Abstract
Purpose
To investigate live birth rate (LBR) and cumulative live birth rate (CLBR) to achieve the first newborn per blastocyst transferred and oocyte retrieved in the first complete IVF cycle of autologous and donated oocytes and identify the possible success factors.
Methods
This was a retrospective cohort study of a private IVF center. There were 1867 cycles, 1241 of which were fresh transfers and 626, their subsequent thawing transfers.
Results
We found significant variables by binary logistic regression. For LBR, female infertility and the day of blastocyst transferred were relevant; however, for CLBR, the numbers of blastocysts available for future transfers, oocyte age, and maternal age were more critical. Oocyte age is a negative factor that begins to affect CLBR gradually beyond 36 years; from that age, there are significant worse results in polycystic ovary syndrome and poor responder patients.
Conclusion
The LBR and CLBR were optimized for oocyte recipients when eight oocytes were retrieved (63.6%; 87.9%); at most, fourteen oocytes should be assigned to avoid freezing surplus blastocysts. Thirteen autologous oocytes (69.2%; 92.3%) were ideal for optimization. CLBR optimized after three blastocysts in donor oocytes (81.8%) and four for autologous oocyte patients (80.9%). Our outcomes are valuable for doctors and infertile couples, and they give us information on what we can expect from a first complete IVF cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
For recipients and autologous oocytes, eight oocytes or three blastocysts and thirteen oocytes or four blastocysts, respectively, optimized LBR and CLBR in the first complete IVF cycle. |
Introduction
In most countries, it has been estimated that more than 50% of couples seek medical attention for their infertility problems. Advanced maternal age (AMA) is one of the most common causes of infertility; AMA has led to an increase in the average age of the first pregnancy and a reduction in the cumulative live birth rates (CLBR) [1]. Live birth rate (LBR) and CLBR are defined as the possibility of achieving a live newborn after using fresh embryos and all frozen embryos resulting from an in vitro fertilization (IVF) stimulation cycle of the same oocyte collection [2]. Although there has been a decrease in the number of embryos transferred, several studies indicate an improvement in the success rate of IVF accumulated in recent decades [3] due to consistent clinical results between fresh and frozen–thawing embryo transfers (FET) that have been reported with high survival rates of blastocysts with the vitrification method [4].
An important counseling aspect in assisted reproductive technology (ART) is identifying failure risk and the limit of treatments [2]. Each couple should consider either renouncing or changing their gametes according to their chances of success, beliefs, and values regarding the cost–benefit that will be obtained. Thus, when couples start their first cycle, they often want to know their chance of having a newborn with their fresh or frozen surplus embryos. Although there have been several studies that have examined live birth and cumulative live birth rates in different scenarios [5] and other articles that studied the impact of various factors on live birth rates, this research examined them considering all aspects simultaneously and at all ages; because to our knowledge, more information is necessary to be focused their effects on LBR and CLBR in the first complete IVF cycle, especially for the number of blastocysts transferred and oocytes retrieved.
Materials and methods
A retrospective cohort study of a private ART center was investigated, where we analyzed 1867 cycles, of which 1241 were first IVF fresh transfer, and 626 were their subsequent FET, of autologous (AO) and donated oocytes (DO), from January 1, 2009 to January 30, 2023. Both fresh and FET were included. The study was designed by the ARL and approved by the Institutional Review Board of ANU, number 201911; the data were anonymous, following all rules regarding protecting personal information.
For the predictive ability, it was essential to include both low- and high-prognosis couples when considering the social realities of the population studied. All patients had primary infertility (no newborn before).
Inclusion criteria were established
Patients between 30 and 45 years old, diagnosed with polycystic ovary syndrome (PCOS) and poor ovarian responder (POOR), tubal obstruction, grade I of endometriosis, and all patients who received fresh oocyte donation.
