Abstract
Purpose
To evaluate the clinical outcomes after fresh transfer of blastocysts cultured from vitrified–thawed cleavage embryos (VTCE) compared with conventional frozen–thawed blastocysts transfer (FBT), or with the usual fresh blastocysts transfer (FRBT).
Methods
A total of 155 cycles undergoing fresh transfer of VTCE blastocysts, 4904 cycles undergoing FBT, and 1014 cycles undergoing FRBT were retrospectively analyzed from August 2014 to July 2017. Pregnancy, delivery, and neonatal outcomes were compared after propensity score matching.
Results
VTCE blastocysts’ transfer resulted in a lower risk of early miscarriage (8.82% versus 19.70%, P < 0.05) and a decreased fetal birth weight (2611.90 ± 618.65 g versus 2931.86 ± 546.52 g, P < 0.01) compared to FBT. No significant difference was found regarding live birth rate, gestational age, and cesarean section. Correspondingly, VTCE blastocysts’ transfer led to significantly compromised pregnancy outcomes regarding clinical pregnancy rate and implantation, and even a slightly compromised live birth rate when compared with FRBT. Moreover, a higher occurrence of cesarean Section (88.89% versus 71.29%, P < 0.05) and a shorter gestational age (262.04 ± 14.99 days versus 268.06 ± 14.07, P < 0.05) were also found. Nevertheless, the risk of small for gestational age and large for gestational age, and the neonatal birth weight were comparable.
Conclusions
VTCE blastocysts’ transfer results in a comprehensively moderate outcome, which is an acceptable option for patients. Our results can provide efficient value for patients’ counseling. Furthermore, these findings indicate directions for exploring the mechanisms of low birth weight and short gestational age.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In assisted reproductive technology (ART), cryopreservation of human embryos has become a routine procedure in clinical practice. Freezing surplus embryos produced in one controlled ovarian stimulation (COS) cycle provides more opportunities for patients to accept repeated embryo transfers and subsequently improves the cumulative pregnancy rate. Moreover, in cases where the response to ovarian hyperstimulation syndrome or where embryo transfer may be considered inadvisable for other reasons, it offers the option of freezing all the available embryos for thawing and transfer later [1]. The application of the frozen–thawed embryo transfer (FET) has continuously increased over the last few years worldwide, and at present it constitutes more than 50% of all embryo transfers [2, 3]. However, embryos have been successfully cryopreserved at all stages such as pronuclear, cleavage, morula, and blastocyst stage [1]. Cryopreservation of cleavage embryo on day 3 after fertilization is the most common approach in clinical practice from the early years until recently, when blastocyst culture, cryopreservation, and transfer become more and more popular in both in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) cycles [4].
Blastocyst culture is considered an efficient approach to select the most viable cleavage embryo for transfer, which may result in several theoretical advantages including a better temporal synchronization between embryo and endometrium, a higher implantation rate, a potential decrease in the number of embryos transferred, and sequentially a lower risk of multiple gestations [5, 6]. Although no evidence of a difference was observed in cumulative pregnancy rate between the group following cleavage-stage embryo transfer and the one following blastocyst transfer, the latter achieved an increased clinical pregnancy rate and live birth rate per transfer [7]. Therefore, there are more and more patients willing to accept extended culture in vitro to select the viable embryo transfer at the blastocyst stage, which may avoid invalid embryo transfer attempts and reduce the time to remain pregnancy. However, paradoxically many patients vitrified one carrier or more carriers of cleavage-stage embryos derived from previous COS cycle(s) in tanks. On the other hand, with the improvement of ART, the number of embryos transferred was compulsorily reduced to one or two by some governments to minimize the risk of multiple gestations. However, there are some cases in which three cleavage embryos of a patient were vitrified in one carrier device in the early years. Therefore, extended culture of vitrified–thawed cleavage embryos (VTCE) and subsequently transfer at the blastocyst stage seems a promising alternative to solve the problems mentioned above. Nevertheless, the clinical outcomes after transfer of blastocysts derived from frozen–thawed embryos are still obscure. These particular types of blastocysts were subjected to a vitrified–thawed procedure at the stage of cleavage but were transferred as fresh once the blastula stage was reached, and they were called as VTCE blastocysts in following descriptions. Therefore, the purpose of the present study was to evaluate the clinical outcomes after fresh transfer of the VTCE blastocysts in comparison to conventional frozen–thawed blastocysts transfer (FBT) and fresh blastocysts transfer (FRBT). Since reliable criteria to identify embryos destined to develop viable blastocysts in vitro were not established [6], the evaluation of better outcomes between the transfer of thawed cleavage embryos after extended culture until blastocyst formation and transfer at cleavage stage post-thawing was not investigated in this study.
