Abstract
Purpose
Laparoscopic appendectomy (LA) for acute appendicitis (AA) remains controversial during pregnancy. We aimed to determine surgical and obstetrical outcomes of LA in pregnant women.
Methods
Pregnant women who underwent LA for AA (G1) between 2006 and 2019 were included and matched by gender, age, white blood cells, ASA score, and presence of peritonitis in a 1:2 ratio with non-pregnant women who had undergone LA (G2). Demographics and surgical outcomes were compared between groups. Preterm delivery and fetal loss rate were also analyzed.
Results
From a total of 2009 LA, 18 (0.9%) were included in G1 and 36 (1.8%) in G2. There were no intraoperative complications or converted surgeries. Length of hospital stay was longer in G1 (G1: 2.6 vs G2: 1.4 days, p < 0.01). There was no difference in overall morbidity and readmission rates. Fetal loss and preterm delivery rates were both 11%.
Conclusion
LA in pregnant women has similar intraoperative and postoperative outcomes as those achieved in non-pregnant patients. In addition, the laparoscopic approach does not seem to jeopardize obstetrical outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute appendicitis (AA) is the most common non obstetric indication for surgery during pregnancy [1]. The wide variety of obstetric and non-obstetric causes of abdominal pain and leukocytosis, along with the inability to use computed tomography (CT) scan make the diagnosis of AA challenging in pregnant women.
Laparoscopic appendectomy (LA) is the cornerstone treatment for AA treatment in non-pregnant women, mostly due to its proven benefits, such as decreased postoperative pain, shorter hospital stay, faster return to normal activities, and fewer abdominal wall complications as compared to the open approach [2,3,4]. However, safety of LA for both the mother and fetus is still a matter of debate. While some studies have shown that LA was safe in pregnant women [5,6,7,8,9,10], others reported an increased fetal loss rate without significant advantages in AA resolution [11, 12]. Current guidelines state that LA should only be considered during pregnancy when vast laparoscopic expertise is available [2, 13].
The aim of this study was to assess the surgical and obstetrical outcomes of LA in pregnant women.
Materials and methods
Study design and population
Data were collected prospectively from all patients who underwent LA between 2006 and 2019. Pregnant women over 16 years old who underwent LA for AA were included for analysis (group 1) and compared with a matched series of LA in non-pregnant women (group 2).
Diagnosis of acute appendicitis was based on clinical, laboratory and imaging findings. Patients were admitted for surgery within 12 h after diagnosis. All pregnant patients were assessed by an obstetrician to exclude any complications of pregnancy. Obstetric ultrasound (US) was also performed in all patients to establish gestational age and to confirm fetal vitality.
All patients received a single preoperative intravenous infusion of broad-spectrum antibiotics 30 min before the surgical incision. A laparoscopic three-port technique was used as previously described [14]. Trocars’ placement was modified in pregnant patients according to their gestational age. During the first or second trimester of pregnancy, we placed a 10 mm umbilical port for the camera and one 10 mm suprapubic and 5 mm left lower quadrant for laparoscopic instruments (similar port placement locations as non-pregnant patients). For patients in the third trimester, the initial 10 mm port was placed 2 cm cephalad to the gravid uterus in the upper midline between the umbilicus and xiphoid process. The second port (5 mm) was placed in the left upper quadrant, and the third port (10 mm) was placed in the right upper quadrant. After identification of the appendix, mesoappendix was cauterized and sectioned with bipolar coagulation. An endoloop was placed at the base of the appendix and sectioned leaving a 5-mm stump. The appendix was removed through the suprapubic port. Peritoneal fluid was irrigated with normal saline and drained, when present. A surgical Blake drain was placed during surgery according to surgeon’s criteria. No prophylactic tocolysis was performed in any of our pregnant patients.
Complicated appendicitis was defined intraoperatively as perforation of the appendix, gangrene, empyema, or abscess formation. A normal appearing appendix in laparoscopy plus absence of histological findings of AA was defined as normal appendix. The severity of peritonitis was classified as mild (turbid/purulent fluid localized in one, two or three quadrants) or severe (fecal peritonitis or turbid/purulent fluid in four quadrants).
