Abstract
Purpose
The aim of this study was to determine the autoimmune effects of ankylosing spondylitis (AS) on the fertility potential of women by evaluating ovarian reserves of AS patients.
Methods
A total of 104 patients, 52 in the AS group (study group) and 52 in the control group were included in the study. Ovarian reserve was evaluated by serum anti-Müllerian hormone (AMH) levels, antral follicle count (AFC) and baseline serum follicle-stimulating hormone (FSH) levels.
Results
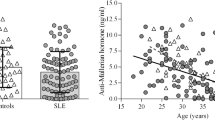
The mean serum AMH levels were significantly lower in the study group when compared to the controls (2.203 ± 1.110 vs. 1.188 ± 0.891, p < 0.001). In addition, the mean AFC was also significantly lower in the study group. (10.67 ± 1.81 vs. 9.54 ± 2.50, p = 0.009). Mean FSH levels were calculated to be 6.72 ± 1.14 in the study group and 7.21 ± 1.22 in the control group. The difference was not statistically significant (p = 0.781).
Conclusion
This study shows that AS like several other autoimmune conditions has an adverse effect on the female fertility potential. Therefore, an early start and long-term management of AS patients who have fertility desire is recommended. Serum AMH levels can be used in monitoring ovarian reserve and in early detection of reproductive decline of AS patients.
ClinicalTrial Number
NCT04209881.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Female fertility is affected by genetic, endocrinological, environmental, psychological, and iatrogenic factors. Ovaries play two important roles in female fertility and their proper function is essential for a healthy female reproductive function. They produce mature eggs for fertilization and produce hormones essential for the menstrual cycle as well as for the maintenance of a pregnancy [1]. The adverse effects of autoimmune disorders on the ovarian function have already been reported [2]. An autoimmune etiology can be found in approximately one-third of the premature ovarian failure (POF) cases [3]. Furthermore, a condition called autoimmune oophoritis, where the immune system attacks the ovaries, develops in the presence of autoimmune polyendocrine syndromes [4].
Ankylosing spondylitis (AS) is characterized as an autoimmune and a chronic inflammatory disease. It primarily affects the axial skeleton. Involvement of peripheral joints as well as extra-articular manifestations is also observed. The mean age of diagnosis is within the reproductive years, commonly during late teens and early 20′s [5]. A prevalence ranging from 0.7 to 49 per 10,000 has been reported [6]. AS is associated with several autoimmune disorders, including inflammatory bowel disease, anterior uveitis and psoriasis, which indicates the possibility of a common genetic and pathophysiological basis. Furthermore, it has been reported that patients diagnosed with an autoimmune disorder develop another autoimmune condition with a risk of 25% [7]. According to literature, autoimmune disorders, such as rheumatoid arthritis, familial Mediterranean fever, systemic lupus erythematosus, autoimmune thyroiditis, and Sjögren’s syndrome, reduce ovarian reserve, the capacity of ovaries to produce of eggs for fertilization [8,9,10,11,12].
Fertility potential can be characterized by the assessment of the ovarian reserve [13]. Ovarian reserve is determined by the antral follicle count (AFC), serum anti-Müllerian hormone (AMH) levels and basal follicle-stimulating hormone (FSH) levels [14, 15]. It is known that assessment of serum AMH levels in sub-fertile population has a high predictive value for fertility potential [16].
The adverse effects of several autoimmune disorders on the ovarian reserve have already been established. However, the effects of AS on the female fertility potential have not yet been evaluated. The aim of this study was to determine the autoimmune effects of AS on the fertility potential of AS patients by evaluating their ovarian reserves through determination of AFC, serum AMH levels and serum FSH levels.
Materials and methods
This prospective cohort study was conducted at the gynecological outpatient clinic of Health Sciences University Istanbul Kanuni Sultan Suleyman Training and Research Hospital, between June 2019 and January 2020. The study protocol was approved by the institution’s Ethics Committee (2019/456 14.10.2019) and was registered to ClinicalTrials.gov (NCT04209881). Written informed consent was obtained from subjects before they were enrolled in the study.
52 patients who were diagnosed with AS according to the Modified New York criteria, who visited our gynecological clinic during the study period and who met the inclusion criteria were included in the study group [17]. Women with regular menstrual cycles (21–35 days), with cycle length variations of < 4 days, with both ovaries intact, non-smokers, who had no history of liver failure, malignant diseases, chronic renal failure, infertility or other gynecological conditions, such as abnormal uterine bleeding, and who had not been on hormonal or herbal medication during the previous 3 months met the inclusion criteria. Women diagnosed with other autoimmune and/or chronic inflammatory disorders were excluded from the study. 52 healthy patients who visited the gynecological outpatient clinic during the same period for routine gynecological control were included in the control group.