Methods
Patients
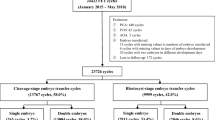
A total of 374 patients undergoing embryo transfer at blastocyst stage after extended culture of VTCE in our fertility center between August 1, 2014 and July 31, 2017 were retrospectively included. Only patients who harvested available blastocysts and underwent embryos transfer as fresh were included. The patients in whom one new formed blastocyst was transferred with another cryopreserved blastocyst were excluded. Finally, a total of 155 VTCE blastocysts transfer cycles were included for analysis. Correspondingly, a total of 5190 conventional FBT cycles and a total of 1017 FRBT cycles at the same time interval were included as references for propensity score matching (PSM). For convenience, these three types of cycles aforementioned were categorized as three groups: VTCE group, in which VTCE blastocysts were transferred as fresh; frozen group, in which frozen blastocysts were transferred about 2 h after thawing; and fresh group, in which fresh blastocysts were transferred on day 5 after oocyte retrieval. The slow-freezing cycles, preimplantation genetic testing cycles, cycles in which frozen–thawed oocytes were used, and cycles in which two blastocysts came from different COS cycles were excluded from the frozen group, and cycles that were missing any data for matching were excluded from both frozen and fresh group. A flowchart of the study is shown in Fig. 1. A written informed consent to the study was obtained from each patient and the program was approved by the Research Ethics Committee of our Hospital (Approval Number: SZZSECHU-20180020).
Flowchart of cycles included in the study. A few of patients in the VTCE group matched only one counterpart in reference groups because of the strict matching conditions which resulted in the final ratio was not 1:2 after propensity scores matching. VTCE group: cycles underwent blastocysts’ transfer as fresh after extended culture of vitrified–thawed cleavage embryos (VTCE)
PSM
Clinical outcomes after transfer of VTCE blastocysts were evaluated through the comparison with FBT and FRBT. A PSM method was adopted to screen the counterparts of patients in the VTCE group from the frozen group using the nearest neighbor matching (caliper 0.2). The matching ratio was 1: 2 (Fig. 2). The matching factors included patient characteristics at the time of transfer such as maternal age, body mass index (BMI), endometrial thickness, number of blastocysts transferred, and number of high-quality blastocysts transferred. In addition, it included the patient characteristics at the time of oocyte retrieval such as maternal age, infertility years, gravidity, number of oocytes retrieved, fertilization methods, number of high-quality embryos on day 3, and maternal infertility diagnosis. After achieving a balanced cohort through PSM, 134 patients in the VTCE group matched with 237 patients in the frozen group. Among them, 31 patients successfully matched only one counterpart because of the strict matching conditions and 103 patients successfully matched two counterparts. The distributions of patient characteristics before and after PSM are shown in Supplemental Table S1 and Table 1, respectively. Next, a similar method was carried out to match the VTCE group with the fresh group. The matching factors included maternal age, BMI, number of blastocysts transferred, number of high-quality blastocysts transferred, number of oocytes retrieved, fertilization methods, number of high-quality embryos on day 3, infertility years, gravidity, and maternal infertility reasons. After matching, a total of 101 patients in the VTCE group matched with 179 patients in the fresh group. Among them, 23 patients matched only one counterpart and 78 patients matched two counterparts. The distributions of patient characteristics before and after PSM are shown in Supplemental Table S2 and Table 2, respectively.
Propensity score matching for VTCE group versus frozen group and VTCE group versus fresh group. The distributions of the propensity scores (a) and standardized differences (b) before and after matching indicated balance between the compared cohorts. VTCE group: cycles undergoing blastocysts’ transfer as fresh after extended culture of vitrified–thawed cleavage embryos (VTCE); frozen group: cycles undergoing vitrified–thawed blastocysts transfer; fresh group: cycles undergoing fresh blastocysts transfer on day 5 after fertilization
Stimulation, embryo culture, and blastocysts transfer in fresh cycles
In fresh cycles, common stimulation protocols were applied by clinicians in our center based on the individual ovarian reserve and response, including long down-regulation protocols, antagonist protocols, and clomiphene-based mild stimulation protocols. Human chorionic gonadotropin (HCG) or gonadotropin-releasing hormone agonist was injected as a trigger when there were two leading follicles larger than 18 mm. Ovum picking-up (OPU) was performed by vaginal ultrasound-guided follicular aspiration 36 h after trigger, and conventional IVF or ICSI was carried out 3–4 h after OPU. Fertilization was assessed and confirmed by the presence of two pronuclei and two polar bodies at 17 ± 1 h after insemination. Quinn's Advantage Sequential Media (SAGE BioPharma, USA) containing 10% (v/v) serum protein substitute (SAGE BioPharma, USA) was used to culture the embryos according to the manufacturer’s specification. The cleavage embryos were subjected to single culture in one drop and then, subjected to group culture in Quinn’s Advantage Blastocyst Medium (SAGE BioPharma, USA) on day 3. The quality of the blastocyst was examined and evaluated as high quality (AA, AB, BA, and BB), fair quality (AC, CA, BC, and CB) and poor quality (CC) on day 5 and 6 based on the Gardner system [8]. Only high-quality and fair-quality embryos were available for transfer and cryopreservation. FRBT was performed on day 5 after OPU under the guidance of ultrasound.
