Abstract
Purpose
Vaginal brachytherapy reduces the risk of local recurrence and was shown to be equieffective in preventing vaginal vault recurrence, but less toxic compared to external-beam radiotherapy in a subset of high intermediate-risk endometrial cancer patients and is administered as single adjuvant treatment in those patients. Different radiotherapeutic approaches with various dosing schemes exist toward brachytherapy. The aim of this study was to compare the outcome and long-term quality of life after brachytherapy with two different high-dose-rate dosing schemes.
Methods
Retrospective analysis was conducted of the recurrence and survival rates of 104 patients with endometrial cancer FIGO stage I–II that underwent adjuvant brachytherapy with three times 5 Gy or four times 5 Gy to the upper two-thirds of the vaginal vault in two different institutions between January 2010 and December 2013. Quality of life was assessed by EORTC QLQ-30 questionnaire and EN 24 module.
Results
The vaginal vault recurrence rates were 4.9% and 5.0% for patients treated with 3 × 5 Gy and 4 × 5 Gy, respectively (p = 0.98). We did not observe a difference in pelvic recurrence (p = 0.96), overall survival (p = 0.33) or quality of life between the different radiotherapy regimens. Metastatic recurrence and the use of chemotherapy contribute to impairment on quality of life. Younger patients (< 70 years) reported worse emotional functioning (p = 0.02) and higher symptom scales of diarrhea (p = 0.01) and financial problems (p = 0.03). Sexual activity was lower in patients younger than 70 years (p = 0.05).
Conclusions
Further prospective studies are needed to evaluate the effect of dosing schemes on recurrence rates and quality of life. Younger patients (< 70 years) seem to experience greater reduction in quality of life due to endometrial cancer diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometrial cancer is the most common gynecological malignancy with an annual incidence of approximately 11,000 cases each year in Germany. Incidence rates have increased notably, most likely due to a rise in risk factors such as obesity, diabetes and smoking. It usually occurs in postmenopausal women with a mean age at diagnosis of 69 years [1, 2]. Prognosis is considered to be favorable as 5-year overall survival lies around 79% in Germany [2]. Standard treatment consists of hysterectomy and bilateral salpingectomy with or without lymphonodectomy. The adjuvant treatment depends on the tumor stage and grading [2, 3].
The role of adjuvant radiotherapy in early-stage endometrial cancer has been investigated by several studies. PORTEC-I and COG-99 demonstrated a benefit in locoregional recurrence for patients treated with external-beam radiotherapy of around 10% [4, 5]. At the same time, quality of life was impaired by a rise in side effects of around 26%, especially in terms of urinary and bowel symptoms [4]. Particularly, patients with high intermediate risk of recurrence defined by higher age, grade 2–3, lymphovascular invasion and deep myometrial invasion profited from adjuvant radiotherapy [4, 6, 7].
In the PORTEC-II trial, vaginal brachytherapy was compared to external-beam radiotherapy (EBRT) as adjuvant treatment for FIGO stage I–IIA patients with high intermediate-risk endometrial cancer providing evidence for its equal efficacy [8]. Therefore, vaginal brachytherapy is now used as sole adjuvant treatment in high intermediate-risk endometrial cancer and may be combined with chemotherapy and or external-beam radiotherapy in high-risk cancer [9, 10]. Different radiotherapeutic approaches with various dosing schemes exist toward brachytherapy. Some centers prefer high-dose rate (HDR) techniques, while intermediate-dose rates are applied in other centers. Few studies have been conducted to identify differences in quality of life, oncologic outcome or toxicities [11].
Patients and methods
This retrospective study was approved by the Ethics Committee at the University of Regensburg, Bavaria. Patient data were treated in conformity with the Declaration of Helsinki.
