Abstract
Purpose
To summarize available evidence from randomized-controlled trials which have evaluated triggering of final oocyte maturation with concomitant GnRH agonists and hCG in patients undergoing IVF, and to analyze whether dual triggering is as efficacious as hCG triggering in terms of oocyte and pregnancy outcomes.
Methods
A comprehensive literature search was performed to identify randomized-controlled trials comparing IVF outcomes between women receiving combined administration of hCG with GnRH agonists and those receiving hCG alone for triggering of final oocyte maturation.
Results
Four studies including 527 patients eligible for inclusion in meta-analysis were identified. No significant difference in the number of mature oocytes or fertilized oocytes retrieved was found between groups. Clinical pregnancy rate with dual triggering was significantly higher as compared with hCG-alone triggering (pooled OR = 0.48, 95% CI 0.31–0.77, P = 0.002), but there was no significant difference in the ongoing pregnancy rate between groups.
Conclusion
Results of meta-analysis indicate comparable or significantly improved outcomes with the use of GnRH agonists plus hCG as compared with hCG alone for triggering of final oocyte maturation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triggering of oocytes to go through the last stage of maturation prior to retrieval and fertilization during in vitro fertilization (IVF) is conventionally accomplished with a single bolus of human chorionic gonadotropin (hCG) as a surrogate luteinizing hormone (LH) surge. However, due to the prolonged luteotrophic effect of hCG, hCG triggering is associated with increased risk of ovarian hyperstimulation syndrome (OHSS), a complication that can lead to severe adverse events involving multiple organ systems in addition to IVF cycle cancellation [1]. Multiple randomized-controlled trials (RCTs) have evaluated gonadotropin-releasing hormone agonists (GnRHa) as an alternative to hCG for triggering final follicular maturation in IVF with controlled ovarian stimulation using GnRH antagonists [2,3,4,5,6]. Triggering with GnRHa has been shown to be beneficial in GnRH antagonist protocols in preventing OHSS, likely due to the shorter duration of LH surge that is induced, which ends 24 h after onset, compared with the surge induced by hCG, which lasts several days into the luteal phase [7, 8]. Findings from a recent Cochrane review provide further support that triggering with GnRHa instead of hCG in GnRH antagonist IVF cycles effectively prevents OHSS; however, the meta-analysis also revealed that GnRHa triggering in fresh autologous cycles was associated with a lower live birth rate, a lower ongoing pregnancy rate, and a higher rate of early miscarriage [1]. It has been postulated that the poorer pregnancy outcomes observed after GnRHa triggering might be associated with its possible negative effect on luteal phase function or endometrial receptivity [7].
Numerous studies have investigated variations in ovulation triggering, including concomitant administration of GnRHa and a reduced or standard dose of hCG (dual triggering) to optimize oocyte maturity, blastulation rates, and pregnancy outcomes [9,10,11,12,13]. It is hypothesized that triggering with GnRHa minimizes the risk of OHSS, while the added hCG rescues luteal function. The purpose of this study is to summarize available evidence from RCTs which have evaluated triggering of final oocyte maturation with concomitant GnRHa and standard dose hCG in patients undergoing IVF, and to analyze whether dual triggering is as efficacious as hCG triggering in terms of oocyte and pregnancy outcomes.
Materials and methods
Search methods
Computerized searches of MEDLINE, EMBASE, Cochrane Controlled Trials Register, and ClinicalTrials.gov databases were conducted in September 2016 using combinations of the following keywords: gonadotropin-releasing hormone, human chorionic gonadotropin, oocyte maturation, and in vitro fertilization. The search was not restricted by language or publication time. In addition, the bibliographies of relevant publications were manually searched for potentially eligible articles. Included articles were RCTs published in full that compared the outcomes of dual triggering versus hCG triggering of final oocyte maturation in women undergoing GnRH antagonist IVF treatment cycles. Duplicate search results were excluded. Publications were excluded if clearly not relevant based on review of title and/or abstract. Abstract publications were excluded due to missing information and inability to assess methodological quality.
