Abstract
To assess the prognostic factors for local control in patients with early glottic cancer, we retrospectively analyzed the data of 130 consecutive patients who were treated by definitive radiation therapy (RT) or concurrent chemoradiotherapy (CRT) for early glottic squamous cell carcinoma (UICC sixth edition T1N0M0 and T2N0M0) at Kanagawa cancer center between 1999 and 2011. There were 63 patients with T1 cancer and 67 patients with T2 cancer. Twenty-one patients with T2 tumors were treated by chemoradiotherapy (CRT). The median follow-up period was 73 months (range, 22–165 months). The 5-year local control (LC) rate in all patients was 81 %. The 5-year LC rates in the patients with T1 and T2 cancer were 89 and 74 %, respectively. Univariate analysis showed that a higher T stage (T2) (p = 0.0301), anterior commissure involvement (p < 0.000001), and habitual drinking (p = 0.054) were correlated with decreased local control rate. Multivariate analysis identified only anterior commissure involvement as a significant prognostic factor for local control (LC rate 91 vs. 51 %, risk ratio 5.3, 95 % CI 2.3–12, p < 0.001). In the patients with T2 cancer, there was no statistically significant difference in the LC rate between patients who received RT alone and those who received CRT (RT alone 76 % vs. CRT 67 %; p = 0.832). The findings of this study suggest that anterior commissure involvement is a significant factor influencing the prospect of local control. CRT was not found to be effective for T2 patients in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early glottic cancer is a highly curable disease with radiation therapy (RT). The 5-year local control (LC) rate in early glottic cancer patients treated by RT alone is in the range of 85–95 % for T1N0M0 and in the range of 60–80 % for T2N0M0 [1–4]. Patients can also be treated by conservative surgery such as transoral laser microsurgery (TLM), partial laryngectomy, or SCPL-CHEP [1, 5–8]. The reported 5-year local control rate following TLM is 85–95 % for patients with T1 cancer and 65–85 % for those with T2 cancer. The treatment of first choice for early glottic cancer remains controversial. The goals of treatment are preservation of the larynx and voice and an optimal voice quality, in addition to eradiation of the tumor. It is generally accepted, based on the results of retrospective studies, that voice preservation is better following RT than after any surgical procedure [9–11].
According to several reports, the prospect of local control of early glottic cancer depends mainly on the T stage [12], the tumor size [13, 14], and the presence/absence of anterior commissure involvement [3, 15–17]. However, the optimal treatment approach for such high-risk patients remains unclear.
Although a meta-analysis of randomized controlled trials did not reveal any conclusive evidence of the efficacy of chemoradiotherapy (CRT) for early head and neck cancer [18], some retrospective studies have indicated that CRT improves the local control and larynx preservation rates in patients with early glottic cancer [19–22].
At our hospital, we treat early glottic cancer mainly by RT in the hope of better function preservation. In addition, we administer CRT to improve LC rate in T2 patients with bulky tumor or anterior commissure involvement which are thought to be the risk factors for local recurrence. In this retrospective single-center experience, we attempted to assess the prognostic factors for local control, including anterior commissure extension, and the effectiveness of CRT for early glottic cancer.
Method
Patients
The data of a total of 130 previously untreated patients with T1N0M0 or T2N0M0 squamous cell carcinoma of the glottis who underwent definitive radiotherapy at Kanagawa Cancer Center between 1999 and 2011 were retrospectively studied. In this study, the stage of the tumor was determined on the basis of the clinical findings and classified according to the criteria of the Union for International Cancer Control (UICC), sixth edition. The tumor status was determined routinely by computed tomography (CT), magnetic resonance imaging (MRI) and direct laryngoscopy performed under general anesthesia, which allows evaluation of the extension of the tumor and involvement of the anterior commissure.
Because Kanagawa Cancer Center is a referral center, all patients were restaged prior to the administration of radiotherapy.