Embryo vitrification, thawing, extended culture, and transfer
With regard to vitrification, cleavage embryos or blastocysts were immersed into the equilibration solution composed of 7.5% ethylene glycol (EG), 7.5% dimethylsulfoxide (DMSO), and 5% (m/v) human serum albumin (HSA) (SAGE BioPharma, USA) for 6 min or 10 min separately. Next, the embryos were placed into the vitrification solution composed of 15% (v/v) EG, 15% (v/v) DMSO, 0.5 M sucrose, and 5% (m/v) HSA, and subsequently placed on an opened cryotop carrier device (Kitazato, Japan) within 1 min. With regard to thawing, the cleavage embryos or blastocysts were immersed into 1 M, 0.5 M, and 0 M sucrose containing 5% (m/v) HSA sequentially for 1 min, 3 min, and 6 min, respectively. The whole freezing and warming steps were carried out at room temperature except for the first one of thawing, which was performed at 37 °C. The cleavage embryos after thawing were continually cultured in Quinn’s Advantage Blastocyst Medium (SAGE BioPharma, USA) for 72 h. Blastocysts’ transfer was performed once available stage 3–6 blastocysts were observed. Correspondingly, regarding conventional FBT, the blastocysts underwent laser-assisted hatching immediately after thawing (ZILOS-tk laser, Hamilton Thorne, US) [9] and were transferred after 2–3 h under ultrasound guidance.
Endometrial preparation
In conventional FBT cycles and VTCE blastocysts transfer cycles, endometrium was prepared by either natural protocols for women with spontaneous ovulation or artificial protocols for women with ovulation disorders. Follicle monitoring was carried out in natural protocol and embryo transfer was performed on day 5 after ovulation if the endometrial thickness exceeded 7 mm. Comparatively, estradiol at consecutive oral doses of 4, 6, and 8 mg was prescribed for patients in artificial protocol and progesterone was injected when the endometrial thickness reached 7–8 mm. Embryo transfer was performed 6 days later. Comparatively, the blastocysts derived from the extended culture of VTCE were transferred on the day of blastulation, which was generally 6–7 days after progesterone injection.
Outcomes’ evaluation
Maternal serum was collected to measure HCG on day 11 after blastocysts’ transfer, and the level higher than 5 IU/L indicated positive. The patient with a positive HCG level was confirmed as clinical pregnancy by the presence of intrauterine gestational sac on transvaginal ultrasound 30 days after transfer. The number of gestational sacs represents the implantation potential of embryos. Ongoing pregnancy was defined when the pregnancy completed ≥ 3 months of gestation, whereas spontaneous loss of an intrauterine pregnancy before 3 months of gestational age was regarded as an early miscarriage. Live birth rate was calculated as the number of deliveries with at least one live baby among 100 embryo transfer cycles. Low birth weight was defined as birth weight < 2500 g. Preterm birth and very preterm birth were defined as delivery prior to 37 weeks and 34 weeks, respectively [10]. Small for gestational age (SGA) was defined as a birth weight below the 10th percentile for the gestational age and large for gestational age (LGA) was defined as a birth weight larger than the 90th percentile for gestational age [11].
Statistical analysis
Statistical analysis was performed using SPSS version 24 (IBM company, USA). PSM was implemented on SPSS with the cooperation of the PS Matching plug-in [12, 13]. After PSM, the baseline characteristics and clinical outcomes of the matched data were compared using the unpaired two-tailed Student’s t test for continuous variables and Chi-squared test for categorical variables. All categorical variables were expressed as number and frequency, and all quantitative data were expressed as mean ± standard deviation (SD). Binary logistic regression was adopted to evaluate the effect of blastocysts from different sources on pregnancy and delivery outcomes by adjusting the factors used in PSM. The results were presented as odds ratio (OR) with 95% confidence interval (CI). A P value < 0.05 was considered statistically significant.
Results
Patients’ characteristics
A total of 1680 vitrified cleavage embryos from 374 patients were thawed for extended culture in vitro. The survival rate post-thawing was 97.38% since 1636 embryos had half or more of their blastomeres intact. After the extended culture, 766 blastocysts were finally formed in 246 patients and the blastulation rate was 46.82%. Among them, 155 patients accepted the transfer of blastocysts entirely derived from extended culture of VTCE and were included in the VTCE group. After achieving a balanced cohort through PSM, 134 patients in the VTCE group matched with 237 patients in the frozen group. The baseline characteristics between the two groups were comparable, which included maternal age, BMI, endometrial thickness, number of blastocysts transferred, and number of high-quality blastocysts transferred at the time of transfer, then, maternal age, infertility years, gravidity, number of oocytes retrieved, fertilization methods, number of high-quality embryos on day 3, and finally, maternal infertility diagnosis at the time of OPU (Table 1). Correspondingly, 101 patients in the VTCE group matched well with 179 patients in the fresh group, both with similar baseline characteristics in terms of maternal age, BMI, number of blastocysts transferred, number of high-quality blastocysts transferred, infertility years, gravidity, number of oocytes retrieved, fertilization methods, number of high-quality embryos on day 3, and maternal infertility diagnosis (Table 2).