Data extraction and quality assessment
Two reviewers independently extracted data from the included studies. The methodological quality of each study was assessed using the risk-of-bias assessment tool outlined in the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0) [14]. Two reviewers subjectively reviewed all studies and assigned a value of “low risk”, “high risk”, or “unclear” to the following: (a) random sequence generation, (b) allocation concealment, (c) blinding (of patients, personnel, and assessor), (d) incomplete outcome data, (e) selective reporting, and (f) inclusion of an intention-to-treat analysis. Any discrepancies were resolved after discussion with a third reviewer.
Outcome measures and data analysis
The primary outcomes for this meta-analysis were the number of mature (MII) oocytes, number of fertilized (2PN) oocytes, pregnancy rate in completed cycles, and ongoing pregnancy rate. Secondary outcomes were implantation rate, the total number of oocytes retrieved, and number of good-quality embryos. Odds ratios with 95% confidence intervals (CIs) were calculated for dichotomous outcomes (pregnancy rate and implantation rate) between patients in the hCG plus GnRHa group and those in the hCG group for each individual study and for all the studies combined. Difference in means (DIM) with 95% CIs between the two groups was calculated for continuous outcomes (the number of mature oocytes, fertilized oocytes, oocytes retrieved, and good-quality embryos). A χ2-based test of homogeneity was performed and the inconsistency index (I2) and Q statistics were determined. Heterogeneity determined using the I2 statistic was defined as follows: 0–24% = no heterogeneity; 25–49% = moderate heterogeneity; 50–74% = large heterogeneity; and 75–100% = extreme heterogeneity. Because the number of studies included in the meta-analysis was small, heterogeneity tests had low statistical power [15]. When tests for heterogeneity are underpowered, random-effects models are routinely used [16]. The National Research Council report recommends the use of random-effects approaches for meta-analysis and the exploration of sources of variation in study results [17]. Pooled effects were calculated, and a two-sided P value < 0.05 was considered to indicate statistical significance. Sensitivity analyses for primary outcomes were carried out using the leave-one-out approach. Publication bias analysis was not performed, because the number of studies was too few to detect an asymmetric funnel [18]. All analyses were performed using Comprehensive Meta-Analysis statistical software, version 2.0 (Biostat, Englewood, NJ, USA).
Results
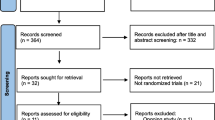
The article selection process is summarized in Fig. 1. After the removal of duplicates, 368 references were identified and screened. Articles were excluded based on title or upon abstract or full-text review due to non-randomized study design, publication not in full, or comparisons not of interest. Four RCTs were deemed eligible and included in the meta-analysis [9,10,11,12].
Study characteristics
The four RCTs included a total of 527 infertile women: 263 patients were triggered with hCG alone and 264 patients with concomitant hCG and GnRHa. Baseline demographics and triggering interventions of the studies are presented in Table 1. All patients were treated with a GnRH antagonist protocol. Oocyte maturation was triggered when at least one follicle reached a diameter ≥ 19 mm [11], when ≥ 1 follicles reached a mean diameter of ≥ 18 mm [10], when 3 follicles reached a diameter of ≥ 17 mm [9], or when ≥ 3 follicles reached a diameter of > 18 mm [12]. For hCG-alone triggering, a standard dose of hCG (5000 or 10,000 IU) was administered in 3 trials [9, 11, 12], and 250 μg of recombinant hCG (rhCG; equivalent to standard dose hCG) was used in 1 trial [10]. For dual triggering, triptorelin 0.1 or 0.2 mg [9,10,11] or leuprolide acetate 1 mg [12] was administered concomitantly with hCG. Embryo transfer was performed on day 3 by Decleer et al., Kim et al., and Mahajan et al. Day of embryo transfer was not reported by Schachter et al. Pregnancy outcomes were reported after fresh embryo transfer [9,10,11]. Mahajan et al. [12] did not report pregnancy outcomes. Dosages of progesterone administered vaginally for luteal phase support included low dose (400 mg micronized progesterone daily) [11], standard dose (90 mg gel form daily) [10], and high dose (200 mg micronized progesterone 3 times daily) [9].