Exclusion criteria established
The presence of any additional female pathology, use of any extraction of sperm technique from patient, body mass index BMI ≥ 30, severe endometriosis, hydrosalpinx, patients with some indicator of ovarian hyperstimulation syndrome (OHSS), and couples who have a morphological sperm selection technique (IMSI) or chemical sperm selection technique (PICSI) before intracytoplasmic sperm injection (ICSI) or who performed preimplantation genetics test for aneuploidies (PGTA) or the used of frozen sperm or oocytes.
All patients of their AO received GnRH antagonist protocol in conventional IVF stimulation. Oocyte donors were healthy women aged 18–30, selected by international consensus. For all oocyte recipients’ transfers and FET, the endometrial preparation was with exogenous administration of estrogen and progesterone. At the time of embryo transfer, the mean endometrial thickness was 8.7 ± 1.9 mm, and the mean estradiol concentration was 332.3 ± 361.6 pg/ml. Clinical pregnancies were confirmed when a gestational sac with a fetal heartbeat was detected by ultrasound at seven weeks of pregnancy. Live birth (LB) was defined as the delivery of any live-born infant (at least 24 weeks or more of gestation) in the fresh or the subsequent FET cycles. Only the first delivery was considered in the analysis. Embryos were cultured under 5% oxygen and the same culture medium (Vitrolife). Those blastocysts reaching an inner diameter of over 160 m on day 5 or 6 were transferred or vitrified. In our clinic, all transfers and vitrification [4] are made in the blastocyst stage with viable potential blastocysts in their different combinations of internal cell mass and trophectoderm, according to the Gardner classification [6]. Regardless of the semen sample, we included patients only with a diagnosis of terato-, astheno-, or asthenoteratozoospermia, according to the criteria of the World Health Organization, with fresh seminal samples, a density gradient centrifugation procedure, recovery of at least 2 million progressive motile sperm for ICSI and/or IVF, and DFI per sperm chromatin dispersion tests (SCD) [7]. Reproductive success was measured per blastocyst transfer, per number of blastocysts transferred, and per number of oocytes retrieved needed to achieve a live birth.
Statistical analysis
The selecting variables were based on their statistical frequency and scientific relevance. The nominal and ordinal candidate predictor variables were subjected to bivariable analysis by chi-square test to evaluate statistical relationships between each candidate predictor and the outcomes, LB and CLBR. For the binary logistic regression analysis (BLRA), the determinants involved were identified and selected for their frequency relevance, according to the knowledge by literature and the information provided by the data [8]. Maternal and oocyte age were grouped into three age groups: ≤ 35, 36 to 39, and ≥ 40 years old [9]; in this study, maternal age is the age of the woman who received the transfer, and oocyte age is the age of the oocyte at the time of retrieval; for DFI were grouped in ≤ 15, 16 to 29, and ≥ 30% [7]. Prognostic power was evaluated with internal validation using data from the same study cases; the study population was randomly divided into two groups: 70% of the validation set and 30% of the training set and bootstrapping test. We used the standard method of entry for exploratory analysis and backward stepwise; we also verified the results with p = 0.157, a detention rule equivalent to using the AIC criterion [10].
We calculated the area under the curve (AUC) of the receiver operating characteristics (ROC) in training and test groups to evaluate the factors’ discriminating capacity. The Hosmer–Lemeshow test evaluated regression calibration. The uncertainties uncovered by the instrument were evaluated by pseudo-coefficients r2, Nagelkerke, and Cox–Snell [10]; the p value considered statistically significant was 0.05. Beta coefficients were used to calculate the predicted probability of live birth rate for each observation in the validation data set. The Kaplan–Meier estimator was used to evaluate the CLBR per blastocyst transferred through Log-rank, Breslow, and Tarone–Ware tests according to each categorical group. Microsoft Excel software was used for some analyses and graph generation. Statistical analysis was performed using the Statistical Package for the Social Sciences 26 SPSS and MedCalc 20 Software.
