Abstract
Propose
Endometriosis is a benign disease characterized by implantation and the growth of endometrial tissue outside the uterine cavity and it shares similarities with cancer. Lamin B1, p16 and p21 play a role on cell cycle regulation, development, cell repair and its activities are related to cancers. Considering the similarities between endometriosis and cancer, the aim of the present cross-sectional study is to detect p16, p21 and Lamin B1 in the ectopic endometrium of patients with endometriosis (n = 8) with eutopic (n = 8) and control endometrium (n = 8) and relate them to the maintenance and development of endometriosis.
Methods
Biopsies were obtained from both eutopic and ectopic, from deep infiltrating lesions, endometrium frozen and used for immunofluorescent (p16) or immunohistochemistry procedures (p16, p21, lamin B1).
Results
Detected higher lamin B1 in the eutopic endometrium when compared with ectopic endometrium, with no differences between endometriosis tissue with control endometrium. Similar presence of p16 in all groups of patients and no p21 detection was observed.
Conclusion
We observed reduced detection of lamin B1 in the ectopic endometrium raising the possibility that the presence of senescent cells might be contributing to the maintenance and progression of endometriosis by apoptosis resistance and peritoneal stress inherent of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a major gynecological cause of hospital admissions [1] which affects 6–10% of the female population at reproductive age [2], endometriosis etiopathogenesis is not fully understood. Because of its impact on physical and psychological health of patients [3, 4] and of its socioeconomic impact [5], endometriosis can be considered a public health problem and deserves further investigation [1, 6,7,8,9]. It is characterized by implantation and growth of endometrial tissue outside the uterine cavity [10], and it is often associated with dysmenorrhea, deep dyspareunia, acyclic pelvic pain, infertility and alterations of the intestinal and urinary habits [11, 12].
There seems to be a similarity between endometriosis and cancer [13,14,15,16], particularly deep infiltrating endometriotic lesions, shares features with neoplastic processes, such as self-sufficiency in growth signals, insensitivity to anti-proliferative signals, resistance to apoptosis, sustained angiogenesis, tissue invasion, and genomic instability [17, 18]. Additionally, endometriosis and some types of cancer have alterations in the expression of oncogenes and tumor suppressor genes [19, 20] that are related to features that are commonly observed in both diseases, such as proliferation, apoptosis and cell cycle arrest [21, 22].
There is a correlation of a few oncogenes involved in cell cycle regulation, cell repair mechanism, replication and DNA repair and programmed cell death [23,24,25,26] such as cyclins p16INK4a (p16) and p21WAF1 (p21), with the progression of tumors [27]. Some studies suggest the involvement of increased p16 in tumor proliferation, invasiveness and angiogenesis by inhibiting cellular proliferation thus protecting cells from malignant transformation [28, 29]. Increased p21 and p16 expression have been described in endometrioma and endometrial polyps, respectively, suggesting a role of cyclins in some endometrial disorders [30,31,32]. Even though the involvement of p21 and of p16 in the more invasive types of endometriosis should be expected, none study has yet described an altered expression of such cell cycle biomarkers in deep infiltrating endometriosis.
Other proteins involved with cell cycle regulation in tumorigenesis are lamins. Lamins (A, B and C) are a group of proteins found inside the inner layer of the nuclear membrane of all cells [33] and are associated with the nuclear membrane structure maintenance. Lamin B1 is particularly involved with proliferation [34]. Moreover, higher lamin B1 seems to be associated with prostate and liver cancer, whereas lower lamin B1 was seen in breast, pancreas, and others types of cancer [35,36,37,38], commonly connected to an increased proliferative capacity and apoptosis instability of tumors, as that protein is able to prevent or delay apoptosis depending on the amount of lamin present [39].
Although there is a robust amount of evidence showing the role of p16, p21 and lamins in cancer and other diseases, there are hitherto no reports of their involvement on the development and maintenance of endometriosis, particularly in the deep infiltrating phenotype. Due to the clear involvement of cell cycle regulation in proliferative, invasiveness and apoptotic processes, the present study aims to test the hypothesis that p16, and p21 are higher and lamin B1 is lower in endometriotic lesions as compared to the eutopic endometrium, suggesting the presence of a higher number of senescence cell in endometriotic tissue.
