Abstract
Objective
To evaluate fetal ventricular diastolic function in pregnancies of women with gestational diabetes (GD), to determine whether minimal anomalies of glucose metabolism may influence fetal cardiac function.
Study design
Fetal ventricular filling time was measured by transabdominal ultrasound in singleton pregnancies between 34 and 37 weeks of gestation. We used a measurement which consists in the ratio between the diastolic time and the whole cardiac cycle time.
Results
The study included 35 women with a GD and 217 non-diabetic. Right ventricular filling time (RVFT) was significantly lower in the GD group (mean of RVFT = 39.2 ± 4.4 vs 43.6 ± 4.6; p < 0.01). Likewise, left ventricular filling time (LVFT) was shorter in the GD group compared to the non-GD group, though the difference was not significant (mean of LVFT = 43.6 ± 4.6 vs 44.6 ± 5.5; p = 0.33).
Conclusions
Fetal right cardiac function is altered also in pregnancies where gestational diabetes is well controlled.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes (GD) is associated both with short- and long-term adverse outcomes in the offspring, such as large-for-gestational-age fetuses, macrosomia and perinatal morbidity. Later in life, children of mothers with GD are at higher risk of developing overweight, diabetes and metabolic syndrome [1, 2]. The fetal heart is among the main organs which are susceptible to poor glycemic control in the mother. Fetal myocardium dysfunction is a known condition which develops in fetuses of women with pre-gestational diabetes as a consequence of the hyperinsulinemia due to a poorly controlled glucose metabolism [1,2,3,4,5]. However, a significant impairment of the cardiac function has also been observed in women with poorly controlled GD. Still, the effects of GD on fetal cardiac function remain unclear, especially in cases of minimally increased levels of maternal glycaemia [3, 5,6,7,8,9,10,11,12,13,14,15,16,17].
The aim of our study was to evaluate fetal ventricular diastolic function in singleton pregnancies of women with well-controlled gestational diabetes, to determine whether minimal modifications of glucose metabolism may influence fetal cardiac function.
Methods
Study design and setting
Between January 2019 and September 2019, we recruited all consecutive women with singleton pregnancies attending our outpatient clinic. Gestational diabetes was diagnosed according to the International Association of Diabetes and Pregnancy Study Group’s (IADPSG) recommendations [18]: fasting plasma glucose > 92 mg/dl and a positive 75-g oral glucose tolerance test (OGTT) between 24 and 28 weeks of gestation. Women with fasting plasma glucose ≥ 92 mg/dl (first trimester GD) or ≥ 126 mg/dl (pre-gestational diabetes) during the first trimester of gestation were excluded from the study. In the same period of time, we recruited the control group which included all consecutive women with singleton pregnancies screened negative at the OGTT.
All patients underwent an ultrasound scan between 34 and 37 weeks of gestational age. This exam is routinely performed in our center for the assessment of fetal and maternal well-being. Gestational age was determined on the basis of the first trimester ultrasound [19]; pregnancies with uncertain dating were excluded. Cases of chromosomal or structural fetal abnormalities, including intrauterine growth fetal restriction (IUGR) and large-for-gestational-age (LGA) with an estimated fetal weight above the 90th percentile, were also excluded. Polyhydramnios, defined as a deepest vertical pocket greater than 8 cm, also represented an exclusion criteria [20].
Further exclusion criteria included ongoing medication which may have affected fetal heart rhythm such as amiodarone, digoxin, beta blockers, flecainide, sodium and calcium channel blockers; a diagnosis of type 1 diabetes mellitus, type 2 diabetes mellitus, first trimester GD as well as preeclampsia, chronic hypertension, and renal, liver or hematological disorders.
Maternal and fetal characteristics assessment and ultrasound measurements
Sonologists were not aware of OGTT results. Thirty-five women were diagnosed with GD and as such underwent the protocol in use in our institute for monitoring mothers’ and fetuses’ well-being in pregnancies complicated by GD. In particular, women were asked to self-monitor blood glucose levels six times per day. Normal fasting glucose levels were defined as: < 95 mg/dl (i.e., measurements were taken approximately 30 min before meals), while post-prandial measurements were considered normal when < 120 mg/dl (i.e., approximately 120 min after meals). In pregnancies with a fetal abdominal circumference exceeding the 75th centile of local reference values, normal fasting glucose levels were defined as < 90 mg/dl, while normal post-prandial measurements were defined as < 110 mg/dl [21]. All patients diagnosed with GD were initially treated with a personalised diet. Insulin was prescribed (starting with 0.1 U/kg IM before meals) when glucose levels were sub-optimal (i.e., when 20% of measurements or more were above reference values) [22]. This scheme was customized to account for patients’ glycemic control and fetal growth [23]. Well-controlled GD was diagnosed, independent of the type of treatment patients were assigned, when 80% or more glucose measurements where within range, estimated fetal weight was appropriate for gestational age and the amniotic fluid index was normal [22]. Women tested negative for the glucose-tolerance test were considered non-diabetic (non-GD).