Treatments
All patients received definitive radiotherapy, consisting of conventionally fractionated doses of 2 Gy in 5 weekly sessions. In all patients, the radiotherapy was given with 60Co γ-rays or high-energy photons of 4MV X-rays from a linear accelerator. Parallel opposed fields were used, irrespective of the radiation device employed. Since 2006, CRT is given patients with T2 cancer, mainly those with bulky tumor and/or anterior commissure involvement, who are under 80 years and have no renal dysfunction, in the expectation of obtaining improved local control. The chemotherapeutic regimen consisted of two cycles of S-1 given at the dose of 80–120 mg/day (80 mg/day for BSA < 1.3, 100 mg/day for 1.3 ≤ BSA < 1.6, and 120/mg for BSA ≥ 1.6) on days 1–14 and days 29–42. S-1 is an oral antitumor agent consisting of tegafur, 5-chloro-2, 4-dihydroxypyridine (CDHP) and potassium oxonate in a molar ratio of 1:0.4:1. Tegafur is a prodrug of 5-fluorouracil (5-FU), and CDHP and potassium oxonate prolong the half-life in the blood of 5-FU and diminish the toxicity of 5-FU, respectively [23]. The dose of TS-1 was determined according to previous study [22].
Evaluation
The response during the radiotherapy period was evaluated weekly by fiberoptic laryngoscopy, by both the head and neck surgeon and the radiation oncologist. Local response was estimated 2 months after the completion of radiotherapy by CT or MRI of the head and neck. After the completion of treatment, the patients were followed up monthly during the first year, bimonthly in the second year, and every 3–6 months thereafter. Detection of the evidence of tumor at or within 2 months of completion of radiation therapy was defined as persistent disease; the evidence of tumor detected 2 months or more after completion of therapy was defined as local recurrence. Local control was defined as the freedom from persistent or locally recurrent disease. Salvage treatment for persistent disease or local recurrence usually consisted of TLM or total laryngectomy.
Statistical analysis
Local control rates were estimated by the Kaplan–Meier product limit method. Comparisons between subgroups divided according to patient, tumor and treatment variables were performed by the log-rank test. Cox proportional hazard regression models were applied for multivariate analysis.
Results
The median duration of follow-up was 73 months (range, 22–165 months). The patient characteristics are summarized in Table 1. The male-to-female ratio was 128:2 and the median age was 68 years (range, 42–91). It is noteworthy that the majority were smokers (95 %) and drinkers (76 %), and that 36 patients (28 %) had double cancers. There were 63 patients with T1 tumors (T1a; 38, T1b 25) and 67 with T2 tumors; 13 patients (21 %) with T1 cancer and 19 patients (28 %) with T2 cancer had anterior commissure involvement.
The treatment results are summarized in Table 2; 77 patients were treated with 60Co and 53 with 4MV X-rays. The median total radiation dose was 66 Gy (range, 60–70 Gy). Of the 67 patients with T2 cancer, 21 (31 %) were treated by CRT; 18 of these 21 received two cycles of S-1, while the remaining 3 received only one cycle of treatment because of early adverse events; 2 of the patients had grade 2 diarrhea and 1 had grade 3 mucositis.
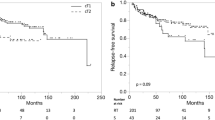
The 5-year overall survival (OS) rate was 79 % and the 5-year disease-specific survival (DSS) rate was 94 %. Of the total, 6 patients (5 %) had persistent disease and 18 patients (14 %) had recurrent disease. The recurrence was classified as local recurrence in all cases. The 5-year local control (LC) rate in all patients was 81 % (Fig. 1) and the 5-year laryngeal preservation (LPS) rate was 82 %. The LC rates according to the T stage are shown in Fig. 2. The 5-year LC rates in patients with T1 and T2 cancer were 89 and 74 %, respectively, with statistically significant difference between the two groups (p = 0.0301). The decrease in the local control rate associated with anterior commissure involvement (from 91 to 51 % at 5 years) is shown in Fig. 3. Anterior commissure involvement was found to be a significant prognostic factor (p < 0.000001).
The results of the univariate analysis conducted to identify factors influencing the LC are shown in Table 3. Higher T stage, history of drinking (p = 0.054) and anterior commissure involvement were found to be unfavorable factors for local control.
Of the several multivariate analysis models applied here, the model depicted in Table 4 was chosen because of its high statistical power. According to this model, only anterior commissure involvement was identified as a statistically significant factor influencing the prospect of local control (risk ratio 5.3, 95 % CI 2.3–12, p < 0.001).