Results
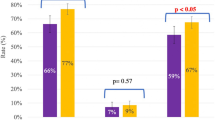
In Table 1, we show the general baseline characteristics of the population. All the clinical characteristics of the factors are shown in Table 2; the probability of having a child for DO and AO in LBR was 68.06% and 50.95%; for CLBR, it was 84.58% and 79.66%, respectively. In oocyte age, women ≥ 40 had the lowest LBR (40.15%) and CLBR (67.04%). By analyzing the number of oocytes retrieved by groups of oocytes, the highest rates were when recovered between 11 and 15 oocytes, LBR (56.6% or 70.7%), and CLBR (86.26% or 87.50%). For DFI, ≥ 30% had the lowest rates among all factors studied, including LBR (30.05%) and CLBR (55.95%). For female infertility, the lowest rates were for the POOR patients, LBR (42.33%) and CLBR (71.16%), followed by PCOS and endometriosis grade I, respectively. For the day of blastocyst transfer, day 5 or 6, there were significant differences in favor of day five. There was no significant difference between the numbers of blastocysts transferred for FET, but yes, for fresh transfers. Our overall rate of one blastocyst transfer was 32%. Regarding the number of blastocysts available in AO, LBR plateaued and optimized after two blastocysts (80%) and CLBR for four blastocysts (87.60%). Per oocyte age, we found that in patients between 36 and 39 and ≥ 40 years, the average number of those who did not have a newborn was 8.8% and 20.1% for POOR and 7.1% and 7.3% for PCOS.
For the BLRA, we analyzed these variables: number of blastocysts transferred, day of transferred, oocyte age, maternal age, DFI, number of oocytes retrieved, female infertility, sperm diagnostic, number of available blastocysts, and oocytes’ origin AO or DO. The variables selected as significant predictors of LBR and CLBR are presented in Table 3. We found five essential factors in each rate. The r2, Nagelkerke, and Hosmer–Lemeshow calibration were satisfactory for training and validation sets. Concerning reference groups, BLRA showed common factors that were associated with LBR and CLBR, like oocyte age, DFI, and the number of available blastocysts. Additionally, only female infertility and the day of transfer were important for LBR, and maternal age and the number of blastocysts transferred were more critical for CLBR. Table 3. The area under the AUC of the ROC curves were 0.784 and 0.826, indicating how much the analysis could discriminate between LBR and non-LBR couples. Figure 1, comprising data, shows that the discriminatory capacity of this predictive analysis could be considered adequate for these factors [11].
Success rates were evaluated per blastocyst transferred to create Kaplan–Meier curves Fig. 2. Except for maternal age (long-rank test: X2 5.5, < 0.0613), there are significant differences between oocyte origin, female infertility, DFI, and oocyte age in all subgroups, 95%CI (2.804–3.031) p < 0.001 for all Log-rank, Breslow, and Tarone–Ware tests comparisons. These curves show CLBR depending on the total blastocysts used until the first newborn. This allowed us to calculate the blastocysts for each variable; we show all graphs and descriptive results only for oocyte origin. It demonstrates blastocysts per blastocyst that CLBR increased in DO after three blastocysts at 81.8% (95% CI 80.8–82.8), after four at 88.7% (95% CI 87.7–86.7), representing an increase of 6.9% per every additional blastocyst; in AO, after four blastocysts at 80.9% (95% CI 79.9–81.9), after five at 86.4% (95% CI 85.4–87.4), representing an increase of 5.5% per every additional blastocyst.
The LBR and CLBR were optimized for oocyte recipients when eight oocytes (63.6% CI 62.6–64.6; 87.9% CI 86.9–88.9) were obtained, for fourteen oocytes (79.4% CI 78.4–80.4; 97.10% CI 96.1–98.1) it was higher rate. Thirteen autologous oocytes (69.2% CI 68.2–70.2; 92.3% CI 91.3–93.3) were ideal for optimization. Figure 3.