All women enrolled in the study underwent a transabdominal ultrasound scan which was performed by an expert sonologists using a RAB 4–8 MHz probe, Voluson S10, GE Medical Systems, Milwaukee, WI, USA. Prior and during examination, maternal and fetal characteristics were collected. Maternal characteristics included age, parity, height, weight and blood pressure at time of inclusion in the study; fetal characteristics included gestational age, estimated fetal weight calculated using the Hadlock formula [24] and umbilical artery pulsatility index.
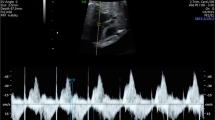
Fetal cardiac function was evaluated combining two-dimensional ultrasound imaging and targeted pulsed wave Doppler. A four-chamber view of the fetal heart in an anterior apical projection was obtained, with the interventricular septum aligned with the Doppler beam. Once the atrio-ventricular valves were identified, the Doppler sample gate was positioned immediately below them. The angle of insonation was manually corrected when > 20°. The Doppler sample gate ranged between 2 and 3 mm to avoid contamination of the signal. Measurements were performed in absence of fetal movement. Pulse repetition frequency was adjusted to visualize stable Doppler traces for at least 5 cardiac cycles. The right ventricular filling time (RVFT) and the left ventricular filling time (LVFT) were then calculated on frozen Doppler traces. Figure 1 shows the opening and closing of the atrio-ventricular valves. The first component of the wave is named E wave (early or passive ventricular filling) and is related to the distension of the ventricular chamber and to the subsequent negative pressure in the ventricles. The following component is named A wave (atrial, active or late) and results from the atrial contraction during ventricular filling. The two waves together constitute the total ventricular diastolic time, from the opening of the atrio-ventricular valves to the closure of mitral and tricuspid valves. We considered the ventricular filling time (VFT) as the time between the beginning of E and the end of A. To assess the contribution of ventricular diastolic function to the entire cardiac cycle (CC), we calculated the ratio between VFT and CC percentage, i.e., VFT/CC*100 (Fig. 1).
Statistical analysis
Categorical variables are reported in terms of absolute frequencies (n) and percentages (%), whereas continuous variables are reported in terms of mean and standard deviation (sd). Differences in means and proportions regarding GD were evaluated using Student’s test and Chi-squared t test, respectively. We compared the agreement of RVFT and LVFT measurements for a single examiner and between two different examiners using Bland–Altman analysis on a sample of study participants (n = 31 women) [25].
We evaluated the relation between LVFT and RVFT (respectively) and selected maternal and fetal characteristics using univariate and multivariate linear regression models. The final models included terms for the following maternal characteristics: age (years, in continuous), BMI (Kg/m2, in continuous), mean blood pressure (mmHg, in continuous) and GD (present or absent); and for the following fetal characteristics: percentile of estimated fetal weight (in continuous), gestational age at fetal echo-cardiography (weeks, in continuous), umbilical artery pulsatility index (in continuous) and heart rate (bpm; in continuous).
All statistical tests were two-sided with a significance level set at < 0.05. Statistical analyses were performed using the SAS 9.4 statistical software (SAS Institute, Cary, NC, USA).
Results
Descriptive analysis on maternal and fetal characteristics
Overall, 285 women were identified as potential participants. Among these, 33 were excluded as they did not meet the inclusion criteria. In particular, nine pregnancies were complicated by hypertension, six women failed to perform the OGTT due to side effects connected with the procedure, and six patients did not satisfy the definition of well-controlled GD. Following the 34–37-week ultrasonographic screening, other 12 women were excluded from the study: 7 pregnancies were complicated by fetal growth anomalies, 3 by polyhydramnios, and 2 women were lost to follow-up. Thus, the present analysis was based on 252 women, 35 of whom were part of the GD group and 217 of the non-GD group. Among the GD group, five women (14.2%) were prescribed insulin with a daily mean of 20 international units (min = 8 and max = 34 units). Maternal and fetal characteristics of GD and non-GD women are reported in Table 1.