Patients’ biometric data including age, body mass index (BMI), fertility and menstrual history were recorded. Venous blood samples were obtained during the follicular phase (days 2–6) of the menstrual cycle during the morning hours (08:00–09:00). Serum samples were stored at − 20 °C and assayed for FSH, luteinizing hormone (LH), estradiol (E2), prolactin (PRL) and thyroid-stimulating hormone (TSH). Serum AMH levels were measured with fully automated AMH electrochemiluminescence assay (ECLIA; ElecsysVR AMH assay, Roche Diagnostics, Basel, Switzerland).
All patients received a thorough gynecological examination. The AFC was performed using transvaginal ultrasound by counting the antral follicles with a diameter of 2–10 mm. All AFCs were carried out by the same gynecologist who was blinded to the medical data of the patients. Ovarian volume was calculated using the formula postulated by Orsini et al. (length × width × thickness × 0.5235) [18].
Sample size calculations were performed according to an estimated prevalence of 0.5% for AS. The estimation was done based on the reported prevalence of 0.1–1.4% in the western countries [6]. A sample size of 51 was calculated with a 95% confidence interval and a 5% desired precision. Data analysis was performed using SPSS version 24.0 for Windows (SPSS Inc., Chicago, IL). Pearson normality test was used for the normality and the distribution of variables. Numerical variables were compared using the independent samples t test or the Mann–Whitney U test. The data are expressed using means with standard deviations. A p value of < 0.05 was considered statistically significant.
Results
A total of 104 patients, 52 in the AS group (study group) and 52 in the control group were included in the study. The demographic data of the patients are displayed in Table 1. There are no significant differences between the groups in terms of age, BMI, and in the levels of follicular phase hormones (FSH, LH, E2 and PRL) and TSH. Therefore, the two groups are comparable in nature.
Mean serum AMH levels, mean AFC, and mean ovarian volumes are presented in Table 2. The mean ovarian volumes for both right and left ovaries were similar in both groups. However, the mean serum AMH levels were significantly lower in the study group when compared to the controls (2.203 ± 1.110 vs. 1.188 ± 0.891, p < 0.001). In addition, the mean AFC was also significantly lower in the study group. (10.67 ± 1.81 vs. 9.54 ± 2.50, p = 0.009). However, this difference in terms of AFC between the two groups did not reflect on to their clinical presentation. Both groups were clinically similar. Mean FSH levels were calculated to be 6.72 ± 1.14 in the study group and 7.21 ± 1.22 in the control group (Table 1). The difference was not statistically significant (p = 0.781).
Discussion
In the current study, the ovarian reserves of AS patients were assessed to determine whether AS had an adverse effect on the female fertility potential. Serum AMH level is proportional to the number of developing follicles and it is an indicator of the follicle pool. Therefore, it is commonly used to monitor ovarian reserve in patients who are at a higher risk for infertility, such as women with endometriosis [19]. Furthermore, a strong correlation between serum AMH levels and the AFC has already been reported [20, 21]. Therefore, serum AMH levels and AFC are commonly used in evaluating the ovarian reserve during fertility treatments [22]. Recent studies demonstrated that serum AMH levels are lower in women of reproductive age with autoimmune disorders [8,9,10,11,12]. The significantly low levels of serum AMH and AFC observed in the AS group of our study were in accordance with the literature and the results were interpreted as a reduction in the ovarian reserves of AS patients compared to the healthy subjects. The results indicated that the autoimmunity associated with AS, as with several other autoimmune disorders, has an adverse effect on the female ovarian function. Furthermore, in literature, AMH levels lower than 1.2 are accepted as low ovarian reverse [23]. Thus, these patients including the ones in our study group could be referred to oocyte or embryo cryopreservation.
In 4–30% of patients with an autoimmune disease, gonadal failure has been reported [24, 25]. Gooren et al. conducted a study with 22 male AS patients evaluating their testicular function. Reduced levels of testicular testosterone reserve, elevated levels of LH, inversion of estradiol testosterone ratio and slightly increased E2 levels were reported. The study showed the negative effects of AS related autoimmunity on the testicular function [26]. In a study conducted by Gooren et al., the differences in female sex hormones were reported between menstruating and menopausal patients with active and inactive AS. They reported that E2 levels in menstruating patients with active AS were significantly lower than those with inactive AS indicating that the increased autoimmunity during the active phase of the disease affects the hormonal regulation of women in reproductive ages negatively. The early onset of AS, and a commonly encountered fulminant course during pregnancy imply that sex hormones play a role in pathophysiology of AS [27].