Patients
The study population consisted of 104 patients with pathologically proven endometrial carcinoma stage I–II treated with post-operative vaginal brachytherapy between January 2010 and December 2013 in two institutions in the region of Upper Palatine, Germany. 63 patients were treated at the University Hospital Regensburg, and 41 patients were treated at the regional hospital St. Marien Amberg. Both institutions have different standard dosing approaches for vaginal brachytherapy that were compared in this study. All patients previously underwent hysterectomy and bilateral salpingectomy, and in one patient additional pelvic lymphadenectomy was performed. 75 patients underwent additional pelvic and para-aortic lymphonodectomy. Patients that underwent EBRT were excluded from the analysis. Histologically, 16 patients suffered from a grade 1 tumor, 49 from a grade 2 tumor and 39 from a grade 3 tumor. The cancer was type I in 92 cases (i.e., endometrioid, mucinous, adenocarcinoma without specific subtype) and type II in 12 cases (i.e., serous, clear cell or mixed histology). 39 patients presented with FIGO stage IA at initial diagnosis, 46 patients with FIGO stage IB, and 19 patients with FIGO stage II. 12 patients, 41 patients, 17 patients and 34 patients can be classified as low risk, intermediate risk, high intermediate risk and high risk, respectively, according to the risk stratification of the ESMO–ESGO–ESTRO consensus guidelines [12]. Information on recurrence and survival was obtained from the patients’ hospital files as well as from the regional cancer registry. Five patients received chemotherapy. Patients’ characteristics are stated in Table 1.
Radiation technique
61 patients had been treated with a total dose of 15 Gy administered in three fractions. 40 patients received 20 Gy in four fractions; 1 patient received 16 Gy in 4 fractions. Two patients did not complete treatment and received 10 Gy in weekly doses of 5 Gy (one in each treatment group). In all of the cases, radiation was applied once weekly in the afterloading technique to 5 mm tissue depth to the upper 2/3 of the vaginal stump with an Ir192 source and a vaginal applicator with a diameter of 2.5–3.5 cm (Nucletron, Elekta) depending on the women’s anatomy.
Quality of life assessment
Quality of life was assessed by standardized European Organization for Cancer (EORTC) QLQ-30 questionnaire and the validated endometrial cancer module EORTC EN 24. Questionnaires were sent to the current known address of all patients that were stated to be alive at one time point after radiotherapy. Median time from end of radiation therapy to quality of life query was 41 months (range 22–69 months).
Statistical analysis
Continuous data are expressed as means and standard deviation. Categorical data are described using absolute frequencies and relative percentages. Statistical comparisons were made using Student’s unpaired t test or Chi-squared test for continuous data with normal distribution, and Mann–Whitney U test for data without normal distribution. Log-rank test was used for categorical variables. Multivariate linear regression was performed to identify factors independently associated with QoL and to adjust for confounding parameters. All statistical tests were calculated two-sided. A p value of < 0.05 was considered the threshold of statistical significance. All statistical analyses were performed using SPSS Statistics version 23.0 (Chicago, USA).
Overall survival (OS) and disease-specific survival (DSS) were estimated by means of the Kaplan–Meier method and Cox-regression model from the date of cancer diagnosis until the date of death of any cause, until the date of cancer-related death, or last date recorded alive, respectively. Recurrence rates were calculated from the date of cancer diagnosis until the date of recurrence. Follow-up time was calculated by reversed Kaplan–Meier method.
Results
Recurrence
At the time of analysis, three patients (4.9%) treated with 3 × 5 Gy and two patients (5.0%) treated with 4 × 5 Gy experienced a vaginal recurrence (p = 0.98). There was no local recurrence in the patients that refused further radiotherapy after 2 × 5 Gy or in the patient treated with 4 × 4 Gy. The overall local recurrence rate in the whole cohort was 4.8%. One patient with local recurrence suffered from simultaneous metastatic recurrence. Recurrence in a locoregional lymph node occurred in only one patient (0.9%) 28 months after cancer diagnosis which was treated with 3 × 5 Gy. Pelvic recurrence rates did not differ significantly (p = 0.96). Mean time to locoregional recurrence was 73.3 ± 1.4 months. There was no significant correlation between grading (p = 0.11), risk group (p = 0.27), chemotherapy or FIGO stage (p = 0.94) and local recurrence. None of the recurrences were in grade 1 or low-risk tumors.