VTCE group versus frozen group
The clinical outcomes after transfer of blastocysts in the VTCE group were assessed in comparison with those after transfer of frozen–thawed blastocysts in the frozen group. The outcomes corresponding to pregnancy, delivery, and neonatal outcomes are shown in Tables 3 and 4. The clinical pregnancy rate and implantation potential were found slightly lower in the VTCE group compared with the frozen group, although not significant. However, the occurrence of early miscarriage was also lower, and consequently the ongoing pregnancy rate and live birth rate of both groups were similar. The delivery outcomes such as gestational days, occurrence of preterm birth and very preterm birth, cesarean section, and pregnancy complications were comparable. The neonatal outcomes including gender ratio, admission to neonatal intensive care unit (NICU), and birth defects were also equivalent. Nevertheless, the birth weight of neonates was significantly lower in the VTCE group in which blastocysts derived from thawed cleavage embryos were transferred (P < 0.01).
VTCE group versus fresh group
The clinical outcomes after transfer of blastocysts in the VTCE group were also evaluated in comparison to the fresh group, because the transferred blastocysts in both groups were newly formed after extended culture in vitro. The outcomes corresponding to pregnancy, delivery, and neonatal outcomes are shown in Tables 3 and 4. VTCE blastocysts transfer resulted in a significantly lower implantation potential and also in a lower clinical pregnancy rate (P < 0.05). Additionally, the ongoing pregnancy rate and the live birth rate were compromised, although no significant difference was found. The delivery outcomes including gestational days, preterm birth and pregnancy complications, and the neonatal outcomes including gender ratio, birth weight, admission to NICU, and birth defects, were similar. Nevertheless, the occurrence of cesarean section was higher, and the gestational age was shorter in the VTCE group after transfer of blastocysts derived from thawed cleavage embryos (P < 0.05).
Discussion
In this work, a retrospective propensity-matched cohort study was conducted to evaluate the pregnancy, obstetric, and neonatal outcomes after fresh transfer of blastocysts derived from VTCE compared to conventional FBT and FRBT. To our knowledge, this is the first analysis on this issue. The findings indicated that extended culture of thawed cleavage embryos to reach the blastocyst stage for transfer resulted in a comprehensively moderate outcome, which was acceptable for patients. Specifically, transfer of these specific blastocysts from thawed cleavage embryos achieved comparable live birth rate when compared with conventional FBT, since a slightly lower clinical pregnancy rate was found in the VTCE group and a lower occurrence of early miscarriage was also found along (Table 3). However, the birth weight of newborns was significantly lower when compared to conventional FBT cycles (Table 4). On the other hand, the pregnancy outcomes after VTCE blastocysts’ transfer were significantly compromised regarding the clinical pregnancy rate and implantation rate, and slightly compromised regarding live birth rate with no significance, compared with the outcomes after transfer of fresh blastocysts on day 5 after fertilization. Furthermore, short gestational age and high risk of cesarean section were also found after delivery (Table 3). The neonatal outcomes including gender ratio, birth weight, admission to NICU, and birth defects were similar in the two groups (Table 4).
Vitrification is an increasingly popular approach for embryo cryopreservation since the 1990s [14, 15], showing better embryo survival rate after warming compared with slow freezing method [16], and results in comparable or even better clinical outcomes after embryo transfer compared to fresh embryo transfer [17,18,19]. Vitrification is proved to be a safety technology in embryo cryopreservation, and vitrified–warmed embryo transfer has recently become an indispensable strategy to maximize the success of ART outcomes [20,21,22]. However, a very limited number of studies investigated the clinical outcomes after transfer of blastocysts derived from extended culture of VTCE. A small retrospective clinical study in 2001 evaluated the development and implantation potential of slow-frozen–thawed cleavage embryos after subsequent culture to the blastocyst stage in comparison to slow-frozen–thawed blastocysts transfer [23]. The results showed that the developmental ability of frozen–thawed cleavage embryos to the blastocyst stage and their implantation potential seemed not to be severely affected by the cryopreservation procedure, which was as same as in our comparison between transfer of blastocysts from VTCE and conventional FBT, even if vitrification method was used for embryo cryopreservation. VTCE blastocysts’ transfer may lead to comparable live birth rate compared with conventional FBT. Specifically, a compromised clinical pregnancy was found in patients after transfer of VTCE blastocysts, while a higher occurrence of early miscarriage was found in women following FBT. The slight differences in clinical pregnancy and implantation were probably related to the time for blastocysts transfer in the VTCE group because it depends on the day of blastulation, which was generally carried out on day 6 or 7 after progesterone injection, while, conventional FBTs were orchestrated on day 6 after progesterone injection. The discrepancy of time regarding the blastocyst transfer may affect the endometrium–embryo synchronization in the VTCE group. In addition, the difference in early miscarriage was probably related to the better selection after the extended culture of cleavage embryos in sequential media, while frozen blastocysts were transferred just 2–3 h after thawing. Prolonged culture of cleavage embryos to blastocyst stage has been proposed as an effective strategy to evaluate the resumption of mitosis of blastomeres [24] and to further selection of chromosomally competent embryos [25]. A similar trend of miscarriage was also previously revealed between the transfer of thawed blastocysts and the transfer of blastocysts from thawed cleavage embryos [26].