Materials and methods
Setting
This study is part of the Women’s Health Program of the Hospital Israelita Albert Einstein, carried out in the Experimental Laboratory Center of the Education and Research Institute of the Hospital. The patients were screened by a single gynecologist and divided into two groups: with and without endometriosis.
This study was approved by the Committee on Human Research of the Hospital Albert Einstein (Number 56229916.9.0000.0071; São Paulo, Brazil), which is part of the Ethics Committee of the Brazilian Ministry of Health (CONEP).
Patients
The endometriosis group (n = 8) consisted of patients with unsatisfactory pelvic pain improvements after clinical treatment, who maintained complaints of dysmenorrhea, deep dyspareunia, chronic pelvic pain and/or cyclic intestinal alterations with proven histological diagnosis of the disease. The control group (n = 8), consisted of women submitted to laparoscopy for benign gynecologic diseases (uterine fibroids or ovarian benign cysts) and had confirmation of absence of any endometriosis focus at the time of the surgical procedure. All patients were between 18 and 45 years old, had eumenorrheic cycles, with intervals of 26–34 days between the cycles and non-use of hormonal therapy 3 months prior to surgery, including GnRH analogues, progestins and combined hormonal contraceptives. In the endometriosis group, 4 patients were in the secretory phase, 3 in the proliferative phase and 1 in the menstrual phase of the cycle. In the control groups, 5 women were in the menstrual phase, 2 in the proliferative phase and 1 in the secretory phase of the cycle. For division criteria, proliferative phase was considered from day 5 after menstruation until day 15, the secretory phase, from day 16 until day 34 of menstrual cycle.
Collection and processing of biopsy samples
Endometriosis group had resection of endometriotic lesions, including deep retrocervical lesions and rectum wall nodules [39]. Endometrium was collected using a Pipelle curette from both groups. The biopsies obtained from both endometrium and endometriotic lesions were transported in sterile cryotube immersed on ice to the experimental laboratory of the Hospital Israelita Albert Einstein. The material was frozen and kept in a freezer at − 80 °C until the experiments were carried out.
The tissue was immersed on Tissue-Tek OTC Compound before cryosectioning. Cryosections of the frozen material were made and fixed on a correctly identified slide and stored at − 80 °C until used for immunofluorescence or immunohistochemistry procedures. For immunofluorescence, and immunohistochemistry techniques, all selected tissue fragments were sectioned in 5 μm thickness.
Immunofluorescence for p16 [25]
The cross-sectional samples were thawed; fixed in 4% paraformaldehyde; washed in PBS with 0.5% Tween; immersed in Triton X-100 solution. Subsequently, the samples were incubated overnight with monoclonal antibody to p16INK4A (1:100, Rabbit monoclonal (EPR1473) to CDKN2A/p16INK4a tag: Abcam) mixed in 0.5% Tween and 5% BSA. The slides were then washed in PBS with 0.5% Tween and the secondary antibody (1:600, Goat Anti-Rabbit IgG H & L (Alexa Fluor®) (ab150116)) was added. The evaluation was performed through photographs using a confocal microscope.
Immunohistochemistry for lamin B1, p16 and p21
Immunohistochemistry was accomplished using the marking platform BenchMarck ULTRA IHC/ISH from Ventana Medical System Inc. (Tucson, Arizona, USA]. Basically, it followed a standard protocol for frozen materials. Briefly, slides were blocked with hydrogen peroxide; incubated with primary antibody Lamin B1 (H-90), Rabbit IgG—200 μg/mL—(1:200, Santa Cruz, SC-20682); monoclonal antibody to p16INK4A (1:150, Rabbit monoclonal (EPR1473) to CDKN2A/p16INK4a tag: Abcam); monoclonal antibody p21 [1:200, Rabbit monoclonal (EPR3993), Abcam]. The samples were incubated with polymer, chromogen and stained with hematoxylin. To finalize the procedure, the slides were dehydrated in alcohol for assembly with coverslip and mounting medium.
Histometry [40, 41]
The samples were cryosectioned 4 times each with 4 cuts in between each section and 4 different fields of photographs were taken for histometry procedure, resulting in 16 digitalized images per samples to be analyzed. The captured images were used to quantify the findings through a computer-based image analysis system (Olympus cellSens Dimension 1.16). Each corresponding protein detected field was histometrically evaluated by area percentage; a blinded experiment for histometric assessment was carried out.