Metastatic recurrence was present in eight patients (7.7%). Higher TNM stage (p = 0.03) was significantly associated with metastatic recurrence rates, not risk group (p = 0.15) or grading (p = 0.89).
Survival
At the time of the analysis 11 patients had died, 92 patients were still alive and 1 patient was lost to follow-up. Median follow-up time was 44.8 months. Mean overall survival in the complete cohort was 70.3 ± 1.9 months. Eight deaths occurred in the 3 × 5 Gy treatment group and three deaths in the 4 × 5 Gy treatment group (p = 0.33). The only predictive factors for overall survival was age (p = 0.05). Lymphadenectomy (p = 0.64), grading (p = 0.23), risk group (p = 0.58), tumor stage (p = 0.16) and chemotherapy (p = 0.46) were not significantly associated with overall survival.
Of the 11 patients that had died, 6 patients died from tumor-related causes and 5 from other diseases. Four of the cancer-specific deaths took place in patients treated with 3 × 5 Gy and two in patients treated with 4 × 5 Gy (p = 0.76). Mean disease-specific survival was 73.4 ± 1.4 months. Grading (p = 0.05), risk group (p = 0.04) and age (p < 0.01) were significantly associated with disease-specific survival. There was no statistically significant influence on cancer-specific survival for FIGO stage (p = 0.98), lymphonodectomy (p = 0.45) or chemotherapy (p = 0.57). In a multivariate model none of the factors remained significant.
Mean recurrence-free survival was 69.4 ± 2.1 months. No significant relationship between recurrence-free survival and radiation scheme (p = 0.12), risk group (0.16), FIGO stage (p = 0.71), grading (p = 0.10), lymphonodectomy (p = 0.73), age (p = 0.10) or chemotherapy (p = 0.53) could be established.
Quality of life
Response rate to the questionnaire was 63% (n = 58). Median age of patients that responded to the survey was 65 years (range 44–86). 81% (n = 47) of the patients completed questions on sexual interest and activity, while only 26% (n = 15) and 18% (n = 16) answered the question on sexual enjoyment and sexual vaginal symptoms, respectively. Other questions were answered by 95–100% of the patients (n = 55–58). The median age of the patients that responded to the questions on sexual activity, interest, enjoyment and sexual/vaginal symptoms was 64 years (range 44–86 and 50–73, respectively).
Univariate analysis
Scores for the quality of life categories are displayed in Table 1. There was no significant difference in quality of life for the radiation groups (Table 1) or lymphadenectomy (p = 0.97) in any of the categories.
Four of the patients that responded to the survey had received chemotherapy. Those patients presented significantly higher symptom scales of tingling and numbness (p = 0.01) and lymphedema (p = 0.03).
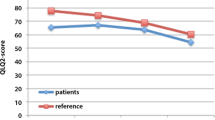
Emotional functioning was significantly worse (p = 0.02) in younger patients (< 70) compared to patients 70 years and older (Fig. 1). Furthermore, they experienced higher symptom scales of diarrhea (p = 0.01) and financial problems (p = 0.03). Sexual activity was higher in patients older than 70 years (p = 0.05) (Fig. 2).
Metastatic recurrence (n = 3) was associated with significantly worse global health status (p = 0.03), physical functioning (p < 0.01) and role functioning (p < 0.01). Patients with metastases had significantly higher symptom scales of fatigue (p = 0.01), lymphedema (p = 0.02), poor body image (p < 0.01), pain in the back and pelvis (p = 0.02), tingling and numbness (p < 0.01) and muscular pain (p = 0.02). Only one of the patients that responded to the survey had a local recurrence.