Discussion
For almost a decade, one of the most suitable and accepted indicators by the scientific community to report the results of ART has been LBR and CLBR for one or several complete cycles of IVF. It has been agreed that the CLBR is more meaningful to patients and clinicians than cycle-based success rates [2] [12,13,14,15]. Hence, to evaluate CLBR for several cycles in time, cohorts of studies with data from the late nineties and early 2000s included variability because of the time of the cohort. i.e., different stimulations protocols, some patients with blastocyst transfers, others with embryo transfer on day two and day three, and frozen embryos with slow freezing technique and vitrification [12, 13, 15,16,17]; some reported CLBR based on the total number of embryos transferred or the number of oocytes retrieved in several consecutive cycles.
Regarding the number of oocytes retrieved to reach the live birth, in the context of traditional ovarian stimulation, there are two positions, one of which the more significant number of eggs has a higher CLBR [16, 17], and the other, which does not necessarily have a more substantial number is better for outcomes [18]. Some authors found an improvement in CLBR with increasing oocyte yield; Polyzos et al. [16] suggest that CLBRs continuously grow with the number of oocytes retrieved in suitable prognosis patient’s ≤ 40 years old. Others report that LBR per cycle does not rise significantly after a certain number of oocytes are retrieved (i.e., between 10 and 12) [18]. Some studies even reported an apparent decline in the LBR with a high oocyte retrieved [19, 20]. Two systematic reviews suggested that retrieving 12–18 oocytes is associated with maximal fresh LBR; they found a positive association between the number of oocytes and the CLBR. However, this association varies according to patients’ age [21] [22] and there is no consensus on the optimum number of oocytes that could balance an optimal CLBR without the risk of ovarian hyperstimulation syndrome OHSS [20] [23].
It is risky to generalize that a great number of oocytes retrieved are better when there is no consideration of oocyte age and female infertility; these factors affect the quality of the oocytes and blastocysts. The concept of oocyte competence is associated with the ability of oocytes to perform reproductive functions [24]. The quality has been defined based on the morphology of the oocyte and often on oocyte age. Using different oocyte competencies, AO and DO, could be more beneficial for evaluating the effect of these on the CLBR. We found that after nineteen oocytes, CLBR had no increase at all. Cycles performed with DO are also consistently associated with higher LBR and CLBR than those achieved with their AO within different age categories [24].
We found the lowest CLBR for the oldest patients when they used AO, 67.04%, similar to other authors, in the first cycle, 64.6% [25] and 69.8% [26]. Our results show that in the group of ≥ 40 years, there were most miscarriages, not only for oocyte recipients but also for POOR and DFI ≥ 30 patients. The impact of female infertility and DFI on the variability of oocyte repair is minor in a young oocyte; in women with AMA over 40 years, samples with a high DFI show the impact on clinical outcomes, embryo development, poor quality, reduced fertilization rate, blastocyst rate, lower implantation and pregnancy rates, and increased miscarriage rates [27]. Our population used the SDC technique for DFI analysis; logistically and economically, no other routine methods were performed for patients. However, a good correlation exists between DFI rates reported with different techniques [7].
Our study did not include patients with PGTA so that chromosomal abnormalities could explain the differences in clinical outcomes between age groups. When maternal age and oocyte age were combined, for oocyte recipients, the highest CLBR was in patients with maternal aged 35–39 years, and the lowest CLBR was in patients ≥ 40 years; even if we use DO, AMA affected the CLBR, this can be one of the explanations of miscarriages and live birth rates reduced, due to impaired cellular senescence and defective endometrial receptivity in these women [28].