Assessment of risk of bias in included studies
Risk of bias in the included studies is summarized in Fig. 2. All four RCTs were rated as having low risk of bias related to random sequence generation. It was unclear whether allocation concealment was used in the Schachter et al. [11] study. The studies conducted by Kim et al. and Schachter et al. clearly reported lack of blinding of investigators and were deemed to be at high risk of bias related to blinding. Three studies did not clearly report on blinding of outcome assessment and were rated as having unclear risk of bias [9, 10, 12]. All four studies were rated as having low risk of attrition bias and reporting bias; however, only one study [11] clearly reported that intention-to-treat analysis was used.
Primary outcomes
Three of the four included studies reported number of MII and 2PN oocytes [9, 10, 12]. The mean number of MII oocytes retrieved ranged from 7.2 to 9.2 after hCG triggering and from 8.4 to 10.3 after dual triggering with no significant difference between groups in each study. The result of the meta-analysis showed no significant difference in the number of MII oocytes retrieved between patients in the hCG plus GnRHa group and those in the hCG-alone group (pooled DIM = − 0.45, 95% CI − 1.34–0.44, P = 0.325) with no heterogeneity in the number of MII oocytes observed (Q statistic = 1.194, I2 = 0%) (Fig. 3a). The mean number of 2PN oocytes retrieved also did not differ significantly between groups in each study, ranging between 5.6 and 8.6 after hCG triggering and between 5.9 and 8.8 after dual triggering [9, 10, 12]. Figure 3b summarizes the difference in the number of 2PN oocytes retrieved between the two groups, with a pooled DIM of − 0.47 (95% CI − 1.26–0.32, P = 0.245). Test of heterogeneity showed no heterogeneity among the three studies (Q statistic = 1.393, I2 = 0%).
Rates of pregnancy in completed cycles with dual and hCG triggering were reported by Kim et al. (53.3% vs 33.3%, respectively; P = 0.027) [10] and Schachter et al. (40.9% vs 28.3%, respectively; P = 0.07) [11]. While Schachter et al. did not describe the definition of pregnancy used, Kim et al. defined clinical pregnancy as a positive hCG serum test with transvaginal ultrasonographic evidence of a gestational sac [10, 11]. In the pooled analysis, the pregnancy rate in completed cycles in the hCG plus GnRHa group was significantly higher as compared with patients in the hCG group (pooled OR = 0.48, 95% CI 0.31–0.77, P = 0.002) with no heterogeneity between the studies (Q statistic = 0.118, I2 = 0%) (Fig. 3c). Ongoing pregnancy rates with dual and hCG triggering were reported by Decleer et al. (31.1% vs 44.1%, respectively; P = NS) [9] and Schachter et al. (36.1% vs 22.3%, respectively; P = 0.046) [11] Decleer et al. defined ongoing pregnancy as a positive hCG serum test with confirmation on ultrasound 23 days after embryo transfer [9]. Definition of ongoing pregnancy was not provided by Schachter et al. [11]. There was no significant difference in the ongoing pregnancy rate between the two groups in the pooled analysis (pooled OR = 0.88, 95% CI 0.29–2.66, P = 0.826) (Fig. 3d). Extreme heterogeneity in the ongoing pregnancy rate between the studies was found (Q statistic = 5.623, I2 = 82.22%).
Secondary outcomes
Implantation rates were not significantly different between the dual trigger group and hCG trigger group as reported by Schachter et al. (21.1% vs 17.1%, respectively) [11] and Decleer et al. (22% vs 34%, respectively) [9]. Kim et al. reported an implantation rate of 24.7% with dual triggering and 14.9% with hCG triggering, with a statistically significant difference between groups (P = 0.006) [10]. In the pooled analysis, no evidence was found of differences in the rate of implantation between groups (pooled OR = 0.83, 95% CI 0.43–1.61, P = 0.579) (Table 2). Extreme heterogeneity in the implantation rate among the studies was found (Q statistic = 8.368, I2 = 76.10%).