We also evaluated the clinical outcomes after transfer of blastocysts derived from VTCE compared with FRBT, because both types of blastocysts were transferred as fresh when the embryos reached the blastocyst stage. The results showed that the pregnancy outcomes after VTCE blastocysts’ transfer were compromised in the rate of clinical pregnancy, implantation, ongoing pregnancy, and live birth. However, the significance was only found in clinical pregnancy and implantation. This compromised trend was probably related to the supraphysiological condition that occurs during or after cryopreservation procedure, which was also indicated by the ability of vitrified cleavage embryo developing to blastocyst stage that was slightly affected in comparison with fresh cleavage embryos (data not shown). In addition, blastocysts transferred in fresh cycles were day 5 blastocysts, which might also contribute to superior pregnancy and implantation outcomes [27, 28], since some studies reported that day 5 expanded blastocysts have a lower aneuploidy rate [29] and higher mitochondrial DNA content [30]. Notably, patients in the VTCE group generally underwent previous transfer cycles, factor that was neglected in this study because it was arduous to balance. High risk of cesarean section and short gestational age were also found in VTCE blastocysts’ transfer cycles when compared with fresh cycles. They were also previously reported in the comparison between FET and fresh embryos’ transfer cycles [31,32,33]. The potential reason for the high risk of the cesarean section could be due to previous cesarean sections in women who accepted blastocysts’ transfer after extended culture [32]. Furthermore, cesarean section operation without medical needs might induce potential bias, because we could not verify if the cesarean sections had medical indications. With regard to the gestational age, although shorter after transfer of VTCE blastocysts, the risk of SGA and LGA remained comparable to the risks obtained with FRBT. The reason for this result was probably related to the cryopreservation procedure because no difference in gestational age was found in the comparison between VTCE blastocysts’ transfer and conventional FBT.
One interesting finding in this study was the live birth weight of newborns delivered after fresh transfer of blastocysts from VTCE, which was nearly equivalent to the fresh cycles, but significantly lower than the conventional FBT cycles. Several previous studies reported newborns from ART pregnancies following FET with higher live birth weight compared to fresh embryo transfer [31, 34, 35]. There is still no consensus on the reasons for this discrepancy. The potential hypothesized mechanisms involve some aspects related to specific treatments of fresh embryos transfer or FET. The primary reason was associated with the operation on women including COS, anesthesia, and needle aspiration of the ovarian follicles [36] because these approaches were performed only in fresh cycles but not in FET. Moreover, the fetal birth weights between cycles after fresh transfer and frozen transfer of embryos generated from donor oocytes indicate no difference [37]. Since the embryos developed from donor oocytes were exposed to a similar intrauterine milieu at the time of fresh or frozen embryo transfer, the ovarian stimulation-induced maternal uterine environment was hypothesized as the potential mechanism contributing to the risk of low birth weight [38]. However, no more direct evidence was provided. Our study compared the live birth weight between the newborns from blastocysts’ transfer after extended culture of vitrified cleavage embryos and those from FRBT. The results showed that the live birth weight was equivalent, even if only women in fresh cycles were subjected to COS. On the contrary, a significantly lower trend of fetal birth weight was found in the delivery after VTCE blastocysts’ transfer as compared to conventional FBT, although both transfers were performed after a similar process of endometrial preparation. These findings challenged the contribution of the intrauterine environment to fetal birth weight. The response of the endometrium to COS was unlikely the reason for the difference in birth weight. Another possible reason originated from the physical effect of the vitrification and thawing process on frozen–thawed embryos, which was different from fresh embryos [36, 39]. However, the VTCE blastocysts also suffered from steps of cryopreservation similar to the frozen–thawed blastocysts. The unique difference was the practice of extended culture in vitro for further selection before transfer, and this is identically performed in fresh blastocyst transfer cycles. Whether this practice could affect birth weight is uncertain, and the reason is also difficult to explain. Our hypothesis is that extended culture in vitro might result in some physical effects induced by vitrification that consequently affect birth weight, such as DNA methylation and imprinting protection caused by cryoprotectants [39].