RVFT was significantly shorter (p < 0.01) in the GD group with a mean of 39.2 ± 4.4 ms compared to the non-GD group (mean = 42.9 ± 5.5 ms). Likewise, LVFT was shorter in the GD group (mean = 43.6 ± 4.6) compared to the non-GD group (mean = 44.6 ± 5.5), without, however, reaching statistical significance (p = 0.33) (Tables 2, 3).
Bland–Altman analysis
The mean difference and the corresponding 95% limits of agreement for paired measurements of LVFT for the same examiner was − 1.4 (− 9.5; 6.7). The same measurement for RVFT was − 0.5 (− 5.3, 4.4). The values in paired measurements performed by two different examiners were 0.6 (− 10.0, 11.3) and 2.2 (− 20.5, 24.9), respectively (Fig. 2).
Right ventricular filling time
In the univariate analysis, gestational diabetes (β = − 3.701; 95% CI − 5.636, − 1.767; p < 0.01) was significantly inversely associated with RVFT. Likewise, heart rate (β = − 0.045; 95% CI − 0.093, 0.002; p = 0.06) showed a borderline inverse association with RVFT. Conversely, gestational age (β = 0.880, 95% CI − 0.063, 1.824; p = 0.07) showed a slight direct relation with RVFT. A similar pattern was observed in the multivariate analysis with gestational diabetes (β = − 3.653; 95% CI − 5.591, − 1.715; p < 0.01) and heart rate (β = − 0.047; 95% CI − 0.094, 0.001; p = 0.05), which were inversely related, whereas gestational age at fetal echo-cardiography (β = 0.884; 95% CI − 0.056, 1.824; p = 0.07) was positively associated with RVFT.
Left ventricular filling time
In the univariate analysis, gestational age at fetal echo-cardiography (β = 1.028; 95% CI 0.115, 1.941; p = 0.03) was significantly positively associated with LVFT, whereas heart rate (β = − 0.095; 95% CI − 0.140, − 0.050; p < 0.01) was significantly negatively associated. In the multivariate analysis, gestational age at fetal echo-cardiography (β = 0.984; 95% CI 0.080, 1.888; p = 0.03) showed a significant direct association with LVFT, whereas heart rate (β = − 0.094; 95% CI − 0.139, − 0.049; p = 0.09) showed a significant inverse association.
Conclusion
Our study clearly indicates that fetal cardiac function is altered in well-controlled GD; in particular, we observed a decrease of the diastolic time (ventricular relaxation and atrial systole) in the right fetal ventricle.
Alterations in fetal cardiac function in pregnancies complicated by pre-gestational diabetes and GD have been documented in several studies [3, 6, 8,9,10,11,12,13,14,15,16]. It is well known that maternal hyperglycemia influences the development of the fetal heart both in terms of embryological organogenesis and in terms of cardiac performance [3,4,5,6,7]. It has been hypothesized that relative intrauterine hyperglycemia and hyperinsulinemia may alter fetal myocardium, modifying cardiac function. According to the ‘Pedersen hypothesis’, fetal pancreatic β-cells are over-stimulated by maternal hyperglycemia, causing fetal hyperinsulinemia, an increased metabolic rate and a tendency to hypoxemia [3,4,5,6,7]. Maternal diabetes also activates placental genes involved in chronic stress and inflammation [8]. Presumably, exposition of myocardial fibers to all these insults increases the risk of cardiovascular morbidity and mortality in the offspring [5, 7].
In patients with pregestational diabetes, the decrease of fetal cardiac function may either be caused by a modification of ventricular compliance due to the thickening of the ventricular walls, or by an alteration of the afterload due to polycythaemia, which has been described in fetuses of diabetic mothers [3, 6, 8,9,10,11,12,13,14,15,16]. Interestingly, Bali et al. [8] showed that the left fetal ventricular myocardial performance index (MPI) is significantly greater in pregnant women with GD; this may add evidence to the hypothesis of a non-hypertrophy related ventricular dysfunction. Similar results were obtained by Figueroa et al. [15] who noticed higher left ventricular MPI values amongst those women with GD who had worse glycemic control and/or larger fetuses. In addition, Bhorat et al. [3] observed a significant impairment of fetal cardiac activity in poorly controlled GD.