Patients with complicated appendicitis underwent antibiotic therapy for 7 days postoperatively. Analgesia based on acetaminophen and opioids was administered postoperatively in pregnant women. Ambulation and oral feeding with clear liquids was resumed when patients were fully awaked. Patients were discharged when they met the following criteria: normal vital signs, adequate oral intake, satisfactory pain control, ability to ambulate and urinate, newborns´ normal examination, and appropriate supervision/assistance at home. In pregnant patients, an obstetric ultrasound was also performed before patients´ discharge.
Follow-up was scheduled at clinics on postoperative day 7, 14 and 30 for pregnant patients and on day 7 and 30 for non-pregnant women. Unless a postoperative complication was clinically suspected, no laboratory or imaging test were performed after surgery. Postoperative intra-abdominal abscesses (IAA) were treated with intravenous antibiotics alone, percutaneous drainage or laparoscopic lavage according to our institution treatment algorithm [15].
This study was approved by the institutional review board (IRB) of our institution. The written informed consent was waived by the IRB owing to the study’s retrospective nature.
Variables and outcomes
Data collected included age, gender, body mass index (BMI), and American Society of Anesthesiologists (ASA) classification. Operative variables such as grade of appendicitis (normal, catarrhal, phlegmonous, gangrenous or perforated), presence of peritonitis, conversion rate, operative time, and intraoperative complications were also registered. Morbidity following Clavien–Dindo classification, mortality, and readmissions were also assessed. The birth records were also reviewed to assess gestational age at delivery, birth weight, and Apgar score.
Preterm delivery was defined as birth on or before the last day of the 37th week (259th day) as defined by the American College of Obstetricians and Gynecologists [16]. Trimesters of pregnancy were divided into first (1–14 weeks), second (15–28 weeks), and third (29–42 weeks).
Statistical analysis
Case–control matching was performed to compare the pregnant group (G1) with a non-pregnant group (G2) with a 1:2 ratio. The matching criteria were sex, age, white blood cells, ASA score, and presence of peritonitis. Categorical data were analyzed with chi-squared test. Continuous variables were compared with Mann–Whitney U test or Student´s T test according to their non-parametric or parametric distribution, respectively.
Statistical analysis was calculated with IBM SPSS v.25 (IBM, Armonk, NY, USA) and a p value < 0.05 was considered significant for all tests.
Results
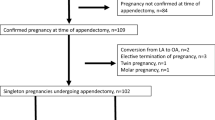
A total of 2009 LA were performed during the study period; of these, 18 (0.9%) patients were included in G1 and 36 (1.8%) in G2.
ASA score and comorbidities such as hypertension, coronary heart disease, chronic obstructive pulmonary disease, diabetes, and obesity had similar distribution between groups. Diagnosis of AA was achieved with ultrasound in 17 (94%) patients in G1 and in 28 (78%) in G2. CT-Scan was performed in 8 patients in G2 (G1: 0% vs G2: 22%, p = 0.03) and magnetic resonance imaging (MRI) in one patient in G1 (G1: 5.55% vs G2: 0%, p = 0.16). Baseline characteristics are shown in Table 1.
Intraoperative characteristics are shown in Table 2. Complicated appendicitis rate was similar in both groups (G1: 22% vs. G2: 19%, p = 0.81). Similar mean operative times (G1: 56 vs. G2: 49 min, p = 0.55) were found in both groups. There were no intraoperative complications or conversions to open surgery in the analyzed series.
Overall morbidity (G1: 22% vs. G2: 17%, p = 0.49) and postoperative IAA rates (G1: 5.55% vs. 2.77%, p = 0.61) were similar in both groups. There was no mortality in the series. Length of hospital stay (LOS) was longer in G1 (G1: 2.6 (1–7) days vs. G2: 1.4 (1–5) days, p < 0.01). One (5.55%) patient was readmitted in G1 and 1 (2.77%) in G2, p = 0.61 (Table 3).
Among pregnant patients, 4 (22%) underwent LA in the first trimester of pregnancy, 12 (67%) in the second trimester and 2 (11%) in the third trimester. There were 7 (39%) patients with high-risk pregnancies due to addiction to illicit substances, uncontrolled hypertension, history of previous fetal losses, anti-phospholipid syndrome, intrahepatic cholestasis of pregnancy, obesity, and threat of preterm labor.
Mean gestational age at LA was 29 (19–39) weeks. Mean Apgar score at 5 min was 9 (8–10) and mean birth weight was 3.52 (2.76–3.95) Kg.