In the patients with T2 cancer, the 5-year LC rates for CRT and RT alone were 67 and 76 %, respectively, the difference not being statistically significant (p = 0.832).
Discussion
Despite the high cure rate achieved by radiation therapy in patients with early glottic cancer, there were some cases with persistent diseases or tumor recurrence. To define this subgroup at high risk of failure of primary treatment, we retrospectively analyzed the influence of different clinical and treatment variables on the local control results. We found that 5 % of patients had persistent disease and 14 % had local recurrence, with an overall 5-year LC rate of 81 %. The LC rates in patients with T1 and T2 tumors were 89 and 74 %, respectively. These figures are consistent with previous reports [2–4]. The result of univariate analysis showed that habitual drinking, high T stage and anterior commissure involvement were predictive of poor local control. When multivariate analysis was performed using the same variables, only anterior commissure involvement remained statistically significant.
T stage is well known as a prognostic factor in patients with glottic cancer [12]. In the present study, it was revealed as a prognostic factor in the univariate analysis, although multivariate analysis did not identify it as a significant factor, probably because of the relatively good local control in the patients with T2 disease in our series.
Anterior commissure involvement is also known as one of the significant prognostic factors, and the reported 5-year LC rate in early glottis cancer patients with anterior commissure involvement ranges from 55 to 70 % [3, 15, 16, 24]. Anatomically, the anterior commissure is attached directly to the thyroid cartilage, which lacks a protective perichondrial lining as a potential tumor barrier [25]. This barrier is a relatively weak area from the point of view of tumor dissemination, where the Broyles’ ligament penetrates into the thyroid cartilage [26].
In our series, anterior commissure extension was found to be an independent prognostic factor in terms of the local control, since we observed 15 cases of relapse out of the 32 with anterior commissure extension, as compared to 9 cases of relapse out of 99 without anterior commissure extension (p < 0.000001). The main and extension parts of recurrent tumors concerning T stage and status of anterior commissure involvement are shown in Table 5. In most patients with anterior commissure involvement, the local recurrence occurred mainly in the anterior portion of the vocal fold or anterior commissure, and most of them had subglottic extension.
On the other hand, some investigators have reported anterior commissure involvement that is not associated with decreased local control following RT [2, 27, 28]. The reasons for such discrepant reports are not clear; there is a possibility of the influence of the irradiation method and technique on the prospect of local control. It is known that the depth where the absorbed radiation dose becomes maximum varies according to the radiation energy used. For example, the absorbed radiation dose becomes greatest at 0.5 cm with 60Co, 1–1.2 cm with 4MV X-rays and 1.5 cm with 6MV X-rays [29]. Sombeck et al. [30] conducted experimental comparisons between 60Co and 6MV beams, and indicated that although 60Co and 6MV X-ray yielded similar doses to the true vocal cords and anterior commissure, the subcutaneous tissues between the anterior commissure and skin surface received a significantly lower dose with 6MV X-rays. They mentioned that patients with anterior commissure involvement might have clinically unappreciated invasion of the anterior subcutaneous tissues and that lower energy beams might be better for patients with anterior commissure involvement.
The radiation fraction size and overall treatment time are also known to be correlated with the success of local control of early glottic cancer. Yamazaki et al. [31] investigated the effect of the radiation fraction size and overall treatment time on the local control of T1 glottic cancer in a prospective randomized study, and concluded that the use of 2.25-Gy fractions with a shorter overall treatment time was associated with superior local control rates as compared to the conventional 2-Gy fractions. Miao-Fen et al. [32] also published the results of a retrospective review of 134 patients of early glottic cancer, and reported that in their study, anterior commissure involvement was a poor prognostic factor in patients with T1 glottic cancer and that further subgroup analysis revealed that a fraction size of >2 Gy could overcome the negative impact of anterior commissure involvement and significantly improve the 5-year local control rate in the patients with T1 disease (100 % at >2 Gy vs. 45 % at ≤ 2 Gy; p = 0.04). Takaya et al. [33] also published data on a series of 48 patients treated with RT for T2 glottic cancer; 21 patients were treated with hyperfractionated RT (twice a day), and 27 patients were treated with conventional RT (once a day). In their series, there were 13 patients with invasion of the anterior commissure in the twice-a-day group, and all the patients were cured and the laryngeal preservation (LP) rate was 100 %. On the other hand, in the once-a-day group, there were 8 patients with invasion of the anterior commissure and the larynx could not be preserved in 5 cases (LP rate 38 %). Thus, the results were worse in the once-a-day RT group.
