Abstract
Unlike laryngoscopy, laryngeal sonography is easily applicable, well tolerated by young children and less exhausting for the examiner. The aim of this study was to determine if sonography can adequately visualize the laryngeal structures and vocal fold movements of children. Synchronic video laryngoscopy and sonography of the larynx were performed in 35 children (aged 2–8 years) without a laryngeal disease. 10 investigators at differing stages of otolaryngological training were tasked with identifying vocal fold movement and specific anatomical structures after seeing (1) the sonography-only video and (2) the sonography video with a laryngoscopy video overlay. The percentage of correct identification of the structures and the vocal cord movement with and without additional video-overlay was compared. A sonographical view of the larynx could be found and the following anatomical structures were identified: subcutaneous fat tissue, left and right sternohyoid and thyrohyoid muscles, thyroid cartilage, left and right vocal folds, left and right paraglottic spaces, left and right side arytenoid cartilages, and rima glottides. Concerning the evaluation of the videos amongst the investigators: they identified >80 % of vocal fold movement and the targeted anatomical structures except the arytenoid cartilages (only up to 63 %), having the sonography only. In combination with the laryngoscopy video-overlay, investigator detection rates significantly improved (>90 % of vocal fold movement, p < 0.001; >90 % in most anatomical structures, (p < 0,001). Laryngeal sonography is a helpful diagnostic tool to identify laryngeal structures and the movement of the vocal folds in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laryngoscopy is the gold standard for pediatric laryngeal examination [1–3]. While direct laryngoscopy poses some risk to children because it is performed under general anesthesia [4], flexible laryngoscopy can be performed without general anesthesia and therefore is often the preferred method to evaluate the function on the infant larynx. Flexible laryngoscopy has additional advantage of being able to visualize the physiological state of the larynx with breathing and phonation. Video can be recorded and watched later in slow motion. Flexible laryngoscopy can, however, be problematic: children have a narrow airway, discomfort can occur, parents can be unhappy with seeing their child undergo such a difficult and invasive procedure, only a little time span is available to evaluate the laryngeal function, the image quality is limited in small endoscopes, and a laryngospasm could occur during examination [5].
Sonography is an alternative method to examine the anatomical structures and the function of the larynx. It is free of radiation, well tolerated by patients and parents, and easy to perform. Although sonography of the neck is a widely used technique, sonography of the larynx is not. Already in 1992 Garel et al. [6] and in 1997 Friedman [7], sonography was described as an important technique for the assessment of the laryngeal structures in adults. Children have special needs and even a flexible endoscopy can be difficult to perform. For that reason, the capability of sonography to assess laryngeal structures and pathologies was evaluated [8, 9]. To the best of our knowledge only one study comparing the two methods for the laryngeal examination in children has been published [9]. But until now a synchronous sonography with a picture in picture image of the laryngoscopic view to analyze the additional value has not been performed.
The primary aim of this study was to identify laryngeal structures and the movement of the vocal folds in short sonography movies of the pediatric larynx and proof them with the simultaneous endoscopy.
Methods and materials
Subjects
35 subjects (aged 2–8 years; median 3 years) without laryngeal pathology participated in the study. 17 were female and 18 male. Synchronous sonography of the larynx and video laryngoscopy via the laryngeal mask were performed during May 2012 and March 2013 in the postoperative period after routine surgical otolaryngological procedures distant from the larynx (adenotomy with or without a myringotomy with possible tube insertion).
