MA (2013) Protective effect of thymoquinone against cisplatin-induced ototoxicity. Eur Arch Otorhinolaryngol 270:2231–2237. doi:10.1007/s00405-012-2254-6
Kapusuz Z, Ozkırıs M, Kala M, Saydam L. Protective Role of Bilberry Extract Against Cisplatin Induced Ototoxicity in Rats. Indian J Otolaryngol Head Neck Surg 65(4):339–344. doi:10.1007/s12070-013-0642-x
Çiçek MT, Kalcioglu MT, Bayindir T, Toplu Y, Iraz M (2014) The effect of lycopene on the ototoxicity induced by cisplatin. Turk J Med Sci 44:582–585. doi:10.3906/sag-1304-66 ©TÜBİTAK
Kelles M, Tan M, Kalcioglu MT, Toplu Y, Bulam N (2013) The protective effect of Chrysin against cisplatin-induced ototoxicity in rats. Indian J Otolaryngol Head Neck Surg. doi:10.1007/s12070-013-0695-x
- Ozturk M, Ucar S, Sarı F, Erdogan S, Topdag M, Iseri M.(2013) Possible protective effect of sertraline against cisplatin-induced ototoxicity: an experimental study. Hindawi Publ Corp Sci World J vol, Article ID 523480, 5 pages 10.1155/2013/523480
Toplu Y, Parlakpinar H, Sapmaz E, Karatas E, Polat A, Kizilay A (2014) The protective role of Molsidomine on the cisplatin-induced ototoxicity. Indian J Otolaryngol Head Neck Surg (July–Sept 2014) 66(3):314–319. doi:10.1007/s12070-014-0718-2
Bayindir T, Iraz M, Kelles M, Kaya S, Tan M, Filiz A, Toplu Y, Kalcioglu MT (2014) The effect of beta glucan on cisplatin ototoxicity. Indian J Otolaryngol Head Neck Surg (Apr–Jun 2014) 66(2):131–134. doi:10.1007/s12070-013-0623-0
Brunton LL, Lazo JS, Parker KI (2006) Goodman and Gilman’s the pharmacological basis of therapeutics. Mc Graw Hill Companies, New York
Gana TJ, Koo J, MacPherson BR (1992) Gross and histologic effects of topical misoprostol on canine gastric mucosa. Exp Toxicol Pathol 44:40–46
Asako H, Kubes P, Wallace J, Wolf RE, Granger DN (1992) Modulation of leukocyte adhesion in rat mesenteric venules by aspirin and salicylate. Gastroenterology 103:146–152
Andrews FJ, Malconenti-Wilson C, O’Brien PE (1994). Effect of nonsteroidal anti-inflammatory drugs on LFA-1 and ICAM-1 expression in gastric mucosa. Am J Physiol 266(4 pt 1):G657–G664
Widomski DL, Walsh RE, Baron DA et al (1991) Effects of the prostaglandin analogue misoprostol on inflammatory mediator release by human monocytes. Agents Actions 34:30–31
Kim HJ, Kwak JY (1996) Effects of misoprostol on renal function and plasma enothelin-1 levels during early postoperative stage of living donor renal transplant recipients. Transplant Proc 1996(28):1207–1209
Kawata R, Urade Y, Tachibana M, Mizukoshi O (1988) Prostaglandin synthesis by the cochlea. Prostaglandins 1988(35):173–184
Briner W, House J, O’Leary M (1993) Synthetic prostaglandin E1 misoprostol as a treatment for tinnitus. Arch Otolaryngol Head Neck Surg 119:652–654
Davies NM, Longstreth J, Jamali F (2001) Misoprostol therapeutics revisited. Pharmacotherapy 21(1):60–73
Ozer MK, Halil A, Moncu MC, Savran M, Calapoglu M, Yesilot S, Candan IA et al (2011) Effects of misoprostol on cisplatininduced renal damage in rats. Food Chem Toxicol 49:1556–1559
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest was declared by the authors.
Funding
This study was funded by Kayseri Training and Research Hospital.
Ethical approval
All applicable international, national and/or institutional guidelines for care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Doğan, M., Polat, H., Yaşar, M. et al. Protective role of misoprostol against cisplatin-induced ototoxicity. Eur Arch Otorhinolaryngol 273, 3685–3692 (2016). https://doi.org/10.1007/s00405-016-4031-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-016-4031-4