Thus, the technique/method of radiotherapy might influence the local control rate. In our series, we administered conventional radiotherapy using fractional doses of 2 Gy once a day for all cases. There were 32 patients with anterior commissure involvement, of whom 13 were treated with 60Co and 19 with 4MV X-rays. The local control rate was 58 % in those treated with 60Co and 27 % in those treated with 4MV X-rays, while the treatment results appeared to be poorer in the patients treated with 4MV X-rays, the difference between the two groups was not statistically significant. However, as the local control rates were low in both groups, we strongly need to reconsider the treatment approach for patients with anterior commissure involvement.
There are several reports on the effectiveness of surgical treatment for early glottic cancer with anterior commissure involvement. Ralph et al. [34] analyzed the influence of anterior commissure involvement on the local recurrence rate in 444 patients with early glottic cancer who underwent transoral laser microsurgery (TLM). They reported that involvement of the anterior commissure affected the local control, but not the survival rates. The Kaplan–Meier 5-year local control rate of carcinomas with and without involvement of the anterior commissure was 73 vs. 89 % for T1a carcinomas, 68 vs. 86 % for T1b carcinomas, and 76 vs. 76 % for T2a carcinomas. Similar results were reported by Sachse et al. [35]. In regard to the usefulness of surgical methods other than TLM, Laccourreye et al. [7] reported the results in 416 patients with early glottic cancer who underwent open vertical partial laryngectomy. The local recurrence rate in the cases with involvement of the anterior commissure was 23 %. Laccourreye et al. [8] also reported a 5-year local control rate of 98 % in 62 patients with T1 or T2 glottic carcinoma with anterior commissure involvement treated by SCPL-CHEP. While this method appeared to yield a superior local control rate, it posed some problems from the aspect of maintenance of function. In their series, 17 patients needed postoperative swallowing training, 4 received temporary, and one, permanent percutaneous endoscopic gastrostomy (PEG). Therefore, this procedure may not be the procedure of first choice for patients with early glottic cancer from the point of view of function preservation.
The effectiveness of CRT for advanced head and neck cancer has been reported from a meta-analysis of randomized controlled trials. [18] The chemotherapeutic regimens contain platinum drugs, such as cisplatin, 5-FU, docetaxel, and combination of these drugs. And recently, cetuximab-based bio-radiotherapy (BRT) became to be one of the treatment choices for advanced head and neck cancer. [36] On the other hand, effectiveness of CRT or BRT for early stage of head and neck cancer is not yet proved by randomized controlled trial. Therefore, standard treatment for early stage head and neck cancer is RT alone or surgery. However, despite the high cure rate achieved by RT alone in patients with early glottic cancer, there were some cases with persistent diseases or tumor recurrence. According to several reports, the prospect of local control of early glottic cancer depends mainly on T stage, the tumor size and presence/absence of anterior commissure involvement [12–14]. Therefore, we treated these high-risk patients by CRT to improve local control.
There are some reports showing effectiveness of CRT for early laryngeal cancer. Kumamoto et al. [21] reported the results of CRT for T2N0 glottic cancer. They administered one bolus i.v. injection of 5-FU and reported 5-year voice preservation and complete laryngeal preservation rates of 91 and 87 %, respectively. Similar results were reported from some other studies [20, 22] suggesting that CRT may be a safe and promising treatment approach for obtaining local control in patients with T2 glottic cancer. On the other hand, there are also reports indicating no differences in the local control rates between patients treated by this and other methods [37].
We employ CRT using of S-1 for patients with T2 cancer, especially those with bulky tumor or anterior commissure involvement. In head and neck cancer, some retrospective studies have indicated that CRT using of S-1 improves the local control and larynx preservation rates in patients with early glottic cancer with low toxicity [22, 38, 39]. We used S-1 because of its low toxicity and good compliance.