Videolaryngoscopy and the simultaneous sonography
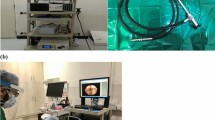
3 authors (A.M., K.K., K.A.) participated in the study as experienced examiners. They performed the videolaryngoscopy and the simultaneous sonography. Recordings, which took 5–10 min, were performed after surgery and in the phase before removal of the larynx mask. The time after finishing the procedure and removal of the laryngeal mask was not prolonged for this study in any patient. The children did not suffer any additional discomfort because the videolaryngoscope had been put down the laryngeal mask and the ultrasound transducer had been applied externally in the usual way. (see Fig. 1)
The recorded videos of the 35 examinations were cut into relevant 30-s long sequences featuring a horizontal and a vertical ultrasound view of the larynx. Firstly, the examiners discussed these sequences and identified the following anatomical structures: subcutaneous fat tissue, left and right sternohyoid and thyrohyoid muscles, thyroid cartilage, left and right vocal folds, left and right paraglottic spaces, left and right side arytenoid cartilages, and rima glottidis (see Fig. 2). These structures were clearly visible for all 35 subjects. Secondly, the video sequences were presented to 10 investigators. The investigators were otolaryngological students or doctors at differing stages of training. The investigators attempted to identify (1) the aforementioned anatomical structures and (2) any degree of vocal fold movement, i.e., not only a complete closure of the vocal folds after watching (1) the sonographical-only video and then (2) the overlay video of sonography plus laryngoscopy (Figs. 3, 4).
Answers were compiled via a questionnaire (see Table 1). All of the 10 investigators watched all of the 35 videos, yielding a maximum possible identification rate of 350/350 for each anatomical structure.
Concerning image quality the examiners defined independently after evaluating all videos that the sequences of patient 1, 15, 17, 18, 20, 22 und 28 were of lower image quality concerning contrast, brightness and clearness as well as visibility of the vocal folds.
Equipment
A XION Video Nasopharyngoscope (EV-N; XION, Berlin, Germany) and a XION EndoStrob-DX processor and GE Logiq P5 sonography machine with the transducer 12L (General Electric Company, Pittsburgh, PA, USA) were used. The B-mode videos from the ultrasound machine were merged with the laryngoscopy video signal of the XION device using a two channel DVI switch with picture-in-picture (PiP) function (SW DVI PLUS, Extron Electronics, Anaheim, CA, USA) and recorded as a PiP image.
Statistics
The statistical evaluation of the questionnaires was done using IBM SPSS version 21.0 statistical software for Windows (Chicago, IL, USA). Analyses consisted of Friedman test and the Mann–Whitney U test. The Bonferroni correction was used. With the Friedman test we analyzed the identification-rate difference between sonography-only and sonography and laryngology. This parametric test could be used as the presented data are only an excerpt of the original data which were configured as a three paired random sample. The influence of the image quality on the identification rate was analyzed with the Mann–Whitney U test.
In general, nominal p values of two-tailed tests were reported. The significance level was set at p < 0.05.
Ethics and consent
The local ethics committee at the Friedrich-Schiller University, Jena approved this study. Subjects’ parents gave their informed consent.
Results
Description of the consensual identified anatomical structures in laryngeal sonography in children
The examiners clearly identified the typical anatomical structures of the larynx (Fig. 2). The subcutaneous fat tissue appeared on the surface as a hyperechoic tissue. The thyroid cartilage was found as a hypoechoic structure surrounded by a hyperechoic inner and outer margin continuing from one side to the other. The strap muscles were identified as a characteristic muscle structure situated on the top of the thyroid cartilage; more precisely, it was also possible to identify the sternohyoid muscle as a continuing structure more anteriorly at the thyroid cartilage and the thyrohyoid muscle more laterally at the thyroid cartilage. The margin of the strap muscles was well defined and interiorly we saw a hyperechoic feathered muscle structure. The paraglottic space and the false vocal folds were found at the inner side of the thyroid cartilage as a paired organ with a hyperechoic appearance between the vocal folds and the curve of the thyroid cartilage. The vocal folds appeared as a hyperechoic paired structure in a v-shape that surrounded the rima glottidis, which was hypoechoic configured or, if there was any mucous between the vocal folds, fluctuating hyperechoic. The arytenoid cartilages appeared as hypoechoid structures not always well defined by a hyperechoid surrounding margin but usually visible with an accented posterior hyperechoic line.