Statistical analysis
Primarily, data analysis was done qualitatively. From the results produced by histometry (quantification of cells or amount of stained areas per field) percentage values were obtained and compared with the groups of the binary questions (lesion vs. eutopic endometrium of patients with endometriosis and endometrium of patient with endometriosis vs. patient free disease endometrium). All data analyses were performed using SAS software, version 6.11 (Statistical Analysis System Institute Inc., Cary, NC, USA). A multilevel linear mixed effect regression model was used to analyze the histometric results of p16 and lamin B1 in the groups. Results are presented as least square mean ± standard error. Statistical significance was defined as p < 0.05.
Results
No significant differences between control and endometriosis groups concerning mean age (38.25 ± 6.54 and 38.38 ± 6.80 years, respectively) or mean body mass index (21.99 ± 2.79 and 22.48 ± 4.21 kg/m2, respectively) were found out.
Validation studies were conducted using standard immunohistochemistry and immunofluorescence protocols and adjusted according to the obtained results [25]. For lamin B1, p16 and p21 antibodies, uterine tubal, ovarian carcinoma, and rectum tissue were used as positive control, respectively.
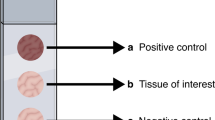
Histometric measurement revealed detection of lamin B1 with a reduced presence in endometriotic lesions when compared with eutopic endometrium (13.06% ± 5.60 and 20.94% ± 5.60, respectively). Endometrium from control patients (36.28% ± 5.60) had no lamin B1 detection significantly different when compared to endometriotic lesions nor endometrium from patients with endometriosis (Fig. 1). Data are presented as mean ± standard error mean (SEM).
Lamin B1 protein detection on ectopic and eutopic endometrium. Representative immunohistochemistry images of lamin B1 staining [brown] of uterine tubal as positive control (a); normal endometrium (b); eutopic endometrium (c) and ectopic endometrium (d). Graphical representation of the percentage of lamin B1 stained cells analyzed by histometry (e). Significance is indicated with an *p < 0.001
Results of p16 immunofluorescence and immunohistochemistry analysis (Fig. 2a–h) generated no significant differences among those three groups (Fig. 2i). After all validations, the p21 was not identified in lesions and eutopic endometrium from both groups (Supplementary Fig. 1), thus this enzyme was not used for any comparisons in this study.
P16 protein detection on ectopic and eutopic endometrium. Representative immunohistochemistry images of p16 staining [brown]: ovarian carcinoma as positive control (a); normal endometrium [33,29 ± 3,72 (b); eutopic endometrium [22,52 ± 3,72 (c); ectopic endometrium [21,31 ± 3,72] (d). Representative immunofluorescence images of p16 staining [red]: ovarian carcinoma as positive control (e); normal endometrium (f) eutopic endometrium (g); ectopic endometrium (h). Graphical representation of the percentage of p16 stained cells analyzed by histometry (i). Data are presented as mean ± standard error mean [SEM]
For the P16 analysis, the sample size (n = 8) was suitable with a 80% statistical power, and 5% of significance, considering a difference of at least 5 ± 3.52% (absolute value ± standard deviation) between groups (women with endometriosis and control without endometriosis) [42].
Discussion
In the present study, no differences in cyclins commonly associated with cancer were observed; however, lamin B1, an important protein responsible for structural integrity of the nucleus, cellular apoptosis and senescence [43, 44], was reduced in deep infiltrating endometriosis lesions (Fig. 1). Reduced lamin B1 has also been described in gastric and breast cancer [38, 45]. These changes can cause cell cycle arrest and proliferation inhibition, leading to apoptosis or senescence, and senescence could help the maintenance of some types of cancers [46]. Proliferation inhibition associated to lamin B1 loss and was found explicitly in senescent fibroblasts, keratinocytes and human diploid cells [43, 47,48,49,50,51]. In addition, the direct knockdown of lamin B1 triggers cells to exhibit premature senescence phenotypes [52]. Dutta et al. evaluating oxidative stress and preterm premature rupture of the membranes, using lamin B1 as one of the markers, suggest that lower lamin B1 expression in preterm premature rupture of the patient membranes was associated with loss of nuclear envelope integrity and with cellular senescence, pointing the latter as a potential mechanism involved in premature rupture [53]. In the endometriosis scenario, a reduced lamin B1 can be related to apoptosis resistance observed in endometriosis [22, 54, 55], even though the possibility of the involvement of senescent cells in the endometriosis pathology should not be ruled out.