In this study, a PSM method was adopted to evaluate the efficacy of blastocysts transfer after extended culture of VTCE compared with conventional FBT and FRBT. The results found in our study showed the feasibility of transfer after extended culture of vitrified cleavage embryos. Although the clinical pregnancy rate and live birth were poorer than FRBT, they were equivalent to conventional FBT cycles. In the process of prolonged culture in vitro, competent embryos were selected for transfer, which might minimize the time for achieving a live birth. Eftekhar et al. also reported that transfer of blastocysts from VTCE can improve ongoing pregnancy rate compared with transfer directly after thawing [40]. The reason for this finding was owing to the discrepancy between the blastocysts and cleavage embryos, which was reported in numerous studies [41,42,43]. However, there was still no consensus on patient selection criteria for blastocyst culture in ART. An unselected blastocysts’ transfer has not been shown to increase the live birth rate compared with the cleavage-stage embryo transfer, which may conversely result in fewer or no embryos available for transfer [6]. Moreover, there is still an ongoing global discussion regarding the optimal timing of embryo transfer, and especially the cumulated pregnancy was taken into consideration [44, 45]. Therefore, the evaluation of better outcomes between transfer of thawed embryos after extended culture until blastocyst formation and transfer at cleavage stage post-thawing was not demonstrated in this study. The focus of this study was to provide data for those couples who are willing to choose blastocysts’ transfer after extended culture.
Our results showed similar clinical outcomes compared to conventional FBT and slightly poorer outcomes than FRBT, under the premise that available blastocysts were harvested. Even if the fetal birth weight in VTCE blastocysts transfer cycles resulted lower when compared with conventional FBT, it was similar to fresh cycles. Furthermore, the gestational age was shorter when compared to FRBT, but it was comparable to conventional FBT. These results suggested that the two parameters were not severely resulting in adverse outcomes for patients undergoing VTCE blastocysts’ transfer. Moreover, our findings also suggested an underlying reason for the discrepancy in birth weight between fresh and frozen embryo transfer, which was probably not associated with COS, and also indicated the cause of short gestational age, which might result from the cryopreservation procedures.
References
Choudhary M, Soni R, Swarankar M, Garg S (2017) Comparison of vitrification and slow freezing for cryopreservation of cleavage stage embryos (day 3) and its impact on clinical outcome. Int J Res Med Sci 10(3):2751–2756
Dyer S, Chambers GM, de Mouzon J, Nygren KG, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, Adamson GD (2016) International committee for monitoring assisted reproductive technologies world report: assisted reproductive technology 2008, 2009 and 2010. Hum Reprod 31(7):1588–1609. https://doi.org/10.1093/humrep/dew082
Pereira N, Rosenwaks Z (2016) A fresh(er) perspective on frozen embryo transfers. Fertil Steril 106(2):257–258. https://doi.org/10.1016/j.fertnstert.2016.06.028
Meldrum DR (1999) Blastocyst transfer—a natural evolution. Fertil Steril 72(2):216–217
Sundhararaj UM, Madne MV, Biliangady R, Gurunath S, Swamy AG, Gopal IST (2017) Single blastocyst transfer: the key to reduce multiple pregnancy rates without compromising the live birth rate. J Hum Reprod Sci 10(3):201–207. https://doi.org/10.4103/jhrs.JHRS_130_16
Practice Committees of the American Society for Reproductive Medicine, the Society for Assisted Reproductive Technology (2013) Blastocyst culture and transfer in clinical-assisted reproduction: a committee opinion. Fertil Steril 99(3):667–672. https://doi.org/10.1016/j.fertnstert.2013.01.087
Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D (2016) Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev 6:CD002118. doi:10.1002/14651858.CD002118.pub5.
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB (2000) Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril 73(6):1155–1158
Wan CY, Song C, Diao LH, Li GG, Bao ZJ, Hu XD, Zhang HZ, Zeng Y (2014) Laser-assisted hatching improves clinical outcomes of vitrified-warmed blastocysts developed from low-grade cleavage-stage embryos: a prospective randomized study. Reprod Biomed Online 28(5):582–589. https://doi.org/10.1016/j.rbmo.2014.01.006
Cobo A, Serra V, Garrido N, Olmo I, Pellicer A, Remohi J (2014) Obstetric and perinatal outcome of babies born from vitrified oocytes. Fertil Steril 102(4):1006–1015 e1004. https://doi.org/10.1016/j.fertnstert.2014.06.019