Patients that did not complete brachytherapy treatment reported higher symptom scales of pain (p = 0.05), poor body imaging (p = 0.05) and tingling and numbness (p = 0.02).
Multivariate analysis
Multivariate regression was conducted to assess the impact of radiotherapy scheme on quality of life. As in the univariate model, no significant correlation between RT scheme and any of the variables in the EORTC QLQ-C30 questionnaire or EN24 module could be identified. Higher symptom scales in the categories pain, poor body imaging and tingling and numbness in patients that did not complete brachytherapy treatment did not prevail in the multivariate model.
In a multivariate regression analysis, metastatic recurrence remained the single predictor for global health status (p = 0.03), physical functioning (p < 0.01), fatigue (p = 0.01), poor body image (p < 0.01) and pain in the back and pelvis (p = 0.02). Symptom scales for lymphedema and tingling and numbness were associated with metastatic recurrence (p = 0.01; p < 0.01) and chemotherapy (p = 0.02; p < 0.01). Muscular pain was significantly associated with chemotherapy (p < 0.01) and metastatic recurrence (p = 0.01).
Younger age remained significantly associated with emotional functioning (p = 0.02), diarrhea (p = 0.02), and lower sexual activity (p = 0.03). Financial problems were significantly associated with lower age (p = 0.02).
Secondary cancer
Eleven patients (10.6%) experienced a secondary cancer after radiotherapy. None of these cancers was located in the pelvis. There was no significant difference between the radiotherapy groups (p = 0.31).
Discussion
The indications for adjuvant radiotherapy in endometrial cancer have been examined by several studies identifying a subset of patients in which the reductions of local recurrences outweighed the side effects [4, 8, 13]. While the effectiveness and quality of life of patients receiving brachytherapy were studied in comparison to external-beam radiotherapy, little is known about different dosing schemes used for delivering brachytherapy to the vaginal stump. In the PORTEC-II trial, two different concepts of radiotherapy were included (21 Gy in 3 fractions high-dose rate vs. 30 Gy low-dose rate) [7]. Other treatment regimens are 24 Gy in six fractions, 22 Gy in four fractions, 40 Gy in four fractions in 5 mm tissue depth or 34 Gy in four fractions at the vaginal mucosa [14,15,16]. Fayed et al. retrospectively compared LDR brachytherapy with a total surface dose of 60–70 Gy to HDR therapy with six fractions of 2 Gy and found no significant difference [17]. Another retrospective analysis compared low-dose rate radium brachytherapy to HDR brachytherapy and found no significant difference in terms of effectiveness, but a higher rate of late toxicities in the HDR group treated with 10–12 Gy per fraction. More than 50% of patients in this study were treated with radiotherapy before surgery and a number of patients did not receive hysterectomy at all. Furthermore, EBRT was additionally applied in medically fit patients and treatment with radium has become outdated for various reasons [18]. As treatment has evolved over the years, further studies are needed to compare modern brachytherapy techniques among each other. To our knowledge, no comparison of different HDR dosing schemes has been evaluated so far. Brachytherapy is generally thought to be well tolerated and to be associated with little late toxicity. The aim of this study was to compare two different dosing schemes in terms of effectiveness (above all local control) and patients’ quality of life.
We confirmed a high level of local control after vaginal brachytherapy with a locoregional recurrence rate of 5.8% [5, 7]. Vaginal recurrence rate in the brachytherapy group of three randomized trials was below 3%, whereas it was 4.9% in our cohort. This may be attributed to the higher risk profile of some of our patients which included high-risk endometrial cancer patients. In the PORTEC-2 trial, the 5-year vaginal recurrence rate was 1.8% and 1.6% for patients treated with brachytherapy and pelvic irradiation, respectively. However, the study excluded patients with serous and clear cell histology as well as patients with FIGO stage II G3 cancers that demonstrate substantially higher aggressiveness. A trial by Sorbe et al. that included only low-risk endometrial cancer patients (endometrioid histology, G1–2, FIGO IA–IB) reported a vaginal recurrence rate of 1.2% for patients treated with brachytherapy that did not significantly differ from the recurrence rate of 3.1% in patients without adjuvant therapy [19]. Another study done by the same group comparing external-beam radiotherapy plus brachytherapy versus brachytherapy alone found a recurrence rate of 2.7% in the brachytherapy group in a cohort of medium-risk endometrial cancer patients defined as stage I, endometrioid histology and one risk factor (G3, > 50% myometrial invasion and nuclear grade 1–2) [20].