Kim et al., Schachter et al., and Mahajan et al. provided data of the total number of oocytes retrieved and good-quality embryos and were included in the meta-analysis [10,11,12]. Good-quality embryo was defined as an embryo good for transfer and cryopreservation (usable embryo) by Mahajan et al. [12] and as an embryo of grade 1 or 2 by Kim et al. [10]. No heterogeneity between the studies was found (I2 = 35.29% for the total number of oocytes retrieved, I2 = 0% for the number of good-quality embryos) (Table 2). The result of the meta-analysis revealed that there was no significant difference in the total number of oocytes retrieved between patients triggered with hCG alone and those receiving hCG plus GnRHa (pooled DIM = − 0.32, 95% CI − 1.26–0.62, P = 0.507). However, patients treated with hCG plus GnRHa had a significantly larger amount of good-quality embryos as compared with patients in the hCG group (pooled DIM = − 0.24, 95% CI − 0.46 to − 0.02, P = 0.032).
Sensitivity analysis
Sensitivity analyses were performed using the leave-one-out approach in which the meta-analysis of primary outcomes reported in at least three studies was performed with each study removed in turn (Table 3). The direction and magnitude of combined estimates on MII oocytes and 2PN oocytes did not vary markedly with the removal of the studies, indicating that the meta-analysis data were robust and not overly influenced by any one study.
Discussion
Meta-analysis of data from RCTs found that the use of dual triggering in women undergoing IVF following a GnRH antagonist protocol was similarly efficacious in terms of oocyte and pregnancy outcomes as compared with hCG-alone triggering. Pooled analyses showed no significant difference between groups in terms of the number of total oocytes retrieved, the number of MII oocytes retrieved, the number of 2PN oocytes retrieved, implantation rate, or ongoing pregnancy rate. Pooled analysis showed a significantly higher number of good-quality embryos and a significantly higher rate of pregnancy in completed cycles with dual triggering than with hCG-alone triggering.
Several studies have reported the retrieval of a higher total number and higher number of mature oocytes and better quality embryos after triggering with GnRHa than after the conventional triggering with hCG [3, 5, 19, 20]. This might be explained by the ability of GnRHa to induce the release of both endogenous LH and follicle stimulating hormone (FSH), which more physiologically mimics the natural cycle surge [21]. In an RCT conducted by Iñarra et al. [13], which was not published in full and, therefore, was excluded from the current analysis, a significantly higher oocyte recovery rate was reported with dual triggering (0.2 mg triptorelin plus 250 μg rhCG) than with hCG-alone triggering. While the number of total, mature, and fertilized oocytes retrieved was numerically higher with dual than hCG-alone triggering in the studies included in the current analysis, the differences did not reach statistical significance within the individual studies or in the pooled analysis. The number of good-quality embryos, however, was found to be significantly higher with dual triggering in the pooled analysis. The comparable or improved oocyte outcomes reported in the included RCTs are supported by the results from retrospective studies comparing dual and hCG-alone triggering, which have shown that the number of total, mature, and fertilized oocytes retrieved and good-quality embryos were similar or significantly higher with dual triggering [22,23,24,25]. Thus, evidence suggests that the beneficial effects of GnRHa triggering on oocyte outcomes are maintained with the addition of hCG in dual triggering.
Though controversial, some studies have suggested reduced implantation and pregnancy rates in IVF cycles following a GnRH antagonist protocol as opposed to one using a GnRHa [26]. Due to the relatively stronger binding affinity to GnRH receptors of GnRHa than GnRH antagonists, it has been hypothesized that in GnRH antagonist protocols, the administration of GnRHa can displace the antagonist from the receptor in the endometrium, and activation of the previously blocked GnRH receptor could lead to improvement in implantation [11, 27]. The results of meta-analysis, however, do not strongly support the implantation-enhancing effects of triggering with GnRHa plus hCG over hCG alone. Of the included RCTs, only Kim et al. [10] reported a significantly higher implantation rate with dual triggering than with hCG triggering. Schachter et al. [11] reported a numerically higher implantation rate, while Decleer et al. [9] reported a numerically lower implantation rate with dual triggering, though the difference in rate with hCG triggering did not reach significance in either study. Interestingly, dual triggering resulted in a significantly higher implantation rate than hCG triggering in retrospective studies conducted by Lin et al. (29.68% vs 18.43%; P < 0.001) [22] and Seval et al. (41.0% vs 27.4%; P = 0.01) [24]. However, owing to the possibility of bias inherent in studies with retrospective design, these findings would be considered less reliable than those of RCTs.