In our study, patients were selected according to a crucial inclusion criterion: an appropriate estimated fetal weight for gestational age. In the suspect of IUGR or LGA, patients were not admitted to the study. We found no significant differences in terms of maternal age, maternal BMI, offspring’s birth weight or gestational age at delivery between patients enrolled in our study. Therefore, women with gestational diabetes were apparently in good metabolic control and differences found in right ventricular filling time should be attributed to minimal glycemic changes which are undetectable with standard clinical tests. Our paper reinforces the hypothesis that even minimal changes in levels of maternal glycemia can modify fetal cardiac function.
In contrast to previous papers, our data show an alteration of cardiac performance limited to the right fetal heart. This observation may be due to the low sensitivity of the system we adopted to evaluate cardiac function. On the other hand, we cannot exclude that long-term changes observed in the fetal heart of patients with poorly controlled diabetes start right from the right ventricle. This hypothesis is supported by the fact that the right heart is predominant in the fetus [16]. We believe that our contrasting results may be due to the fact that several of the above mentioned studies included patients with DM or with GD treated with insulin at time of diagnosis [3, 6, 11, 13, 15]. In contrast, our study only includes women with GD with a good glycemic control, who started treatment and close monitoring at time of diagnosis, between 24 and 28 weeks of gestation; only 15% of our patients subsequently received treatment with insulin. Our hypothesis is that the right ventricle is more susceptible than the left ventricle to slight variations in maternal and intrauterine glycemic state.
Detecting early modifications in apparently well-controlled diabetic mothers may be fundamental in fetal surveillance. At the state of art, prenatal screening for adverse fetal outcomes in pregnancies complicated by diabetes is still receding. Although umbilical artery Doppler velocimetry is widely accepted as a technique for the screening of high-risk pregnancies, the efficacy of this test for the evaluation of fetal well-being in diabetic pregnancies has not been shown useful. Similarly, ductus venosus Doppler velocimetry has not been proven effective in fetuses of diabetic women. Borat suggested MPI as a prognostic indicator of fetal outcome in mothers with non-controlled diabetes. Thus, the adoption of VFT as a new standardized ultrasound technique may represent an easily obtainable and immediate parameter, a valid alternative for more complex and less intuitive ultrasound cardiac measurements. Moreover, the intra-operator and inter-operator difference in measurements has been proven to be acceptable.
In conclusion, this study clearly documents that mild well-controlled diabetes, as is defined by our abovementioned criteria, may influence right fetal cardiac function. The adoption of a simple ultrasonographic technique allows clinicians to identify an early alteration of the fetal cardiac function in terms of a reduced right ventricular filling time, which is an index of the diastolic phase. This measurement, which is characterized by an acceptable intra-operator and inter-operator difference, could represent an innovative technique for the assessment of fetal well-being in pregnancies complicated by GD. However, a greater number of patients, a more uniform methodology for GD screening and diagnosis, as well as a greater consensus regarding the definition of well-controlled GD, are necessary to identify reference values for the prediction of fetal and neonatal adverse outcomes. We believe these represent the main limitations of our study.
References
Fehlert E, Willmann K, Fritsche L, Linder K et al (2017) Gestational diabetes alters the fetal heart rate variability during an oral glucose tolerance test: a fetal magnetocardiography study. BJOG 124:1891–1898
Linder K, Schleger F, Kiefer-Schmidt I et al (2015) Gestational Diabetes Impairs Human Fetal Postprandial Brain Activity. J Clin Endocrinol Metab. 100:4029–36
Bhorat IE, Bagratee JS, Pillay M, Reddy T (2014) Use of the myocardial performance index as a prognostic indicator of adverse fetal outcome in poorly controlled gestational diabetic pregnancies. Prenat Diagn 34:1301–1306
Salvesen DR, Brudenell JM, Proudler AJ, Crook D, Nicolaides KH (1993) Fetal pancreatic beta-cell function in pregnancies complicated by maternal diabetes mellitus. Am J Obstet Gynecol 168:1363–1369
Pedersen J (1952) Diabetes and pregnancy; blood sugar of newborn infants during fasting and glucose administration. Nord Med 47:1049