Bartmann C, Segerer SE, Rieger L, Kapp M, Sutterlin M, Kammerer U (2014) Quantification of the predominant immune cell populations in decidua throughout human pregnancy. Am J Reprod Immunol 71(2):109–119. https://doi.org/10.1111/aji.12185
Thoemmes F (2012) Propensity score matching in SPSS. arXiv:1201.6385
Thoemmes F, Liao W (2013) Propensity score matching (with multi level data) using SPSS and R. In: Modern modeling methods conference, Storrs, Connecticut, USA
Fasano G, Fontenelle N, Vannin AS, Biramane J, Devreker F, Englert Y, Delbaere A (2014) A randomized controlled trial comparing two vitrification methods versus slow-freezing for cryopreservation of human cleavage stage embryos. J Assist Reprod Genet 31(2):241–247. https://doi.org/10.1007/s10815-013-0145-4
Gordts S, Roziers P, Campo R, Noto V (1990) Survival and pregnancy outcome after ultrarapid freezing of human embryos. Fertil Steril 53(3):469–472
Loutradi KE, Kolibianakis EM, Venetis CA, Papanikolaou EG, Pados G, Bontis I, Tarlatzis BC (2008) Cryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysis. Fertil Steril 90(1):186–193. https://doi.org/10.1016/j.fertnstert.2007.06.010
Maheshwari A, Raja EA, Bhattacharya S (2016) Obstetric and perinatal outcomes after either fresh or thawed frozen embryo transfer: an analysis of 112,432 singleton pregnancies recorded in the Human Fertilisation and Embryology Authority anonymized dataset. Fertil Steril 106(7):1703–1708. https://doi.org/10.1016/j.fertnstert.2016.08.047
Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, Zhu Y, Deng X, Qi X, Li H, Ma X, Ren H, Wang Y, Zhang D, Wang B, Liu F, Wu Q, Wang Z, Bai H, Li Y, Zhou Y, Sun M, Liu H, Li J, Zhang L, Chen X, Zhang S, Sun X, Legro RS, Chen ZJ (2018) Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med 378(2):126–136. https://doi.org/10.1056/NEJMoa1705334
Wong KM, van Wely M, Mol F, Repping S, Mastenbroek S (2017) Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst Rev 3:CD011184. https://doi.org/10.1002/14651858.CD011184.pub2
Fauque P, Jouannet P, Davy C, Guibert J, Viallon V, Epelboin S, Kunstmann JM, Patrat C (2010) Cumulative results including obstetrical and neonatal outcome of fresh and frozen-thawed cycles in elective single versus double fresh embryo transfers. Fertil Steril 94(3):927–935. https://doi.org/10.1016/j.fertnstert.2009.03.105
Le Lannou D, Griveau JF, Laurent MC, Gueho A, Veron E, Morcel K (2006) Contribution of embryo cryopreservation to elective single embryo transfer in IVF-ICSI. Reprod Biomed Online 13(3):368–375
Lundin K, Bergh C (2007) Cumulative impact of adding frozen-thawed cycles to single versus double fresh embryo transfers. Reprod Biomed Online 15(1):76–82
Pantos K, Stefanidis K, Pappas K, Kokkinopoulos P, Petroutsou K, Kokkali G, Stavrou D, Tzigounis V (2001) Cryopreservation of embryos, blastocysts, and pregnancy rates of blastocysts derived from frozen-thawed embryos and frozen-thawed blastocysts. J Assist Reprod Genet 18(11):579–582
Wang H-b, Li Y-h (2009) Extended culture of early stage embryos in frozen-thawed cycles. Journal of Reproduction and Contraception 20(1):11–18
Butterworth S (2001) Blastocyst culture: myth or magic? Hum Fertil (Camb) 4(2):109–116
Wang YA, Costello M, Chapman M, Black D, Sullivan EA (2011) Transfers of fresh blastocysts and blastocysts cultured from thawed cleavage embryos are associated with fewer miscarriages. Reprod Biomed Online 23(6):777–788. https://doi.org/10.1016/j.rbmo.2011.07.023
Haas J, Meriano J, Laskin C, Bentov Y, Barzilay E, Casper RF, Cadesky K (2016) Clinical pregnancy rate following frozen embryo transfer is higher with blastocysts vitrified on day 5 than on day 6. J Assist Reprod Genet 33(12):1553–1557. https://doi.org/10.1007/s10815-016-0818-x
Wirleitner B, Schuff M, Stecher A, Murtinger M, Vanderzwalmen P (2016) Pregnancy and birth outcomes following fresh or vitrified embryo transfer according to blastocyst morphology and expansion stage, and culturing strategy for delayed development. Hum Reprod 31(8):1685–1695. https://doi.org/10.1093/humrep/dew127
Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, Nagy ZP, Ubaldi FM (2014) Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod 29(6):1173–1181. https://doi.org/10.1093/humrep/deu033
Klimczak AM, Pacheco LE, Lewis KE, Massahi N, Richards JP, Kearns WG, Saad AF, Crochet JR (2018) Embryonal mitochondrial DNA: relationship to embryo quality and transfer outcomes. J Assist Reprod Genet 35(5):871–877. https://doi.org/10.1007/s10815-018-1147-z
Vidal M, Vellve K, Gonzalez-Comadran M, Robles A, Prat M, Torne M, Carreras R, Checa MA (2017) Perinatal outcomes in children born after fresh or frozen embryo transfer: a Catalan cohort study based on 14,262 newborns. Fertil Steril 107(4):940–947. https://doi.org/10.1016/j.fertnstert.2017.01.021