Fetal loss occurred in 2 (11%) patients after LA. One was diagnosed at postoperative day 15 after LA, in a patient with major depression and addiction to illicit substances (8 weeks pregnancy). The second fetal loss occurred in a patient with a complicated AA at presentation. A re-laparoscopy was performed at postoperative day 6 due to the presence of three intra-abdominal abscesses. Subsequently, the patient was admitted in the ICU due to septic shock and fetal loss was diagnosed four days after the re-laparoscopy (17 weeks pregnancy).
Preterm delivery following LA occurred in 2 (11%) patients. One birth was induced at 35 weeks due to a refractory intrahepatic cholestasis of pregnancy and the other one was delivered by cesarean section at 34 weeks because the patient was in preterm labor at AA presentation (Table 4).
Discussion
The aim of this study was to assess the surgical and obstetrical outcomes of LA in pregnant women. Except for a longer LOS, we did not find differences in postoperative outcomes between pregnant and a matched-cohort of non-pregnant patients. In addition, obstetrical outcomes were favorable in the majority of patients.
The diagnosis of surgical emergencies such us AA during pregnancy remain challenging, and a late diagnosis can lead to serious complications. Historically, pregnant women had a higher negative appendectomy rate, ranging from 23 to 55% compared with 18% in non-pregnant women [17]. This could be explained by the low threshold for surgical intervention in this population due to the high incidence of fetal and maternal mortality from appendiceal perforation [18, 19]. However, this approach is not exempt of complications, exposing patients with normal appendix to an unnecessary higher risk of fetal loss [20]. In our series, negative appendectomy rate was 5%, similar to the rate of general population with AA diagnosis [21].
The abdominal ultrasound plays a central role in the diagnosis of AA diagnosis among pregnant women. A recent study has demonstrated no difference in the accuracy of US to diagnose AA between pregnant and non-pregnant women at reproductive ages [22]. Interestingly, in our series 94% of pregnant women were successfully diagnosed by US. Although current guidelines recommend the use of CT scan in patients with suspected AA and negative ultrasound findings, its use in pregnant women is limited due to the deleterious effects of radiation on the fetus. The American College of Radiology Appropriateness Criteria for pregnant women recommend MRI as a second-line imaging method in inconclusive cases. Although MRI appears to have similar sensitivity and specificity as CT, higher costs and lack of availability hinder the use this diagnostic method in many institutions [23]. In our series, we did not use CT for the diagnosis of AA in pregnant women, and MRI was used in only one patient with a negative US.
The use of laparoscopy provides better intraoperative visualization, less postoperative pain, shorter LOS, quicker return to normal activities, and fewer abdominal wall complications as compared to the open approach [3, 4]. LA is technically feasible in all trimesters of pregnancy and is associated with the same benefits of laparoscopic surgery seen in non-pregnant patients [17]. The potential deleterious effect of pneumoperitoneum, which may provoke uterus contractions and possible fetal loss is a common concern. However, a study evaluating the fetal response to pneumoperitoneum showed the lack of adverse effects of CO2 insufflation on the fetal placental perfusion and blood gases [24]. Although recent studies have demonstrated the safety and feasibility of LA in pregnancy [5,6,7,8,9,10, 25], a systematic review [26] and four meta-analysis [12, 27,28,29] concluded that LA during pregnancy might be associated with a greater risk of fetal loss. Therefore, the optimal surgical approach in pregnant women remains uncertain. In our series, all pregnant women with AA were treated with LA and, despite a longer LOS, postoperative outcomes were similar to those observed in non-pregnant women. Furthermore, the longer hospital stay seen in pregnant patients was likely related to the closer follow-up of this population after the operation. In non-pregnant patients, we follow a fast-track protocol and patients with non-complicated appendicitis are often discharge within 12–24 h from the operation [30]. In pregnant patients, however, we prefer to prolong the hospital stay to rule out pregnancy-related complications. The rate of fetal loss was 11% which is in agreement with previous reported rates of fetal loss in pregnant women with AA (3–15%) [20]. It is worth mentioning, however, that both patients were at high risk for fetal loss (one with major depression and addiction to illicit drugs and the other one with complicated appendicitis and septic shock).