Alterations in lamin B1 expression itself may not lead to cellular senescence, but additional stress on lamin B1 depleted cells may enhance and accelerate cellular senescence [44, 46, 50, 51]. It is known that endometriosis patients have higher pro inflammatory cytokines, macrophages and metabolites in the peritoneal cavity [12, 54]. Oxidative stress and increased estrogen concentration are also endometriosis features [56,57,58,59] which are considered pro-senescence factors. Thus, we speculate that the lower lamin B1 detection in endometriotic lesions described in the present study, coupled with additional peritoneal stress inherent of endometriosis patients, potentially indicate the presence and involvement of senescent cells in ectopic endometrium, therefore, helping the lesion maintenance and progression of the disease. This situation would aid in the maintenance of the characteristic disease inflammatory microenvironment through the senescence secretory phenotype, inducing the establishment of more senescent cells in the endometriotic lesions. However, it is unclear if the reduced lamin B1 detection indicates cellular senescence in endometriosis and whether this finding is a cause or consequence of the disease.
Besides commonly associated with malignant diseases [28], p16 is a robust marker of senescence cells and aging [27]. Here, we demonstrated p16 detection in deep endometriosis lesions, but no difference was seen among the studied groups, possibly because of the small and heterogeneous group of patients, since no distinction was made regarding the menstrual cycle phase. p16 could be a more sensitive marker and estrogen menstrual cycle variation could be regulating p16 expression [60]. Few studies correlate endometriosis with p16 expression. p16 expression was measured in two groups of polypoid endometriosis. One group had p16 equally expressed in comparison with non-polypoid endometriosis patients, in the other group, p16 was similarly expressed as in normal endometrium polyps, a disease that usually affects older individuals, with age-associated senescent cells [31]. The authors suggest that these results reflect the activation of cellular senescence pathways in polypoid endometriosis, corroborating with our hypotesis. Similarly, Moritani et al. assessed the p16 expression in endometrial polyps and endometrial hyperplasia, describing increased p16 expression in stromal cells of endometrial polyps that can be associated with the senescence state of these cells [61].
Conclusion
In conclusion, although using a limited but well characterized group of samples, this report was able to describe for the first time a reduced presence of lamin B1 in endometriosis lesions compared with eutopic endometrium from patients with deep infiltrating endometriosis, associated with no differences in p16 and with the absence of p21. Even though endometriosis has common features with cancer, as proliferation and invasion, the oncogenes [p21 and p16] that regulate these features in tumorigenesis were not dysregulated in our groups. The present results indicate that the role of lamin B1 and p16 should be further elucidated in the context of endometriosis pathogenesis and in the involvement of senescent cells in endometriosis.
References
Fuldeore M, Yang H, Du EX, Soliman AM, Wu EQ, Winkel C (2015) Healthcare utilization and costs in women diagnosed with endometriosis before and after diagnosis: a longitudinal analysis of claims databases. Fertil Steril 103(1):163–171. https://doi.org/10.1016/j.fertnstert.2014.10.011
Bulletti C, Coccia ME, Battistoni S, Borini A (2010) Endometriosis and infertility. J Assist Reprod Genet 27:441–447
De Graaff AA, Dirksen CD, Simoens S, De Bie B, Hummelshoj L, D’Hooghe TM et al (2015) Quality of life outcomes in women with endometriosis are highly influenced by recruitment strategies. Hum Reprod 30(6):1331–1341
De Graaff AA, D’hooghe TM, Dunselman GAJ, Dirksen CD, Hummelshoj L, Simoens S et al (2013) The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod 28(10):2677–2685