We did not observe a difference in local recurrence rates between the three or four fractions of 5 Gy each. Arguably, small patient numbers might have concealed minimal differences in local control between the two fractionation schemes. As the crude recurrence percentages (4.9% vs. 5.0%) were almost identical, this seems unlikely. Locoregional lymph node recurrence was observed in only one patient, confirming the results of the PORTEC-I trial that most pelvic recurrences occur in the vagina [8].
Metastatic recurrence was more common and occurred in 7.7% of patients. One patient presented with both a local and metastatic relapse. In this patient, prognosis will most likely be defined by metastases, as prognosis after salvage treatment for local relapse is favorable [4].
Three retrospective studies have compared the quality of life of patients that underwent brachytherapy to endometrial cancer patients that did not undergo adjuvant treatment. Damast et al. did not report a difference in sexual functioning in an evaluation of 205 patients 4–10 years after treatment using the female sexual functioning index. Gastrointestinal or urological symptoms were not evaluated. Prescribed cumulative doses were 18–21 Gy [21]. Similar findings were reported in a retrospective study by Becker et al. on 55 patients [22]. Another retrospective analysis comparing 16 patients after brachytherapy to a surgical control group of 53 patients found higher rates of patients reporting problems in controlling their bowels. No differences in sexual functioning were found [23]. In the light of these findings, a difference in quality of life in our treatment groups was improbable. However, in contrast to the above-mentioned studies, this study used the recently developed EN24 module that was specifically designed to measure quality of life in endometrial cancer patients. Using this sensitive tool for the evaluation of QoL, we found no difference between patients that underwent brachytherapy with a cumulative dose of 20 Gy and 15 Gy, in sexual, urological or gastrointestinal functioning. Scores from the QLQ-30 questionnaire in the Becker et al. study were comparable to our findings [22], whereas Quick et al. reported higher scores. This may be due to the fact that patients with recurrent disease, patients with stage III disease and patients with mental or physical handicap were excluded from the study [23]. Quality of life in our patient cohort was lower compared to the published data from the Portec-2 study. 36 months after therapy, the global health status for example was 77.7 in the Portec-2 cohort, whereas it is 66.6 in this publication (p > 0.01). Similar differences exist for physical, role, emotional, cognitive and social functioning. Sexual symptom scales could not be compared, as a different scoring manual was used. Explanations for the higher quality of life may be the higher age and lower recurrence rate in the Portec-2 cohort. Patient selection may be another reason, as only patients with WHO performance scores 0–2 were eligible and some preexisting diagnoses were excluded [24].
Even though the additional fraction of 5 Gy did not influence quality of life, other factors did. Metastatic recurrence at the time of the survey resulted in significant reductions of global health status, physical functioning, role functioning and higher symptom rates of fatigue, lymphedema, poor body image, pain in the back and pelvis, tingling and numbness and muscular pain. The fact that metastasis reduces patients’ quality of life is not surprising, but it is worth noting that only three patients with metastases responded to the survey. This emphasizes the crucial effect a metastatic recurrence has on the quality of life, as absolute effects need to be high to detect a significant difference in such a small cohort. Chemotherapy resulted in higher rates of tingling and numbness as well as lymphedema. The recently published PORTEC-3 trial compared chemoradiotherapy to radiotherapy alone in high-risk endometrial cancer patients and found no benefit for stage I and II cancer patients. Our analysis confirms the devastating impact chemotherapy has on quality of life reported in the trial and demonstrates that these differences remain long term [25].