Maheshwari A, Pandey S, Shetty A, Hamilton M, Bhattacharya S (2012) Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril 98(2):368–377. https://doi.org/10.1016/j.fertnstert.2012.05.019
Aflatoonian A, Karimzadeh Maybodi MA, Aflatoonian N, Tabibnejad N, Amir-Arjmand MH, Soleimani M, Aflatoonian B, Aflatoonian A (2016) Perinatal outcome in fresh versus frozen embryo transfer in ART cycles. Int J Reprod Biomed (Yazd) 14(3):167–172
Shapiro BS, Daneshmand ST, Bedient CE, Garner FC (2016) Comparison of birth weights in patients randomly assigned to fresh or frozen-thawed embryo transfer. Fertil Steril 106(2):317–321. https://doi.org/10.1016/j.fertnstert.2016.03.049
Zhang J, Du M, Li Z, Wang L, Hu J, Zhao B, Feng Y, Chen X, Sun L (2018) Fresh versus frozen embryo transfer for full-term singleton birth: a retrospective cohort study. J Ovarian Res 11(1):59. https://doi.org/10.1186/s13048-018-0432-x
Shih W, Rushford DD, Bourne H, Garrett C, McBain JC, Healy DL, Baker HW (2008) Factors affecting low birthweight after assisted reproduction technology: difference between transfer of fresh and cryopreserved embryos suggests an adverse effect of oocyte collection. Hum Reprod 23(7):1644–1653. https://doi.org/10.1093/humrep/den150
Galliano D, Garrido N, Serra-Serra V, Pellicer A (2015) Difference in birth weight of consecutive sibling singletons is not found in oocyte donation when comparing fresh versus frozen embryo replacements. Fertil Steril 104(6):1411–1418. https://doi.org/10.1016/j.fertnstert.2015.08.013
Kalra SK, Ratcliffe SJ, Coutifaris C, Molinaro T, Barnhart KT (2011) Ovarian stimulation and low birth weight in newborns conceived through in vitro fertilization. Obstet Gynecol 118(4):863–871. https://doi.org/10.1097/AOG.0b013e31822be65f
De Geyter C, De Geyter M, Steimann S, Zhang H, Holzgreve W (2006) Comparative birth weights of singletons born after assisted reproduction and natural conception in previously infertile women. Hum Reprod 21(3):705–712. https://doi.org/10.1093/humrep/dei378
Eftekhar M, Aflatoonian A, Mohammadian F, Tabibnejad N (2012) Transfer of blastocysts derived from frozen-thawed cleavage stage embryos improved ongoing pregnancy. Arch Gynecol Obstet 286(2):511–516. https://doi.org/10.1007/s00404-012-2303-9
Stoop D, Van Landuyt L, Van den Abbeel E, Camus M, Verheyen G, Devroey P (2011) Should a single blastocyst transfer policy be a clinical decision or should it depend on the embryological evaluation on day 3? Reprod Biol Endocrinol 9:60. https://doi.org/10.1186/1477-7827-9-60
Papanikolaou EG, D'Haeseleer E, Verheyen G, Van de Velde H, Camus M, Van Steirteghem A, Devroey P, Tournaye H (2005) Live birth rate is significantly higher after blastocyst transfer than after cleavage-stage embryo transfer when at least four embryos are available on day 3 of embryo culture. A randomized prospective study. Hum Reprod 20(11):3198–3203. https://doi.org/10.1093/humrep/dei217
Papanikolaou EG, Kolibianakis EM, Tournaye H, Venetis CA, Fatemi H, Tarlatzis B, Devroey P (2008) Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Hum Reprod 23(1):91–99. https://doi.org/10.1093/humrep/dem339
Martins WP, Nastri CO, Rienzi L, van der Poel SZ, Gracia C, Racowsky C (2017) Blastocyst vs cleavage-stage embryo transfer: systematic review and meta-analysis of reproductive outcomes. Ultrasound Obstetr Gynecol 49(5):583–591. https://doi.org/10.1002/uog.17327
De Vos A, Van Landuyt L, Santos-Ribeiro S, Camus M, Van de Velde H, Tournaye H, Verheyen G (2016) Cumulative live birth rates after fresh and vitrified cleavage-stage versus blastocyst-stage embryo transfer in the first treatment cycle. Hum Reprod 31(11):2442–2449. https://doi.org/10.1093/humrep/dew219
Funding
Funding has been received form Basic Research Program of Shenzhen with Grant no. (JCYJ20160427113153295), Sanming Project of Medicine in Shenzhen with Grant no. (SZSM201502035), National Natural Science Foundation of China with Grant no. (21807072) and clinical research special fund of Chinese Medical Association with Grant no. (18010120741).
Author information
Authors and Affiliations
Contributions
FX: project development, data collection, manuscript writing. GGL and QS: data analysis, statistical support. SSW: methodology. CYW, PLC, ZHY and HXZ: manuscript editing. YZ: supervision and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
We declared that we have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiong, F., Li, G., Sun, Q. et al. Clinical outcomes after transfer of blastocysts derived from frozen–thawed cleavage embryos: a retrospective propensity-matched cohort study. Arch Gynecol Obstet 300, 751–761 (2019). https://doi.org/10.1007/s00404-019-05236-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-019-05236-3