Vercellini P, Viganò P, Somigliana E, Fedele L (2014) Endometriosis: pathogenesis and treatment. Nat Rev 10(5):261–275. http://www.nature.com/doifinder/10.1038/nrendo.2013.255%5Cn, http://www.ncbi.nlm.nih.gov/pubmed/24366116
Signorile PG, Baldi A (2010) Endometriosis: new concepts in the pathogenesis. Int J Biochem Cell Biol 42(6):778–780. http://www.sciencedirect.com/science/article/pii/S1357272510001196
Nnoaham KE, Hummelshoj L, Webster P, D’Hooghe T, De Cicco Nardone F, De Cicco Nardone C et al (2011) Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 96(2):366–373. https://doi.org/10.1016/j.fertnstert.2011.05.090
Culley L, Law C, Hudson N, Denny E, Mitchell H, Baumgarten M et al (2013) The social and psychological impact of endometriosis on women’s lives: a critical narrative review. Hum Reprod Update. 19(6):625–639
Zubrzycka A, Zubrzycki M, Janecka A, Zubrzycka M (2015) New horizons in the etiopathogenesis and non-invasive diagnosis of endometriosis. Curr Mol Med 15(8):697–713
Agarwal A, Gupta S, Sharma RK (2005) Role of oxidative stress in female reproduction. Reprod Biol Endocrinol 3:28
Bellelis P, Podgaec S, Abrão MS (2014) Fatores ambientais e endometriose: um ponto de vista. Rev Bras Ginecol e Obs 36(10):433–435. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-72032014001000433&lng=en&nrm=iso&tlng=en
Kyama CM, Debrock S, Mwenda JM, D’Hooghe TM (2003) Potential involvement of the immune system in the development of endometriosis. Reprod Biol Endocrinol 1:123. http://www.ncbi.nlm.nih.gov/pubmed/14651748%5Cn, http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC305339
Somigliana E, Vigano P, Parazzini F, Stoppelli S, Giambattista E, Vercellini P (2006) Association between endometriosis and cancer: a comprehensive review and a critical analysis of clinical and epidemiological evidence. Gynecol Oncol 101(2):331–341
Bassi MA, Arias V, Filho ND, Gueuvoghlanian-Silva BY, Abrao MS, Podgaec S (2015) Deep invasive endometriosis lesions of the rectosigmoid may be related to alterations in cell kinetics. Reprod Sci 22(9):1122–1128. http://journals.sagepub.com/doi/10.1177/1933719115574341
Pupo-Nogueira A, de Oliveira RM, Petta CA, Podgaec S, Dias JA, Abrao MS (2007) Vascular endothelial growth factor concentrations in the serum and peritoneal fluid of women with endometriosis. Int J Gynaecol Obstet 99(1):33–37. http://www.ncbi.nlm.nih.gov/pubmed/17602688
Bessa NZ, de Francisco Oliveira D, Paula Andres M, Gueuvoghlanian-Silva BY, Podgaec S, Fridman C (2016) Polymorphisms of ICAM-1 and IL-6 genes related to endometriosis in a sample of Brazilian women. J Assist Reprod Genet 33(11):1487–1492. https://doi.org/10.1007/s10815-016-0796-z
Varma R, Rollason T, Gupta JK, Maher ER (2004) Endometriosis and the neoplastic process. Reproduction 127(3):293–304
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70. http://www.ncbi.nlm.nih.gov/pubmed/10647931
Pavone ME, Lyttle BM (2015) Endometriosis and ovarian cancer: links, risks, and challenges faced. Int J Womens Health 7:663–672
Anglesio MS, Papadopoulos N, Ayhan A, Nazeran TM, Noë M, Horlings HM et al (2017) Cancer-associated mutations in endometriosis without cancer. N Engl J Med 376(19):1835–1848. https://doi.org/10.1056/NEJMoa1614814
Chao C, Herr D, Chun J, Xu Y (2006) Ser18 and 23 phosphorylation is required for p53-dependent apoptosis and tumor suppression. EMBO J 25(11):2615–2622. http://www.ncbi.nlm.nih.gov/pubmed/16757976%5Cn, http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1478190
Hirakawa T, Nasu K, Abe W, Aoyagi Y, Okamoto M, Kai K et al (2016) MiR-503, a microRNA epigenetically repressed in endometriosis, induces apoptosis and cell-cycle arrest and inhibits cell proliferation, angiogenesis, and contractility of human ovarian endometriotic stromal cells. Hum Reprod 31(11):2587–2597
Munoz-Espin D, Serrano M (2014) Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 15(7):482–496. https://doi.org/10.1038/nrm3823