Genetic background constitutes an important part in the pathogenesis of AS as well. One of the most important genetic factors associated with AS is the major histocompatibility complex (MHC) class I allele encoding human leukocyte antigen B27 (HLA-B27) [28]. Among first-degree relatives with positive HLA-B27, a higher prevalence has been observed [29]. Risk ratios of 94%, 25%, and 4% were calculated for first-, second-, and third-degree relatives, respectively [30]. Thus, the presence of AS in a first-degree relative puts one in a higher risk group. Since the clinical manifestations of autoimmunity occur after months or years of a subclinical course and since fertility is already affected at the subclinical stages, an early management of women in reproductive ages with autoimmune disorders has utmost importance [31, 32]. Therefore, women with AS positive mothers or siblings should be advised accordingly in terms of the risk factors on their fertility potential.
To the best of our knowledge, this is the first study evaluating the serum AMH levels and AFC in women with AS. The results show that serum AMH levels and AFC, in other words the ovarian reserves, of AS patients are affected negatively by the autoimmunity of AS. It is possible that autoimmune damage to the ovaries happens over the course of the autoimmune condition. Thus, a progression to POF or early menopause might be possible. Therefore, long-term follow-up is advisable in these patients and serum AMH levels along with AFC can be used to monitor these patients over the course of their disease.
Conclusion
This study shows that AS like several other autoimmune conditions has an adverse effect on the female fertility potential through a reduction in the ovarian reserve, which can be determined by serum AMH levels and AFC. Therefore, an early start and long-term management of AS patients who have fertility desires are recommended. Serum AMH levels can be used in monitoring ovarian reserve and in early detection of reproductive decline of AS patients.
References
Barbieri RL (2014) The endocrinology of the menstrual cycle. Methods Mol Biol 1154:145–169. https://doi.org/10.1007/978-1-4939-0659-8_7
Vannuccini S, Clifton VL, Fraser IS, Taylor HS, Critchley H, Giudice LC, Petraglia F (2016) Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Human reproduction update 22(1):104–115
Cordts EB, Christofolini DM, dos Santos AA, Bianco B, Barbosa CP (2011) Genetic aspects of premature ovarian failure: a literature review. Arch Gynecol Obstet 283(3):635–643
Komorowska B (2016) Autoimmune premature ovarian failure. Przeglad menopauzalny. Menopause Rev 15(4):210
de Winter JJ, van Mens LJ, van der Heijde D, Landewé R, Baeten DL (2016) Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthrt Res Thera 18(1):196
Dean LE, Jones GT, MacDonald AG, Downham C, Sturrock RD, Macfarlane GJ (2014) Global prevalence of ankylosing spondylitis. Rheumatology 53(4):650–657
Mohan MP, Ramesh TC (2003) Multiple autoimmune syndrome. Indian J Dermatol Venereol Leprol 69(4):298
Brouwer J, Laven JS, Hazes JM, Schipper I, Dolhain RJ (2013) Levels of serum anti–Müllerian hormone, a marker for ovarian reserve, in women with rheumatoid arthritis. Arthritis Care Res 65(9):1534–1538
Şahin A, Karakuş S, Durmaz Y, Yıldız Ç, Aydın H, Cengiz AK, Güler D (2015) Evaluation of ovarian reserve with anti-müllerian hormone in familial Mediterranean fever. International journal of rheumatology 5:3
del Carmen Velarde-Ochoa M, Esquivel-Valerio JA, Vega-Morales D, Skinner-Taylor CM, Galarza-Delgado DÁ, Garza-Elizondo MA (2015) Anti-Müllerian hormone in reproductive age women with systemic lupus erythematosus. Reumatología Clínica (English Edition) 11(2):78–82
Tuten A, Hatipoglu E, Oncul M, Imamoglu M, Acikgoz AS, Yilmaz N, Sahmay S (2014) Evaluation of ovarian reserve in Hashimoto’s thyroiditis. Gynecol Endocrinol 30(10):708–711
Karakus S, Sahin A, Durmaz Y et al (2017) Evaluation of ovarian reserve using anti-müllerian hormone and antral follicle count in Sjögren's syndrome: preliminary study. J Obstet Gynaecol Res 43(2):303–307. https://doi.org/10.1111/jog.13216