Depending on the angle of the sonographic probe, different parts of the vocal folds were visible. The best view to the glottic level was achieved by positioning the probe at 45° upward at the midline of the neck (Fig. 6). At the end of the recording, we could identify a characteristic picture (Fig. 5) for the supraglottic appearance. A hypoechoic zone could always be found reaching from the end of the thyroid cartilage to the vocal folds and situated anteriorly to the hypoechoic area of the arytenoid cartilage. Further, the laryngeal mask and its hyperechoic repeated lines were identifiable behind the arytenoid cartilages.
Identification rates of the defined laryngeal structures
In sonography-only, investigators scored >80 % correct for all of the defined anatomical structures, except the arytenoid cartilages. Identification rates for subcutaneous fat tissue (100 %), sternohyoid and thyrohyoid muscles (99.7 %), and the paraglottic space (96.3 %) were particularly high.
After the investigators have seen the laryngoscopy video-overlay the correct identification rates significantly improved >90 % in most of the anatomical structures, if there was a significant difference in the identification rates (p < 0,001 for most structures), p < 0,05 paraglottis space, no significant difference in the subcutaneous fat tissue and sternohyoid/thyrohyoid muscle; and <80 % in the arytenoid cartilages, (p < 0,001).
Investigators’ ability to correctly identify the anatomical structures increased significantly with the addition of the laryngoscopic view (Friedman test; p < 0.001) (see Table 2).
Identification rates of the defined anatomical structures
In sonography-only, investigators’ correct identification rate was ≥81.4 for all anatomical features, except for the left and right arytenoid cartilage. Investigators were particularly successful at identifying subcutaneous fat tissue, the sternohyoid muscles, and the paraglottic spaces (all ≥96.3 % correct).
In sonography and laryngoscopy, investigators’ correct identification rate was ≥88.9 for all anatomical features, except the left and right arytenoid cartilage. Investigators were particularly successful at identifying subcutaneous fat tissue, the sternohyoid muscles, and the paraglottic spaces (all ≥98.0 % correct).
After seeing the laryngoscopy video-overlay, investigator correct detection rates significantly improved >90 % in most anatomical structures (p < 0.001) and >80 % in the arytenoid cartilages (p < 0.001; Friedman test) (see Table 2).
Vocal fold movement
A bilateral vocal fold movement was visible in 33/35 (94 %) subjects. In sonography-only, correct identification of vocal fold movement was 82.0 % (left) and 83.4 % (right); in sonography and laryngoscopy this improved to 92.6 % (left) and 92.9 % (right). The improvement was highly significant (Friedman test, p < 0.001). (Table 2)
Image quality and identification rates
Concerning image quality the examiners defined independently after seeing the videos that the sequences of patient 1, 15, 17, 18, 20, 22 und 28 were of lower image quality concerning contrast, brightness and clearness as well as visibility of the vocal folds (Fig. 7). The image quality had a very significant influence of the identification rate (Mann–Whitney U test, p < 0.001).
Discussion
It goes back up to 50 years that early developments in the field of sonography of the larynx had been reported [10]. Further developments in Japan followed. But it was Garel et al. [6] and Friedman [7] who later used sonography to describe not only the vibration of the vocal folds but also the anatomical structures of the larynx (sternohyoid and thyrohyoid muscles, vocal folds, ventricular bands, thyroid cartilage, arytenoids cartilages). Although the image quality of the sonographical picture was not as sharp and clearly visible as it is today, they described the structures fairly similar as the present study to us. Whereas they only defined the vocal folds as hypoechoic structures, we identified them as hyperechoic figures, as can be seen in Fig. 1. Friedman [7] put the vocal folds in the same position but marked them as a hyperechoid zone; Garel et al. [6] placed the marking in a different position at the inner side of the thyroid cartilage. We defined the position of the arytenoid cartilages similarly to the others but described them more clearly and defined them as hypoechoic with a surrounding hyperechoic margin (which was not always well defined). Friedman [7] described them as echogenic. The structures described in Fig. 1 are self-explanatory and, we believe, easily recognizable as we have described them. The new changes in the B-mode image of the sonographical picture have made it possible to achieve such an image.