Concerning female infertility, some authors consider that even though PCOS patients typically produce more oocytes, these are often of poor quality and show a low rate of fertilization and blastulation [29]; in their studies, they reported it more appropriate to evaluate the CLBR by the number of embryos to be transferred and not by the number of oocytes, [12]. In PCOS patients, high CLBR can be obtained when the number of oocytes retrieved was 15 or more [29]; however, the rate of embryos available and embryo quality was lower when over 18 oocytes were retrieved and even worse if women’s age increased [29]. Oocyte age is a negative factor that begins to affect CLBR gradually beyond 36 years; related to female infertility from that age, we found significantly worse results for POOR and PCOS patients [29, 30]
In a recent study [23], they reported that nine oocytes, or four embryos, can optimize LBR, and there was no increase in the CLBR with more than 12–15 oocytes or nine embryos in the perspective of regular responders with minimal stimulation; at the same time, this research found that thirteen oocytes optimize the LBR and CLBR for AO (traditional stimulation protocol). For DO, eight oocytes optimize the LBR and CLBR; at most, fourteen should be assigned in the first cycle to avoid excess freezing of surplus blastocysts in oocyte recipients.
Given the quick changes in the last 10 years, not only in the management of ovarian stimulations [23] but also in incubation technology, time-lapse, low concentration of oxygen, blastocyst culture for transfer, and vitrification [4] of surplus blastocysts, it has achieved high pregnancy and live birth rates, revolutionizing the deferred handling of transfers, the use of embryo and oocyte banking, single embryo transfer, SET, and the PGTA [31].
Nowadays, we have more blastocysts viable per cycle than 20 years ago, and we believe that in this vitrification era, with blastocyst transfer, we could consider each blastocyst as a single opportunity for achieving a live birth; in the last two decades, the expectations of LBR and CLBR have been changed for the first complete IVF cycle. For example, another study [31] analyzed a period of 10 years (2007 to 2017); during this time, the chance of having a live-born baby increased from 27 to 36.3% per complete IVF cycle, and with low multiple birth rates, because of these technology changes.
Other studies show blastocyst stage transfer was associated with higher CLBR 56.48%, more than cleavage stage [14]. In our research, for AO and DO, LBR was 50.95% and 68.06%, and CLBR was 79.66% and 84.58%, respectively. A Danish fertility clinic [5] found a CLBR of 64.0% after multiple ovarian stimulations, and they considered only using blastocyst transfer, too. Even in single embryo transfers, blastocyst vitrification is essential in improving CLBR. It allows for a lower number of oocytes retrieved needed to achieve an LB and a shortened time to get it [31]; for instance, with the freeze-all strategy, the chance of having a child after the first complete IVF cycle was 50.74% [32].
Another study found that five blastocysts will maximize LBR in SET fresh transfer by ≤ 36 years old patients [33]; there are other positions regarding the CBLR and blastocyst; a Cochrane review found higher LBR after blastocyst more than cleavage stage transfer in fresh cycles, but they reported that the situation remains unclear for CLBR [34].
Blastocyst transfer literature displays discordant results regarding the transfer day for fresh and FET cycles. A recent meta-analysis [35] recommends that ART practitioners should preferably transfer D5 rather than D6 blastocysts in both fresh and FET cycles. Although transferring a D6 vitrified-warmed blastocyst remains a reasonable option, prioritizing a D5 embryo would reduce the time to a successful pregnancy [36]. In our study, all blastocysts vitrified on D5 or D6 were warmed on D5 progesterone and transferred after 2 or 3 h; we found differences in favor of blastocysts transferred on D5 for LBR and CLBR. According to numbers three to four, blastocysts optimized CLBR for DO and AO, respectively. As in previous studies [12] [25], with Kaplan–Meier, we could determine at which point of the curve the couple is and then, in agreement with them, decide to continue or move toward ovum donation, remaining childless, or make another cycle.
The strengths of our study include the robust size of the cohort and the wide range of demographic variables assessed of all the couples with inclusion criteria (14 years had been included), followed by all subsequent blastocyst FET of the same cohort until they got their first newborn.
The discriminatory capacity can be considered adequate with a value of AUC for LBR and CLBR. Optimization of stimulation protocols and the effectiveness of the blastocyst vitrification technique can explain the high CLBR in this study. The main area for improvement is the retrospective design, where unmeasured confounders might play a role. Large prospective controlled trials are needed to validate the current findings. Secondly, it would be helpful in subsequent studies to include the anti-Müllerian hormone test; not all patients had this test because it had been routinely implemented in the clinic since 2012; these hormone values could influence the evaluation of ovarian reserve, which could change the overall percentage prediction. Third, other variables like the trophectoderm and ICM could provide further information in future analysis.