Vural B, Cakiroglu Y, Vural F, Filiz S (2014) Hormonal and functional biomarkers in ovarian response. Arch Gynecol Obstet 289(6):1355–1361
Silva CAA, Brunner HI (2007) Gonadal functioning and preservation of reproductive fitness with juvenile systemic lupus erythematosus. Lupus 16(8):593–599
Fleming R, Seifer DB, Frattarelli JL, Ruman J (2015) Assessing ovarian response: antral follicle count versus anti-Müllerian hormone. Reproduct Biomed Online 31(4):486–496
Karakus S, Sahin A, Durmaz Y, Aydin H, Yildiz C, Akkar O, Cetin A (2017) Evaluation of ovarian reserve using anti-müllerian hormone and antral follicle count in Sjögren's syndrome: preliminary study. J Obstet Gynaecol Res 43(2):303–307
Linden SVD, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. Arthrit Rheum 27(4):361–368
Vignali M, Belloni GM, Pietropaolo G, Barbasetti Di Prun A, Barbera V, Angioni S, Pino I (2020) Effect of Dienogest therapy on the size of the endometrioma. Gynecol Endocrinol 1:1–5
Tomassetti C, D'Hooghe T (2018) Endometriosis and infertility: Insights into the causal link and management strategies. Best Pract Res Clin Obstet Gynaecol 51:25–33. https://doi.org/10.1016/j.bpobgyn.2018.06.002
de Vet A, Laven J, de Jong F, Themmen A, Fauser B (2019) Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril 112(4):E183–E188
Anjum S, Zahra F, Yousuf SM (2019) Ovarian reserve parameters and response to controlled ovarian stimulation in infertile patients. Pak J Med Sci 35(4):958
Practice Committee of the American Society for Reproductive Medicine (2015) Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril 103(3):e9–e17
Porcu E, Cillo GM, Cipriani L, Sacilotto F, Notarangelo L, Damiano G, Roncarati I (2019) Impact of BRCA1 and BRCA2 mutations on ovarian reserve and fertility preservation outcomes in young women with breast cancer. J Assist Reproduct Gene 1:1–7
Schoemaker J, Drexhage H, Hoek A (1997) Premature ovarian failure and ovarian autoimmunity. Endocrine Rev 10:106–123
Silva CA, Yamakami LYS, Aikawa NE, Araujo DB, Carvalho JF, Bonfá E (2014) Autoimmune primary ovarian insufficiency. Autoimmun Rev 13(4–5):427–430
Tapia-Serrano R, Jimenez-Balderas FJ, Murrieta S, Bravo-Gatica C, Guerra R, Mintz G (1991) Testicular function in active ankylosing spondylitis. Therapeutic response to human chorionic gonadotrophin. J Rheumatol 18(6):841–848
Gooren LJ, Giltay EJ, van Schaardenburg D, Dijkmans BA (2000) Gonadal and adrenal sex steroids in ankylosing spondylitis. Rheumatic Dis Clin North Am 26(4):969–987
Brewerton DA, Hart FD, Nicholls A, Caffrey M, James DCO, Sturrock RD (1973) Ankylosing spondylitis and HL-A 27. The Lancet 301(7809):904–907
Carter N, Williamson L, Kennedy LG, Brown MA, Wordsworth BP (2000) Susceptibility to ankylosing spondylitis. Rheumatology 39(4):445–445
Taurog JD (2007) The mystery of HLA–B27: if it isn't one thing, it's another. Arthritis Rheum 56(8):2478–2481
Sen A, Kushnir VA, Barad DH, Gleicher N (2014) Endocrine autoimmune diseases and female infertility. Nat Rev Endocrinol 10(1):37
Yun BH, Kim G, Park SH, Noe EB, Seo SK, Cho S, Lee BS (2017) In vitro fertilization outcome in women with diminished ovarian reserve. Obstet Gynecol Sci 60(1):46–52
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
PYB: study designation and manuscript writing; PK: manuscript writing and statistical analysis; BY: data interpretation; EÜÖ: laboratory analysis; KÇ: statistical analysis; NFTS: critically revised the article and supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethics approval
The Institutional Review Board of Health Science University Istanbul Sadi Konuk Training and Research Hospital, Istanbul, Turkey approved this study (IRB Approval Number: 2019/456,).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yalçın Bahat, P., Kadiroğulları, P., Topbas Selcuki, N.F. et al. Ovarian reserve in patients with ankylosing spondylitis. Arch Gynecol Obstet 303, 189–193 (2021). https://doi.org/10.1007/s00404-020-05824-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05824-8