Inferior pregnancy outcomes have been observed for GnRHa-triggered IVF cycles when compared with hCG triggered cycles in GnRH antagonist-based protocols [3, 4, 28]. A recently updated Cochrane review reported significantly decreased ongoing pregnancy rate (OR 0.70; 95% CI 0.54–0.91) and live birth rate (OR 0.47; 95% CI 0.31–0.70) and a significantly higher rate of early miscarriage (OR 1.74; 95% CI 1.10–2.75) with the use of a GnRHa trigger as compared with hCG trigger in fresh IVF autologous cycles [1]. The poorer outcomes are potentially due to a detrimental effect on endometrial receptivity secondary to defective corpus luteum function or early luteolysis resulting from the shorter LH surge induced by GnRHa; therefore, the co-administration of hCG is believed to restore corpus luteum function and to improve conception rates with GnRHa triggering [29]. Indeed, meta-analysis results are in support of this theory as the pregnancy rate in completed cycles with dual triggering was significantly higher than with hCG triggering, and ongoing pregnancy rate was similar between groups. Similar pregnancy rates with dual and hCG triggering were also demonstrated in the RCT conducted by Iñarra et al. [13]. Furthermore, although baby take-home rate is the gold standard primary outcome in IVF studies, live birth rate per cycle was reported only by Kim et al., and was found to be significantly higher with dual triggering than with hCG triggering (50.0% vs 30.0%, respectively; P = 0.025). The benefit of adjuvant hCG to GnRHa triggering in regard to pregnancy outcomes is further supported by findings from retrospective studies, which have consistently demonstrated significantly higher rates of clinical pregnancy and live birth rates [22,23,24].
Possible corpus luteal deficiency and luteal phase insufficiency, characterized by inadequate secretion of progesterone during the luteal phase, may induce early pregnancy loss when GnRHa is administered for oocyte maturation in GnRH antagonist cycles [3, 10, 28]. Therefore, it has been suggested that adequate luteal phase support appropriate to the triggering regimen used is likely to be a critical variable that affects the pregnancy rate [30]. In an RCT conducted by Humaidan et al., a 1500 IU bolus of hCG administered after GnRHa triggering was shown to rescue the luteal phase [5]. Furthermore, compared with GnRHa-alone trigger followed by standard luteal support, ongoing pregnancy rate was shown to be significantly higher with GnRHa plus low-dose hCG trigger followed by standard luteal support and with GnRHa trigger followed by enhanced luteal support [31]. Interestingly, in the studies included in the current pooled analysis, pregnancy outcome trended in favor of the dual trigger group in studies using low [11] and standard [10] dose of progesterone for luteal phase support, while pregnancy rate was numerically more in favor of the hCG-alone trigger group in the Decleer et al.’s [9] study in which high-dose luteal phase support was used. The lack of correlation between luteal phase support intensity and pregnancy outcome could suggest that with dual triggering, the hCG bolus has a greater role in the rescuing of corpus luteum function than progesterone supplementation. This finding further supports dual triggering as an effective strategy to salvage luteal defect and restore endometrial receptivity.