The data reported here are initial explorations to propose a post-treatment model that could predict the chances of success before starting the second cycle in couples whose first complete cycle was unsuccessful. When predicting the second cycle, all factors mentioned in this study and the outcome from the first complete cycle would be critical to consider before starting over [8] [37].
We found common and different predictor factors between LBR and CLBR. For LBR, female infertility and the day of blastocyst transfer were relevant; however, for CLBR, the number of blastocysts available for future transfers, oocyte age, and maternal age was more critical. Our outcomes are valuable both for doctors and infertile couples who will carry out their first IVF/ICSI cycle with AO or DO; they not only give us information on what we can expect from the complete cycle regarding LBR and CLBR but also help us to decide whether to continue with another cycle after not having a newborn in the first one.
References
Vollenhoven B, Hunt S (2018) Ovarian ageing and the impact on female fertility. F1000Res 7:1835
De Neubourg D et al (2016) How do cumulative live birth rates and cumulative multiple live birth rates over complete courses of assisted reproductive technology treatment per woman compare among registries? Hum Reprod 31(1):93–99
Wade JJ, MacLachlan V, Kovacs G (2015) The success rate of IVF has significantly improved over the last decade. Aust N Z J Obstet Gynaecol 55(5):473–476
Kuwayama M (2007) Highly efficient vitrification for cryopreservation of human oocytes and embryos: the cryotop method. Theriogenology 67(1):73–80
Høyer S, Kesmodel US, Aagaard J (2021) Conditional and cumulative live birth rates after blastocyst transfer. Eur J Obstet Gynecol Reprod Biol 261:46–51
Gardner DK et al (2000) Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril 73(6):1155–1158
Liffner S et al (2019) Diagnostics of DNA fragmentation in human spermatozoa: are sperm chromatin structure analysis and sperm chromatin dispersion tests (SCD-HaloSpermG2(®)) comparable? Andrologia 51(8):e13316
Shingshetty L et al (2024) Predictors of success after in vitro fertilization. Fertil Steril 121(5):742–751
Lean SC et al (2017) Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS ONE 12(10):e0186287
Austin PC, Merlo J (2017) Intermediate and advanced topics in multilevel logistic regression analysis. Stat Med 36(20):3257–3277
Arvis P et al (2013) Predictive models for ART. J Gynecol Obstet Biol Reprod (Paris) 42(1):12–20
Garrido N et al (2011) Cumulative live-birth rates per total number of embryos needed to reach newborn in consecutive in vitro fertilization (IVF) cycles: a new approach to measuring the likelihood of IVF success. Fertil Steril 96(1):40–46
Garrido N et al (2012) Cumulative newborn rates increase with the total number of transferred embryos according to an analysis of 15,792 ovum donation cycles. Fertil Steril 98(2):341
Cameron NJ, Bhattacharya S, McLernon DJ (2020) Cumulative live birth rates following blastocyst- versus cleavage-stage embryo transfer in the first complete cycle of IVF: a population-based retrospective cohort study. Hum Reprod 35(10):2365–2374
Drakopoulos P et al (2019) Cumulative live birth rates in in-vitro fertilization. Minerva Ginecol 71(3):207–210
Polyzos NP et al (2018) Cumulative live birth rates according to the number of oocytes retrieved after the first ovarian stimulation for in vitro fertilization/intracytoplasmic sperm injection: a multicenter multinational analysis including ∼15,000 women. Fertil Steril 110(4):661–670
Fanton M et al (2023) A higher number of oocytes retrieved is associated with an increase in fertilized oocytes, blastocysts, and cumulative live birth rates. Fertil Steril 119(5):762–769