Patey O, Carvalho JS, Thilaganathan B (2019) Perinatal changes in fetal cardiac geometry and function in diabetic pregnancy at term. Ultrasound Obstet Gynecol 54:634–642
Marco LJ, McCloskey K, Vuillermin PJ, Burgner D, Said J, Ponsonby AL (2012) Cardiovascular disease risk in the offspring of diabetic women: the impact of the intrauterine environment. Exp Diabetes Res 2012:565160
Bali S, Pac FA, Ece İ, Oflaz MB, Kibar AE, Kandemir Ö (2014) Assessment of cardiac functions in fetuses of gestational diabetic mothers. Pediatr Cardiol 35:30–37 (Epub 2013 Jun 19)
Rizzo G, Aarduini D, Romanini C (1991) Cardiac function in fetuses of type 1 diabetic mothers. Am J of Obstet Gynecol 164:837–843
Rizzo G, Pietropolli A, Capponi A, Cacciatore C, Arduini D, Romanini C (1994) Analysis of factors influencing ventricular filling patterns in fetuses of type 1 diabetic mothers. J Perinat Med 22:149–157
Sanhal CY, Daglar HK, Kara O, Uygur D, Yucel A (2017) Assessment of fetal myocardial performance index in women with pregestational and gestational diabetes mellitus. J Obstet Gynaecol Res 43:65–72
Arslan D, Oran B, Vatansev H, Cimen D, Guvenc O (2013) The usefulness of plasma asymmetric dimethylarginine (ADMA) levels and tissue doppler echocardiography for heart function in term infants born to mothers with gestational diabetes mellitus. J Matern Fetal Neonatal Med 26:1742–1748
Ren Y, Zhou Q, Yan Y, Chu C, Gui Y, Li X (2011) Characterization of fetal cardiac structure and function detected by echocardiography in women with normal pregnancy and gestational diabetes mellitus. Prenat Diagn 31:459–465
Garg S, Sharma P, Sharma D, Behera V, Durairaj M, Dhall A (2014) Use of fetal echocardiography for characterization of fetal cardiac structure in women with normal pregnancies and gestational diabetes mellitus. J Ultrasound Med 33:1365–1369
Figueroa H, Silva MC, Kottmann C et al (2012) Fetal evaluation of the modified-myocardial performance index in pregnancies complicated by diabetes. Prenat Diagn 32:943–948
Rychik J (2004) Fetal cardiovascular physiology. Pediatr Cardiol 25:201–209
Hernandez-Andrade E, Figueroa-Diesel H, Kottman C et al (2007) Gestational-age-adjusted reference values for the modified myocardial performance index for evaluation of fetal left cardiac function. Ultrasound Obstet Gynecol 29:321–325
International Association of Diabetes and Pregnancy Study Groups Consensus Panel: International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33:676–682.
Robinson HP, Fleming JE (1975) A critical evaluation of sonar crown rump length measurements. Br J Obstet and Gynaecol 82:702–710
Cardwell MS (1987) Polyhydramnios: a review. Obstet Gynecol Surv 42:612–617
Rossi G, Somigliana E, Moschetta M, Bottani B, Barbieri M, Vignali M. Adequate timing of fetal ultrasound to guide metabolic therapy in mild gestational diabetes mellitus. Results from a randomized study. Acta Obstet Gynecol Scand. 2000;79:649–54.
Hochberg A, Pardo A, Oron G, Krispin E, Amikam U, Wiznitzer A, Hadar E, Salman L (2019) Perinatal outcome following induction of labor in patients with good glycemic controlled gestational diabetes: does timing matter? Arch Gynecol Obstet 300:299–303
Bonomo M, Cetin I, Pisoni MP, Faden D, Mion E, Taricco E, Nobile de Santis M, Radaelli T, Motta G, Costa M, Solerte L, Morabito A. Flexible treatment of gestational diabetes modulated on ultrasound evaluation of intrauterine growth: a controlled randomized clinical trial. Diabetes Metab. 2004 Jun;30:237–44.
Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK (1985) Estimation of fetal weight with the use of head, body, and femur measurements–a prospective study. Am J Obstet Gynecol 151:333–337
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Funding
None.
Author information
Authors and Affiliations
Contributions
All the authors were part of the guideline group within: F D’Ambrosi: project development, data collection, and manuscript writing. G Rossi: project development and manuscript writing. CM Soldavini: manuscript writing. GE Cetera: manuscript writing. M Di Maso: data analysis. N Cesano: data collection. IF Carbone: data collection and manuscript writing. E Ferrazzi: project development and manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
None declared. Completed disclosure of interests form available to view online as supporting information.
Ethical approval
The study was approved by the Institutional Review Board of Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Milan, Italy (reference n.2955).
Informed consent
All patients gave their written formal consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
D’Ambrosi, F., Rossi, G., Soldavini, C.M. et al. Evaluation of fetal cardiac function in pregnancies with well-controlled gestational diabetes. Arch Gynecol Obstet 304, 337–344 (2021). https://doi.org/10.1007/s00404-020-05948-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05948-x