Previous studies suggested that irritation of the uterus during an abdominal operation was associated with preterm delivery [31]. A recent meta-analysis reported no significant difference between LA and open approach with respect to preterm delivery. However, a trend towards an increasing risk of preterm delivery was evident in those who underwent open appendectomy [25]. On the other hand, another meta-analysis showed a non-significant increased risk of preterm delivery in those who underwent LA [12]. In our series, 2 (11%) patients had preterm delivery, which is similar to the rates reported in the literature (15–45%) [20].
The main limitation of this study is its retrospective nature. In addition, a relatively low number of pregnant women patients with AA were operated in our institution. Finally, we did not have a control group with conventional approach.
Conclusion
Despite a longer LOS, postoperative outcomes following LA were satisfactory and similar as those achieved in non-pregnant women with AA. In addition, obstetrical outcomes were favorable in the majority of patient. Our results, however, should be interpreted with caution due to the low number of pregnant patients included. Therefore, large scale and well-designed trials are still needed to clarify the inconsistencies in the evidence regarding the safety of LA in pregnant patients.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Vujic J, Marsoner K, Lipp-Pump AH, Klaritsch P, Mischinger HJ, Kornprat P (2019) Non-obstetric surgery during pregnancy—an eleven-year retrospective analysis. BMC Pregnancy Childbirth 19(1):382
Di Saverio S, Podda M, De Simone B et al (2020) Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg 15(1):27
Jaschinski T, Mosch CG, Eikermann M, Neugebauer EA, Sauerland S (2018) Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev 11(11):CD001546
Athanasiou C, Lockwood S, Markides GA (2017) Systematic review and meta-analysis of laparoscopic versus open appendicectomy in adults with complicated appendicitis: an update of the literature. World J Surg 41(12):3083–3099
Maimaiti A, Aierkin A, Mahmood KM et al (2017) Laparoscopic appendectomy in pregnancy with acute appendicitis: single center experience with world review. Surg Laparosc Endosc Percutan Tech 27(6):460–464
Yoo KC, Park JH, Pak KH et al (2016) Could laparoscopic appendectomy in pregnant women affect obstetric outcomes? A multicenter study. Int J Colorectal Dis 31(8):1475–1481
Karaman E, Aras A, Çim N, Kolusarı A, Kızıltan R, Çelik S, Anuk T (2016) Maternal and fetal outcomes after laparoscopic vs. open appendectomy in pregnant women: data from two tertiary referral centers. Ginekol Pol 87(2):98–103
Laustsen JF, Bjerring OS, Johannessen Ø, Qvist N (2016) Laparoscopic appendectomy during pregnancy is safe for both the mother and the fetus. Dan Med J 63(8):A5259
Cheng HT, Wang YC, Lo HC et al (2015) Laparoscopic appendectomy versus open appendectomy in pregnancy: a population-based analysis of maternal outcome. Surg Endosc 29(6):1394–1399
Chung JC, Cho GS, Shin EJ, Kim HC, Song OP (2013) Clinical outcomes compared between laparoscopic and open appendectomy in pregnant women. Can J Surg 56(5):341–346
Winter NN, Guest GD, Bozin M et al (2017) Laparoscopic or open appendicectomy for suspected appendicitis in pregnancy and evaluation of foetal outcome in Australia. ANZ J Surg 87(5):334–338
Wilasrusmee C, Sukrat B, McEvoy M, Attia J, Thakkinstian A (2012) Systematic review and meta-analysis of safety of laparoscopic versus open appendicectomy for suspected appendicitis in pregnancy. Br J Surg 99(11):1470–1478
Gorter RR, Eker HH, Gorter-Stam MA et al (2016) Diagnosis and management of acute appendicitis. EAES consensus development conference 2015. Surg Endosc 30(11):4668–4690
Schlottmann F, Sadava EE, Peña ME, Rotholtz NA (2017) Laparoscopic appendectomy: risk factors for postoperative intraabdominal abscess. World J Surg 41(5):1254–1258
Laxague F, Schlottmann F, Piatti JM, Sadava EE (2020) Minimally invasive step-up approach for the management of postoperative intraabdominal abscess after laparoscopic appendectomy. Surg Endosc. https://doi.org/10.1007/s00464-020-07448-0