A potential disadvantage of dual triggering is the possible increased risk of OHSS due to the use of hCG. With hCG triggering, incidence of OHSS has been reported to be as high as 31% in women at high risk for OHSS, whereas the risk of OHSS is essentially eliminated with GnRHa triggering [2, 29, 32]. Triggering with GnRHa is associated with a significantly lower risk of OHSS of any grade than with hCG alone, as shown in a meta-analysis of 8 RCTs involving patients undergoing fresh autologous cycles (OR 0.15, 95% CI 0.05–0.47) [1]. Furthermore, the risk of OHSS with dual triggering may be related to the dose of hCG used. In a large retrospective cohort analysis including 8790 IVF cycles, Lu et al. reported two cases of moderate-to-severe OHSS in patients triggered with triptorelin 0.1–0.2 mg plus hCG 5000 IU, while there were no cases of OHSS in the dual trigger groups using hCG 1000 or 2000 IU [25]. Only two of the RCTs included in the current analysis, both using standard dose hCG, reported the incidence of OHSS [9, 10]. OHSS did not occur in any patient in the Decleer et al.’s study, and in the Kim et al.’s study, incidence of severe OHSS was 3.3% (2/60) in both the dual triggering and the hCG triggering groups. The low rate of OHSS occurrence may have been due to the exclusion of patients with polycystic ovary syndrome (PCOS) which has been associated with an increased risk of OHSS [33]. The presence of other primary risk factors associated with OHSS, including history of PCOS and elevated anti-Müllerian hormone (AMH) [33], was not reported in these studies. In a retrospective study comparing dual triggering (leuprolide acetate 1 mg plus hCG 1000 IU) and GnRHa-alone triggering in high responders, Griffin et al. reported a low rate of OHSS (1/34; 2.9%) in patients receiving dual triggering, which was not significantly different from the OHSS rate with GnRHa-alone triggering [34]. In another retrospective study of high responders who were at a relatively high risk for OHSS (elevated E2 and large number of follicles on the day of trigger), Shapiro et al. reported 1 case of OHSS in 182 patients (0.55%) with dual triggering (leuprolide acetate 4 mg plus hCG 1000 to 2500 IU) and none with GnRHa-alone triggering [31]. In contrast, a retrospective study conducted by O’Neill et al. found a significantly higher incidence of OHSS in high responders after dual trigger (leuprolide acetate 4 mg plus hCG 1000 IU) than GnRHa-alone trigger (8.6 vs. 0%, P < 0.01), with 4 of the 6 patients who developed OHSS developing severe OHSS [35]. As the existing evidence does not provide a clear association between dual triggering and the risk of OHSS, particularly in patients at high risk of OHSS, further study in controlled trials with more selective patient inclusion is required to better elucidate the risk of OHSS following dual triggering relative to triggering with hCG or GnRHa-alone, and whether the risk is related to the hCG dose used.
The present meta-analysis has a number of limitations that warrant consideration. First, only four studies were eligible for inclusion and at most three studies reported results for each of the analyzed outcomes. Second, the quality of the studies was only moderate overall, as blinding of participants and assessors was not done or not specified. Third, as the included studies did not select for patients at high risk of OHSS and were likely underpowered to detect the difference in the risk of OHSS between groups, these studies do not contribute to further understanding of OHSS risk reduction with dual triggering, which is the primary reason for avoiding the conventional hCG triggering. Finally, live birth rate, undoubtedly the most important outcome measure of IVF treatment, was only reported in one study. In future study, a three-armed randomized trial comparing dual, hCG-alone, and GnRHa-alone triggering with more specific patient selection would provide a clearer understanding into the differences in clinical outcomes and OHSS risk among the three regimens.
Conclusion
All studies published at the time of this review, either of randomized or retrospective design, showed comparable or significantly improved oocyte (number of total, mature, and fertilized oocytes, and good-quality embryos) and pregnancy (implantation, clinical pregnancy, and ongoing pregnancy rates) outcomes with the use of GnRHa plus hCG as compared with hCG alone for triggering of final oocyte maturation. Though the rate of OHSS reported with dual triggering is low, further study is needed to better understand the association between dual triggering and OHSS risk as well as the difference in risk between dual triggering and triggering with GnRHa or hCG alone. Overall, current evidence suggested that dual triggering with concomitant GnRHa and a standard bolus of hCG is a promising option for final oocyte maturation in fresh autologous in GnRH antagonist cycles for IVF.