Younger patients (< 70 years) had a significantly lower emotional functioning scale and experienced higher symptom scales of diarrhea and financial problems. While younger patients are more likely to be financially dependent on their employment, patients older than 70 years are usually retired. Therefore, an occupational incapacity due to the disease has more serious consequences on younger patients leading to a higher perception of financial difficulties. The diagnosis of endometrial cancer may influence younger patients more than older ones, since they have a higher life expectancy before treatment and usually have more responsibilities toward their families (i.e., children, etc.). Besides, sexual activity probably plays a bigger role in their relationships. Therefore, treatment-related side effects to this subject will have a greater impact on them. This may explain the lower emotional functioning scale in younger patients. The higher rate of sexual activity in patients older than 70 years compared to younger patients is surprising. A possible explanation may be that patients over the age of 70 years that responded to this question were the ones that were still sexually active, while others might have regarded questions on their sexual life as irrelevant at their age. Furthermore, as the questionnaire asks for a subjective evaluation whether the sexual activity was high or low, different perceptions of what high or low sexual activity means may play a role. As the absolute values on sexual enjoyment and sexual/vaginal symptoms differ clearly between the age groups, the failure to show a significant difference is most likely attributed to the small number of patients that responded to these questions. The reason for higher symptom scales of diarrhea in younger patients is not yet explicable.
Due to its retrospective design, our study has several limitations that should be discussed. First of all, quality of life was assessed only at a certain time point after radiotherapy. Therefore, no base values are available and statement about the course of quality of life during treatment and follow-up or any acute side effects cannot be made. Furthermore, the number of patients included in this study may not be enough to detect small differences in local recurrence rates and quality of life. Especially, the number of patients that responded to the questions determined to identify vaginal symptom and influences on sexual behavior was small. Since this is the area that receives the highest dose, symptoms in this area are the ones most likely to occur. Hence, a difference in quality of life between the treatment groups cannot be excluded.
Conclusions
Brachytherapy to the vaginal cuff is generally well tolerated. We did not observe a difference in local recurrence or quality of life in patients that were treated with three or four fractions of 5 Gy. Younger patients (< 70 years) seem to experience greater reduction in quality of life due to endometrial cancer diagnosis. Whether vaginal brachytherapy contributes to this impairment remains unclear. Further prospective studies are needed to evaluate the effect of dosing schemes on recurrence and quality of life.
References
AGO e.V. Epidemiologie und Prävention
Hillemanns P (2013) Neue TNM-Klassifikation beim Endometriumkarzinom. Best Practice Onkologie 8(4):22–27
Schmitt G, Carl UM, Pape H et al (1994) The role of adjuvant treatment in endometrial cancer. Strahlenther Onkol 170(10):561–564
Creutzberg CL, Nout RA, Lybeert MLM et al (2011) Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int J Radiat Oncol Biol Phys 81(4):e631–e638
Keys HM, Roberts JA, Brunetto VL et al (2004) A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma. Gynecol Oncol 92(3):744–751
Carl UM, Bahnsen J, Edel B et al (1995) The value of a postoperative radiation therapy in FIGO stage I and II endometrial cancers. Strahlenther Onkol 171(6):322–325
Scholten AN, van Putten WLJ, Beerman H et al (2005) Postoperative radiotherapy for Stage 1 endometrial carcinoma. Int J Radiat Oncol Biol Phys 63(3):834–838