Few years later some studies looked into pathologies of the larynx and their imaging in the ultrasound [11–16] and as well into the function of the larynx and the movements of the vocal folds [17–19]. They all found the sonography very useful in detecting vocal fold paresis. We included only healthy subjects and therefore expected that all subjects would have normal vocal fold movement. After seeing the videos, however, no movement appeared to be visible in 2 subjects. This was because the recording was ended before clear movement would have been visible; these 2 subjects were not awake enough to move their vocal folds. Nevertheless, with sonography, we (the experienced examiners) could easily detect slight vocal fold movement, sometimes more easily than with laryngoscopy. The investigators found identification more difficult than did the examiners but were still able to correctly identify vocal fold movement in ≥82 % of the subjects in sonography-only. The addition of the laryngoscopic view increased the identification rate to ≥92.6 %. We surmise that, since there was almost no visible time shift in the signal, the increased rate of identification is attributable to the addition of the video laryngoscope to the real time sonographical signal. The present study is the first, to our knowledge, which has reported such a find.
During video recording, it became obvious that achieving a good full length image of the vocal folds was not easy. Correspondingly, identifying the full length of the vocal folds was difficult and depended on the angle of the probe and the up and down movement of the entire larynx associated with the subjects’ breathing. Only slight changes could make the vocal folds visible. We found a very typical picture of the supraglottic structures as described in Fig. 5. That has not been described in this form to our knowledge until now. The assumption of having a hypoechoic zone is because of the loss of signal as it penetrated the air until it reached the arytenoid cartilage again. This became our impression from observing the sonographical picture and additionally the laryngoscopic view. There it would be necessary to have an experimental approach for final proof. In terms of the overall results, some videos (especially video 1, 5, 16, 22, and 29, Fig. 7) were more difficult to interpret than others. The mainly supraglottic view to the vocal folds in the above named videos was one reason for this. Additionally, the videos of lower image quality (defined by the examiners) (Fig. 7) were proved to have a significant lower identification rate of the anatomical structures which can be understood clearly as the ability to identify structures can only be as good as the image in front of it.
The idea was to determine if the addition of a laryngoscopic picture to sonography image would increase investigators’ ability to correctly identify anatomical structures in the larynx. In sonography-only, investigators were able to correctly identify the structures, with the exception of the arytenoid cartilage, at least 80 % of the time; a good result for people with some training in otolaryngology but who were formerly without much knowledge of sonographical images of the larynx. The arytenoid cartilages were described as structures identifiable by their in and out movement on abduction and adduction [6, 7]. As we have usually seen more little vocal folds movements, the in and out movement of the arytenoid cartilages were not always very clearly visible, which might be why the rate of identification rate in sonography-only was just over 60 %. The investigators usually put them too far anteriorly. The identification rate improved when the laryngoscopic view was added because the anatomical positions were clearer in the PiP image.
Laryngeal sonography was done under general anesthesia and from knowing how to apply the probe correctly we could now achieve sonographical images and videos in the alert patient as we have practiced it since the study was performed.
Conclusion
Laryngeal ultrasound becomes a more and more important part of the neck sonography. Some identified structures still need experimental proof. But nevertheless the laryngeal sonography in expert hands has a good validity rate and should be used in some cases as the only examination of the larynx especially in children and as well as an additional tool to obtain more information or as a follow-up for certain pathologies.