-American Academy of Pediatrics (2017) American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 8th ed. Elk Grove Village, IL: American Academy of Pediatrics; American College of Obstetricians and Gynecologists
Machado NO, Grant CS (2009) Laparoscopic appendicectomy in all trimesters of pregnancy. JSLS 13(3):384–390
Aggenbach L, Zeeman GG, Cantineau AE, Gordijn SJ, Hofker HS (2015) Impact of appendicitis during pregnancy: no delay in accurate diagnosis and treatment. Int J Surg 15:84–89
Yilmaz HG, Akgun Y, Bac B, Celik Y (2007) Acute appendicitis in pregnancy–risk factors associated with principal outcomes: a case control study. Int J Surg 5(3):192–197
McGory ML, Zingmond DS, Tillou A, Hiatt JR, Ko CY, Cryer HM (2007) Negative appendectomy in pregnant women is associated with a substantial risk of fetal loss. J Am Coll Surg 205(4):534–540
Andersson M, Kolodziej B, Andersson RE, STRAPPSCORE Study Group (2017) Randomized clinical trial of Appendicitis Inflammatory Response score-based management of patients with suspected appendicitis. Br J Surg 104(11):1451–1461
Segev L, Segev Y, Rayman S, Nissan A, Sadot E (2016) The diagnostic performance of ultrasound for acute appendicitis in pregnant and young nonpregnant women: a case-control study. Int J Surg 34:81–85
Garcia EM, Camacho MA, Karolyi DR et al (2018) ACR Appropriateness Criteria® Right Lower Quadrant Pain-Suspected Appendicitis. J Am Coll Radiol 15:S373–S387
Fatum M, Rojansky N (2001) Laparoscopic surgery during pregnancy. Obstet Gynecol Surv 56(1):50–59
Lee SH, Lee JY, Choi YY, Lee JG (2019) Laparoscopic appendectomy versus open appendectomy for suspected appendicitis during pregnancy: a systematic review and updated meta-analysis. BMC Surg 19(1):41
Walker HG, Al Samaraee A, Mills SJ, Kalbassi MR (2014) Laparoscopic appendicectomy in pregnancy: a systematic review of the published evidence. Int J Surg 12(11):1235–1241
Frountzas M, Nikolaou C, Stergios K, Kontzoglou K, Toutouzas K, Pergialiotis V (2019) Is the laparoscopic approach a safe choice for the management of acute appendicitis in pregnant women? A meta-analysis of observational studies. Ann R Coll Surg Engl 101(4):235–248
Prodromidou A, Machairas N, Kostakis ID et al (2018) Outcomes after open and laparoscopic appendectomy during pregnancy: a meta-analysis. Eur J Obstet Gynecol Reprod Biol 225:40–50
Chakraborty J, Kong JC, Su WK et al (2019) Safety of laparoscopic appendicectomy during pregnancy: a systematic review and meta-analysis. ANZ J Surg 89(11):1373–1378
Angeramo CA, Dreifuss NH, Olivero AA, Sadava EE, Schlottmann F (2020) Risk factors for readmission after short-hospital-stay laparoscopic appendectomy. World J Surg 44(12):4006–4011
Soriano D, Yefet Y, Seidman DS, Goldenberg M, Mashiach S, Oelsner G (1999) Laparoscopy versus laparotomy in the management of adnexal masses during pregnancy. Fertil Steril 71(5):955–960
Funding
None.
Author information
Authors and Affiliations
Contributions
Protocol/project development: CAA, MEP, MMV, and FS. Data collection or management: CAA, MEP, MMV, and FS. Data analysis: CAA, MEP, MMV, and FS. Manuscript writing/editing: CAA, MEP, MMV, and FS.
Corresponding author
Ethics declarations
Conflict of interest
Cristian A. Angeramo, María E. Peña, Martín Maqueda Vocos, and Francisco Schlottmann have no conflict of interest, financial ties or funding/support to disclose.
Ethical approval
Ethical approval was waived by the Institutional Review Board (IRB) of our Institution.
Consent for publication
The written informed consent was waived by the IRB owing to the study’s retrospective nature.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Angeramo, C.A., Peña, M.E., Maqueda Vocos, M. et al. Surgical and obstetrical outcomes after laparoscopic appendectomy during pregnancy: a case-matched analysis. Arch Gynecol Obstet 304, 1535–1540 (2021). https://doi.org/10.1007/s00404-021-06201-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-06201-9