References
Youssef MA, Van der Veen F, Al-Inany HG et al (2014) Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist-assisted reproductive technology. Cochrane Database Syst Rev 10:CD008046
Babayof R, Margalioth EJ, Huleihel M et al (2006) Serum inhibin A, VEGF and TNFalpha levels after triggering oocyte maturation with GnRH agonist compared with HCG in women with polycystic ovaries undergoing IVF treatment: a prospective randomized trial. Hum Reprod 21:1260–1265
Humaidan P, Bredkjaer HE, Bungum L et al (2005) GnRH agonist (buserelin) or hCG for ovulation induction in GnRH antagonist IVF/ICSI cycles: a prospective randomized study. Hum Reprod 20:1213–1220
Humaidan P, Bungum L, Bungum M, Andersen CY (2006) Rescue of corpus luteum function with peri-ovulatory HCG supplementation in IVF/ICSI GnRH antagonist cycles in which ovulation was triggered with a GnRH agonist: a pilot study. Reprod Biomed Online 13:173–178
Humaidan P, Ejdrup Bredkjaer H, Westergaard LG, Yding Andersen C (2010) 1,500 IU human chorionic gonadotropin administered at oocyte retrieval rescues the luteal phase when gonadotropin-releasing hormone agonist is used for ovulation induction: a prospective, randomized, controlled study. Fertil Steril 93:847–854
Papanikolaou EG, Verpoest W, Fatemi H, Tarlatzis B, Devroey P, Tournaye H (2011) A novel method of luteal supplementation with recombinant luteinizing hormone when a gonadotropin-releasing hormone agonist is used instead of human chorionic gonadotropin for ovulation triggering: a randomized prospective proof of concept study. Fertil Steril 95:1174–1177
Kasum M, Kurdija K, Orešković S, Čehić E, Pavičić-Baldani D, Škrgatić L (2016) Combined ovulation triggering with GnRH agonist and hCG in IVF patients. Gynecol Endocrinol 8:1–5
Fauser BC, de Jong D, Olivennes F et al (2002) Endocrine profiles after triggering of final oocyte maturation with GnRH agonist after cotreatment with the GnRH antagonist ganirelix during ovarian hyperstimulation for in vitro fertilization. J Clin Endocrinol Metab 87:709–715
Decleer W, Osmanagaoglu K, Seynhave B, Kolibianakis S, Tarlatzis B, Devroey P (2014) Comparison of hCG triggering versus hCG in combination with a GnRH agonist: a prospective randomized controlled trial. Facts Views Vis ObGyn 6:203–209
Kim CH, Ahn JW, You RM, Kim SH, Chae HD, Kang BM (2014) Combined administration of gonadotropin-releasing hormone agonist with human chorionic gonadotropin for final oocyte maturation in GnRH antagonist cycles for in vitro fertilization. J Reprod Med 59:63–68
Schachter M, Friedler S, Ron-El R, Zimmerman AL, Strassburger D, Bern O, Raziel A (2008) Can pregnancy rate be improved in gonadotropin-releasing hormone (GnRH) antagonist cycles by administering GnRH agonist before oocyte retrieval? A prospective, randomized study. Fertil Steril 90:1087–1093
Mahajan N, Sharma S, Arora PR, Gupta S, Rani K, Naidu P (2016) Evaluation of dual trigger with gonadotropin-releasing hormone agonist and human chorionic gonadotropin in improving oocyte maturity rates: a prospective randomized study. J Hum Reprod Sci 9:101–106
Iñarra MJ, Crisol L, Guembe MA (2015) Use of dual trigger with gonadotropin-releasing hormone agonist (GnRH-a) and human chorionic gonadotropin (hCG) to optimize oocyte recovery rates and IVF results. In: European Society of Reproduction and Embryology 2015 Annual Meeting; June 2015; Lisbon, Portugal
Cochrane Handbook for Systematic Reviews of interventions. Version 5.1.0. (updated March, 2011). The Cochrane Collaboration. http://training.cochrane.org/handbook
Hardy RJ, Thompson SG (1998) Detecting and describing heterogeneity in meta-analysis. Stat Med 17:841–856
Takkouche B, Cadarso-Suárez C, Spiegelman D (1999) Evaluation of old and new tests of heterogeneity in epidemiologic meta-analysis. Am J Epidemiol 150:206–215
National Research Council (1992) Combing information: statistical issues and opportunities for research. National Academy Press, Washington, DC
Sterne JA, Sutton AJ, Ioannidis JP et al (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 22(343):d4002
Erb TM, Vitek W, Wakim AN (2010) Gonadotropin-releasing hormone agonist or human chorionic gonadotropin for final oocyte maturation in an oocyte donor program. Fertil Steril 93:374–378