Chen YH et al (2015) Optimum oocyte retrieved and transfer strategy in young women with normal ovarian reserve undergoing a long treatment protocol: a retrospective cohort study. J Assist Reprod Genet 32(10):1459–1467
Magnusson Å et al (2018) The number of oocytes retrieved during IVF: a balance between efficacy and safety. Hum Reprod 33(1):58–64
Steward RG et al (2014) Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertil Steril 101(4):967–973
Law YJ et al (2021) Is there an optimal number of oocytes retrieved at which live birth rates or cumulative live birth rates per aspiration are maximized after ART? A systematic review. Reprod Biomed Online 42(1):83–104
Neves AR et al (2023) Association between the number of oocytes and cumulative live birth rate: a systematic review. Best Pract Res Clin Obstet Gynaecol 87:102307
Datta AK et al (2021) Oocyte or embryo number needed to optimize live birth and cumulative live birth rates in mild stimulation IVF cycles. Reprod Biomed Online 43(2):223–232
Moghadam ARE et al (2022) Oocyte quality and aging. JBRA Assist Reprod 26(1):105–122
Abuzeid MI et al (2014) Cumulative live birth rate and assisted reproduction: impact of female age and transfer day. Facts Views Vis Obgyn 6(3):145–149
Wu CH et al (2015) The influence of female age on the cumulative live-birth rate of fresh cycles and subsequent frozen cycles using vitrified blastocysts in hyper-responders. Taiwan J Obstet Gynecol 54(5):567–571
Lourenço ML et al (2023) Impact of sperm DNA fragmentation on the clinical outcome of assisted reproduction techniques: a systematic review of the last five years. JBRA Assist Reprod 27(2):282–291
Pathare ADS et al (2023) Endometrial receptivity in women of advanced age: an underrated factor in infertility. Hum Reprod Update 29(6):773–793
Jia R et al (2022) The optimal number of oocytes retrieved from PCOS patients receiving IVF to obtain associated with maximum cumulative live birth rate and live birth after fresh embryo transfer. Front Endocrinol (Lausanne) 13:878214
Zhu S et al (2024) Nomogram to predict the probability of clinical pregnancy in women with poor ovarian response undergoing in vitro fertilization/intracytoplasmic sperm injection cycles. Arch Gynecol Obstet. https://doi.org/10.1007/s00404-024-07598-9
Saket Z et al (2021) Cumulative live birth rate after IVF: trend over time and the impact of blastocyst culture and vitrification. Hum Reprod Open 2021(3):hoab021
Zhu Q et al (2018) Live birth rates in the first complete IVF cycle among 20 687 women using a freeze-all strategy. Hum Reprod 33(5):924–929
Smeltzer S et al (2019) Clinical pregnancy and live birth increase significantly with every additional blastocyst up to five and decline after that: an analysis of 16,666 first fresh single-blastocyst transfers from the society for assisted reproductive technology registry. Fertil Steril 112(5):866-873.e1
Glujovsky D et al (2022) Cleavage-stage versus blastocyst-stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev 5(5):Cd002118
Bourdon M et al (2019) Day 5 versus day 6 blastocyst transfers: a systematic review and meta-analysis of clinical outcomes. Hum Reprod 34(10):1948–1964
Ferreux L et al (2024) Fresh or frozen day 6 blastocyst transfer: is there still a question? Reprod Biol Endocrinol 22(1):50
Ratna MB et al (2022) Predicting cumulative live birth for couples beginning their second complete cycle of in vitro fertilization treatment. Hum Reprod 37(9):2075–2086
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cubillos-García, S.P., Revilla-Pacheco, F., Meneses-Mayo, M. et al. Required number of blastocysts transferred, and oocytes retrieved to optimize live and cumulative live birth rates in the first complete cycle of IVF for autologous and donated oocytes. Arch Gynecol Obstet (2024). https://doi.org/10.1007/s00404-024-07712-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00404-024-07712-x