References
Hawkins DB, Clark RW (1987) Flexible laryngoscopy in neonates, infants, and young children. Ann Otol Rhinol Laryngol 96:81–85
Gysin C (2008) Evaluation of the pediatric airway. Rev Med Suisse 4:2089–2090, 2092–2093 [Article in French]
Wood RE (2008) Evaluation of the upper airway in children. Curr Opin Pediatr 20:266–271
Moumoulidis I, Gray RF, Wilson T (2005) Outpatient fibre-optic laryngoscopy for stridor in children and infants. Eur Arch Otorhinolaryngol 262:204–207
Johansson H, Norlander K, Berglund L, Janson C, Malinovschi A, Nordvall L, Nordang L, Emtner M (2015) Prevalence of exercise-induced bronchoconstriction and exercise-induced laryngeal obstruction in a general adolescent population. Thorax 70:57–63
Garel C, Contencin P, Polonovski JM, Hassan M, Narcy P (1992) Laryngeal ultrasonography in infants and children: a new way of investigating. Normal and pathological findings. Int J Pediatr Otorhinolaryngol 23:107–115
Friedman EM (1997) Role of ultrasound in the assessment of vocal cord function in infants and children. Ann Otol Rhinol Laryngol 106:199–209
Wang LM, Zhu Q, Ma T, Li JP, Hu R, Rong XY, Xu W, Wang ZC (2011) Value of ultrasonography in diagnosis of pediatric vocal fold paralysis. Int J Pediatr Otorhinolaryngol 75:1186–1190
Jadcherla SR, Gupta A, Stoner E, Coley BD, Wiet GJ, Shaker R (2006) Correlation of glottal closure using concurrent ultrasonography and nasolaryngoscopy in children: a novel approach to evaluate glottal status. Dysphagia 21:75–81
Mensch B (1964) Analyse par exploration ultrasonique du mouvement des cordes vocals isolées. C R Soc Biol 158:2295–2296
Bisetti MS, Segala F, Zappia F, Albera R, Ottaviani F, Schindler A (2009) Non-invasive assessment of benign vocal folds lesions in children by means of ultrasonography. Int J Pediatr Otorhinolaryngol 73:1160–1162
Baatenburg de Jong RJ, Rongen RJ, Laméris JS, Knegt P, Verwoerd CD (1993) Ultrasound in the diagnosis of laryngoceles. ORL J Otorhinolaryngol Relat Spec 55:290–293
Schade G, Kothe C, Leuwer R (2003) Sonography of the larynx-an alternative to laryngoscopy? Hno 51:585–590 [Article in German]
Shanturov AG, Keiko MV (1991) The role of ultrasonic diagnosis in pediatric laryngology. Vestn Otorinolaringol 4:29–33
Tamura E, Kitahara S, Kohno N (2001) Clinical assessment of intralaryngeal ultrasonography. Laryngoscope 111:1767–1770
Xia CX, Zhu Q, Zhao HX, Yan F, Li SL, Zhang SM (2013) Usefulness of ultrasonography in assessment of laryngeal carcinoma. Br J Radiol 86(1030):20130343
Wang LM, Zhu Q, Ma T, Li JP, Hu R, Rong XY, Xu W, Wang ZC (2011) Value of ultrasonography in diagnosis of pediatric vocal fold paralysis. Int J Pediatr Otorhinolaryngol 75:1186–1190
Vats A, Worley GA, de Bruyn R, Porter H, Albert DM, Bailey CM (2004) Laryngeal ultrasound to assess vocal fold paralysis in children. J Laryngol Otol 118:429–431
Dedecjus M, Adamczewski Z, Brzeziński J, Lewiński A (2010) Real-time, high-resolution ultrasonography of the vocal folds-a prospective pilot study in patients before and after thyroidectomy. Langenbecks Arch Surg 395:859–864
Acknowledgments
We thank for statistical evaluation: Dipl.-Stat. Frederic Klein.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Klinge, K., Guntinas-Lichius, O., Axtmann, K. et al. Synchronous video laryngoscopy and sonography of the larynx in children. Eur Arch Otorhinolaryngol 273, 439–445 (2016). https://doi.org/10.1007/s00405-015-3788-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-015-3788-1