Nout RA, Smit VTHBM, Putter H et al (2010) Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2). Lancet 375(9717):816–823
Koh W-J, Greer B, Abu-Rustum N, Campos SM. NCCN clinical practice guidelines in oncology (NCCN Guidelines®) uterine neoplasms. https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. Accessed 14 May 2017
Marnitz S, Köhler C (2012) Current therapy of patients with endometrial carcinoma. A critical review. Strahlenther Onkol 188(1):12–20
Townamchai K, Lee L, Viswanathan AN (2012) A novel low dose fractionation regimen for adjuvant vaginal brachytherapy in early stage endometrioid endometrial cancer. Gynecol Oncol 127(2):351–355
Colombo N, Creutzberg C, Amant F et al (2016) ESMO-ESGO-ESTRO consensus conference on endometrial cancer. Ann Oncol 27(1):16–41
Nwachukwu CR, Von-Eyben R, Kidd EA (2017) Radiation therapy improves disease-specific survival in women with stage II endometrioid endometrial cancer—brachytherapy may be sufficient. Brachytherapy 17(2):383–391. https://doi.org/10.1016/j.brachy.2017.11.001
Moreau-Claeys M-V, Brunaud C, Hoffstetter S et al (2011) Curiethérapie postopératoire du fond vaginal de haut débit de dose dans le cancer endométrial. Cancer Radiother 15(3):169–175
Nag S, Erickson B, Parikh S et al (2000) The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for carcinoma of the endometrium. Int J Radiat Oncol Biol Phys 48(3):779–790
Zhang H, Donnelly ED, Strauss JB et al (2016) Therapeutic analysis of high-dose-rate (192)Ir vaginal cuff brachytherapy for endometrial cancer using a cylindrical target volume model and varied cancer cell distributions. Med Phys 43(1):483
Fayed A, Mutch DG, Rader JS et al (2007) Comparison of high-dose-rate and low-dose-rate brachytherapy in the treatment of endometrial carcinoma. Int J Radiat Oncol Biol Phys 67(2):480–484
Sorbe B, Kjellgren O, Sténson S (2009) Prognosis of endometrial carcinoma stage I in two Swedish regions. Acta Oncol 29(1):29–37
Sorbe B, Nordström B, Mäenpää J et al (2009) Intravaginal brachytherapy in FIGO stage I low-risk endometrial cancer. Int J Gynecol Cancer 19(5):873–878
Sorbe B, Horvath G, Andersson H et al (2012) External pelvic and vaginal irradiation versus vaginal irradiation alone as postoperative therapy in medium-risk endometrial carcinoma—a prospective randomized study. Int J Radiat Oncol Biol Phys 82(3):1249–1255
Damast S, Alektiar K, Eaton A et al (2014) Comparative patient-centered outcomes (health state and adverse sexual symptoms) between adjuvant brachytherapy versus no adjuvant brachytherapy in early stage endometrial cancer. Ann Surg Oncol 21(8):2740–2754
Becker M, Malafy T, Bossart M et al (2011) Quality of life and sexual functioning in endometrial cancer survivors. Gynecol Oncol 121(1):169–173
Quick AM, Seamon LG, Abdel-Rasoul M et al (2012) Sexual function after intracavitary vaginal brachytherapy for early-stage endometrial carcinoma. Int J Gynecol Cancer 22(4):703–708
Nout RA, Putter H, Jürgenliemk-Schulz IM et al (2012) Five-year quality of life of endometrial cancer patients treated in the randomised Post Operative Radiation Therapy in Endometrial Cancer (PORTEC-2) trial and comparison with norm data. Eur J Cancer 48(11):1638–1648
de Boer SM, Powell ME, Mileshkin L et al (2018) Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3). Lancet Oncol 19(3):295–309
Author information
Authors and Affiliations
Contributions
TP: project development, data collection, and manuscript writing. SS, MH: project development and data collection. AS, MWB, OK: supervision and review. MPL: supervision and editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there are no conflicts of interest.
Informed consent
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Papathemelis, T., Scharl, S., Hipp, M. et al. Quality of life and oncological outcome in endometrial cancer patients after vaginal brachytherapy: comparison of two dosing schemes. Arch Gynecol Obstet 299, 507–514 (2019). https://doi.org/10.1007/s00404-018-4951-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4951-x