In this study, our estimated 5-year LC rates are 72 % for RT alone and 67 % for CRT, with no statistically significant difference in the results between the two treatments. We also examined the effectiveness of CRT for cases with anterior commissure involvement; in our present patient series of 19 T2 cancer patients with anterior commissure involvement, 9 underwent CRT and the remaining 10 received RT alone; the 5-year local control rate was 40 % in both the groups (p = 0.595). Thus, CRT did not improve the LC rate in the cases with anterior commissure involvement.
Thus, no obvious effectiveness of CRT for local control was observed in this study.
However, since this study was a retrospective analysis carried out on a limited number of patients, we cannot arrive at any definitive conclusion on the effectiveness of CRT for early glottic cancer. However, in the absence of clear evidence of the efficacy of CRT for early glottic cancer, we consider that in the very least, all patients with T2 cancer are not suitable candidates for CRT. A prospective randomized controlled trial is needed to evaluate the effectiveness of CRT for early glottic cancer.
Conclusion
This study suggests that the presence/absence of anterior commissure involvement is one of the most significant prognostic factors for local control in patients with early glottis cancer. We need to reconsider the radiotherapy method for cases with anterior commissure involvement, as a difference in the radiotherapeutic approach may influence the prospect of local control in these cases.
CRT for T2 patients was not found to be effective in this study. A prospective randomized controlled trial is needed to evaluate the effectiveness of CRT for early glottic cancer.
References
Ambrosch P, Fazel A (2011) Functional organ preservation in laryngeal and hypopharyngeal cancer. Laryngorhinootologie 90(Suppl 1):S83–S109
Mendenhall WM et al (2001) T1-T2N0 squamous cell carcinoma of the glottic larynx treated with radiation therapy. J Clin Oncol 19(20):4029–4036
Johansen LV, Grau C, Overgaard J (2002) Glottic carcinoma–patterns of failure and salvage treatment after curative radiotherapy in 861 consecutive patients. Radiother Oncol 63(3):257–267
Chera BS et al (2010) T1N0 to T2N0 squamous cell carcinoma of the glottic larynx treated with definitive radiotherapy. Int J Radiat Oncol Biol Phys 78(2):461–466
Motta G et al (2005) CO2 laser surgery in the treatment of glottic cancer. Head Neck 27(8):733
Peretti G et al (2010) Transoral CO(2) laser treatment for T(is)-T(3) glottic cancer: the University of Brescia experience on 595 patients. Head Neck 32(8):977–983
Laccourreye O et al (1991) Vertical partial laryngectomy: a critical analysis of local recurrence. Ann Otol Rhinol Laryngol 100(1):68–71
Laccourreye O et al (1997) Supracricoid partial laryngectomy with cricohyoidoepiglottopexy for “early” glottic carcinoma classified as T1-T2N0 invading the anterior commissure. Am J Otolaryngol 18(6):385–390
Fein DA et al (1993) T1-T2 squamous cell carcinoma of the glottic larynx treated with radiotherapy: a multivariate analysis of variables potentially influencing local control. Int J Radiat Oncol Biol Phys 25(4):605–611
Mendenhall WM et al (1994) Management of Tis, T1, and T2 squamous cell carcinoma of the glottic larynx. Am J Otolaryngol 15(4):250–257
Tombolini V et al (1995) Radiotherapy for T1 carcinoma of the glottis. Tumori 81(6):414–418
Wiernik G et al (1990) The predictive value of tumor classification compared with results of the British Institute of Radiology fractionation trial in the treatment of laryngopharyngeal carcinoma. Laryngoscope 100(8):863–872
Overgaard J et al (1986) Primary radiotherapy of larynx and pharynx carcinoma–an analysis of some factors influencing local control and survival. Int J Radiat Oncol Biol Phys 12(4):515–521
Teshima T, Chatani M, Inoue T (1989) Radiation therapy for early glottic cancer (T1N0M0): I. Results of conventional open field technique. Int J Radiat Oncol Biol Phys 17(6):1199–1202
Zouhair A et al (2004) Decreased local control following radiation therapy alone in early-stage glottic carcinoma with anterior commissure extension. Strahlenther Onkol 180(2):84–90