Imoedemhe DA, Sigue AB, Pacpaco EL, Olazo AB (1991) Stimulation of endogenous surge of luteinizing hormone with gonadotropin-releasing hormone analog after ovarian stimulation for in vitro fertilization. Fertil Steril 55:328–332
Gonen Y, Balakier H, Powell W, Casper RF (1990) Use of gonadotropin-releasing hormone agonist to trigger follicular maturation for in vitro fertilization. J Clin Endocrinol Metab 71:918–922
Lin MH, Wu FS, Lee RK, Li SH, Lin SY, Hwu YM (2013) Dual trigger with combination of gonadotropin-releasing hormone agonist and human chorionic gonadotropin significantly improves the live-birth rate for normal responders in GnRH-antagonist cycles. Fertil Steril 100:1296–1302
Pereira N, Elias RT, Neri QV et al (2016) Adjuvant gonadotrophin-releasing hormone agonist trigger with human chorionic gonadotrophin to enhance ooplasmic maturity. Reprod Biomed Online 33:568–574
Seval MM, Özmen B, Atabekoğlu C et al (2016) Dual trigger with gonadotropin-releasing hormone agonist and recombinant human chorionic gonadotropin improves in vitro fertilization outcome in gonadotropin-releasing hormone antagonist cycles. J Obstet Gynaecol Res 42:1146–1151
Lu X, Hong Q, Sun L et al (2016) Dual trigger for final oocyte maturation improves the oocyte retrieval rate of suboptimal responders to gonadotropin-releasing hormone agonist. Fertil Steril 106:1356–1362
Al-Inany HG, Youssef MA, Ayeleke RO, Brown J, Lam WS, Broekmans FJ (2016) Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev 4:CD001750
Beckers T, Bernd M, Kutscher B, Kühne R, Hoffmann S, Reissmann T (2001) Structure-function studies of linear and cyclized peptide antagonists of the GnRH receptor. Biochem Biophys Res Commun 289:653–663
Kolibianakis EM, Schultze-Mosgau A, Schroer A (2005) A lower ongoing pregnancy rate can be expected when GnRH agonist is used for triggering final oocyte maturation instead of HCG in patients undergoing IVF with GnRH antagonists. Hum Reprod 20:2887–2892
Engmann L, DiLuigi A, Schmidt D, Nulsen J, Maier D, Benadiva C (2008) The use of gonadotropin-releasing hormone (GnRH) agonist to induce oocyte maturation after cotreatment with GnRH antagonist in high-risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: a prospective randomized controlled study. Fertil Steril 89:84–91
Kol S, Humaidan P, Alsbjerg B et al (2015) The updated Cochrane review 2014 on GnRH agonist trigger: repeating the same errors. Reprod Biomed Online 30:563–565
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C (2011) Comparison of “triggers” using leuprolide acetate alone or in combination with low-dose human chorionic gonadotropin. Fertil Steril 95:2715–2717
Humaidan P, Kol S, Papanikolaou EG, Copenhagen GnRH Agonist Triggering Workshop Group (2011) GnRH agonist for triggering of final oocyte maturation: time for a change of practice? Hum Reprod Update 17:510–524
Boothroyd C, Karia S, Andreadis N, Rombauts L, Johnson N, Chapman M, Australasian CREI Consensus Expert Panel on Trial evidence (ACCEPT) group (2015) Consensus statement on prevention and detection of ovarian hyperstimulation syndrome. Aust N Z J Obstet Gynaecol 55:523–534
Griffin D, Benadiva C, Kummer N, Budinetz T, Nulsen J, Engmann L (2012) Dual trigger of oocyte maturation with gonadotropin-releasing hormone agonist and low-dose human chorionic gonadotropin to optimize live birth rates in high responders. Fertil Steril 97:1316–1320
O’Neill KE, Senapati S, Maina I, Gracia C, Dokras A (2016) GnRH agonist with low-dose hCG (dual trigger) is associated with higher risk of severe ovarian hyperstimulation syndrome compared to GnRH agonist alone. J Assist Reprod Genet 33:1175–1184
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
C-HuangC: project development and data analysis. CRT, PHW, WML, HYC, and HHC: Data collection and data analysis. C-HuiC: project development, data collection, data analysis, and manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no known conflicts of interest in this work.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Chen, CH., Tzeng, CR., Wang, PH. et al. Dual triggering with GnRH agonist plus hCG versus triggering with hCG alone for IVF/ICSI outcome in GnRH antagonist cycles: a systematic review and meta-analysis. Arch Gynecol Obstet 298, 17–26 (2018). https://doi.org/10.1007/s00404-018-4751-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4751-3