Le QT et al (1997) Influence of fraction size, total dose, and overall time on local control of T1-T2 glottic carcinoma. Int J Radiat Oncol Biol Phys 39(1):115–126
Marshak G et al (1999) Prognostic factors for local control of early glottic cancer: the Rabin Medical Center retrospective study on 207 patients. Int J Radiat Oncol Biol Phys 43(5):1009–1013
Pignon JP et al (2009) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 92(1):4–14
Akimoto T et al (2006) Radiation therapy for T2N0 laryngeal cancer: a retrospective analysis for the impact of concurrent chemotherapy on local control. Int J Radiat Oncol Biol Phys 64(4):995–1001
Itoh Y, Fuwa N (2006) Retrospective analysis: concurrent chemoradiotherapy using protracted continuous infusion of low-dose cisplatin and 5-fluorouracil for T2N0 glottic cancer. Radiat Med 24(4):277–281
Kumamoto Y et al (2002) “FAR” chemoradiotherapy improves laryngeal preservation rates in patients with T2N0 glottic carcinoma. Head Neck 24(7):637–642
Tsuji H et al (2006) A phase I study of concurrent chemoradiotherapy with S-1 for T2N0 glottic carcinoma. Oncology 71(5–6):369–373
Shirasaka T et al (1996) Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs 7(5):548–557
Bron LP et al (2001) Treatment of early stage squamous-cell carcinoma of the glottic larynx: endoscopic surgery or cricohyoidoepiglottopexy versus radiotherapy. Head Neck 23(10):823–829
Bagatella F, Bignardi L (1981) Morphological study of the laryngeal anterior commissure with regard to the spread of cancer. Acta Otolaryngol 92(1–2):167–171
Shvero J et al (1994) T1 glottic carcinoma involving the anterior commissure. Eur J Surg Oncol 20(5):557–560
Benninger MS et al (1994) Factors associated with recurrence and voice quality following radiation therapy for T1 and T2 glottic carcinomas. Laryngoscope 104(3 Pt 1):294–298
Foote RL et al (1996) Radiation therapy for glottic cancer using 6-MV photons. Cancer 77(2):381–386
Halperin EC et al (2008) Perez and Brady’s principles and practice of radiation oncology. 6 edn. Philadelphia. 1936, p 160
Sombeck MD et al (1996) Radiotherapy for early vocal cord cancer: a dosimetric analysis of 60CO versus 6 MV photons. Head Neck 18(2):167–173
Yamazaki H et al (2006) Radiotherapy for early glottic carcinoma (T1N0M0): results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol Biol Phys 64(1):77–82
Chen MF et al (2003) Radiotherapy of early-stage glottic cancer: analysis of factors affecting prognosis. Ann Otol Rhinol Laryngol 112(10):904–911
Tateya I et al (2006) Hyperfractionated radiotherapy for T2 glottic cancer for preservation of the larynx. Eur Arch Otorhinolaryngol 263(2):144–148
Rodel RM et al (2009) Endoscopic laser surgery of early glottic cancer: involvement of the anterior commissure. Head Neck 31(5):583–592
Sachse F, Stoll W, Rudack C (2009) Evaluation of treatment results with regard to initial anterior commissure involvement in early glottic carcinoma treated by external partial surgery or transoral laser microresection. Head Neck 31(4):531–537
Bonner JA et al (2010) Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 11(1):21–28
Niibe Y et al (2007) Effectiveness of concurrent radiation therapy with UFT or TS-1 for T2N0 glottic cancer in Japan. Anticancer Res 27(5b):3497–3500
Nakayama M et al (2010) Phase I/II trial of concurrent use of S-1 and radiation therapy for T2 glottic cancer. Jpn J Clin Oncol 40(10):921–926
Nonoshita T et al (2010) Concurrent chemoradiotherapy with S-1 for T2N0 glottic squamous cell carcinoma. J Radiat Res 51(4):481–484
Conflict of interest
All author report no conflict of interest related to his manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kitani, Y., Kubota, A., Furukawa, M. et al. Prognostic factors for local control in patients receiving radiation therapy for early glottic cancer: anterior commissure involvement and effect of chemoradiotherapy. Eur Arch Otorhinolaryngol 273, 1011–1017 (2016). https://doi.org/10.1007/s00405-015-3579-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-015-3579-8