Dutto I, Tillhon M, Cazzalini O, Stivala LA, Prosperi E (2014) Biology of the cell cycle inhibitor p21CDKN1A: molecular mechanisms and relevance in chemical toxicology. Arch Toxicol 89:155–178
Ressler S, Bartkova J, Niederegger H, Bartek J, Scharffetter-Kochanek K, Jansen-Dürr P et al (2006) p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell 5(5):379–389
Li J, Poi MJ, Tsai M-D (2011) The regulatory mechanisms of tumor supressor p16INK4 and relevance to cancer. J Biochem 50(25):5566–5582
Davalos AR, Coppe JP, Campisi J, Desprez PY (2010) Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev 29:273–283
Yoon N, Yoon G, Park CK, Kim H-S (2016) Stromal p16 expression is significantly increased in malignant ovarian neoplasms. Oncotarget 7(40). www.impactjournals.com/oncotarget
Svensson S, Nilsson K, Ringberg A, Landberg G (2003) Invade or proliferate? Two contrasting events in malignant behavior governed by p16INK4a and an intact Rb pathway illustrated by a model system of basal cell carcinoma. Cancer Res 63(8):1737–1742
Fauvet R, Poncelet C, Hugol D, Lavaur A, Feldmann G, Daraï E (2003) Expression of apoptosis-related proteins in endometriomas and benign and malignant ovarian tumours. Virchows Arch 443(1):38–43
Stewart CJR, Bharat C (2016) Clinicopathological and immunohistological features of polypoid endometriosis. Histopathology 68(3):398–404
Ohtani N, Hara E (2013) Roles and mechanisms of cellular senescence in regulation of tissue homeostasis. Cancer Sci 104(5):525–530
Dou Z, Xu C, Donahue G, Shimi T, Pan J-A, Zhu J et al (2015) Autophagy mediates degradation of nuclear lamina. Nature 527(7576):1–17. http://www.nature.com/doifinder/10.1038/nature15548
Broers JLV, Ramaekers FCS (2014) The role of the nuclear lamina in cancer and apoptosis. Cancer Biol Nucl Envel 773:27–48. http://springerlink.bibliotecabuap.elogim.com/10.1007/978-1-4899-8032-8
Li L, Du Y, Kong X, Li Z, Jia Z, Cui J et al (2013) Lamin B1 is a novel therapeutic target of betulinic acid in pancreatic cancer. Clin Cancer Res 19(17):4651–4661
Coradeghini R, Barboro P, Rubagotti A, Boccardo F, Parodi S, Carmignani G et al (2006) Differential expression of nuclear lamins in normal\nand cancerous prostate tissues. Oncol Rep 15(3):609–613. http://www.spandidos-publications.com/or/15/3/609/abstract
Sun S, Xu MZ, Poon RT, Day PJ, Luk JM (2010) Circulating lamin B1 (LMNB1) biomarker detects early stages of liver cancer in patients. J Proteome Res 9(1):70–78
Moss SF, Krivosheyev V, de Souza A, Chin K, Gaetz HP, Chaudhary N et al (1999) Decreased and aberrant nuclear lamin expression in gastrointestinal tract neoplasms. Gut 45(5):723–729. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10517909
Abrao MS, Podgaec S, Dias JA, Averbach M, Garry R, Ferraz Silva LF et al (2006) Deeply infiltrating endometriosis affecting the rectum and lymph nodes. Fertil Steril 86(3):543–547
Park YS, Kim S, Oh SH, Park HJ, Lee S, Il Kim T et al (2014) Comparison of alveolar ridge preservation methods using three-dimensional micro-computed tomographic analysis and two-dimensional histometric evaluation. Imaging Sci Dent. 44(2):143–148
El-Domyati M, Hosam W, Abdel Azim E, Abdel-Wahab H, Mohamed E (2016) Microdermabrasion: a clinical, histometric, and histopathologic study. J Cosmet Dermatol 15:503–513
Cohen J (1988) Statistical power analysis for the behavioral sciences. Lawrence Erlbaum, New York
Dreesen O, Ong PF, Chojnowski A, Colman A (2013) The contrasting roles of lamin B1 in cellular aging and human disease. Nucleus 4(4):283–290. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3810336&tool=pmcentrez&rendertype=abstract
Camps J, Erdos MR, Ried T (2015) The role of lamin B1 for the maintenance of nuclear structure and function. Nucleus 6(1):8–14. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4615282/
Wazir U, Ahmed MH, Bridger JM, Harvey A, Jiang WG, Sharma AK et al (2013) The clinicopathological significance of lamin A/C, lamin B1 and lamin B receptor mRNA expression in human breast cancer. Cell Mol Biol Lett 18(4):595–611. http://www.degruyter.com/view/j/cmble.2013.18.issue-4/s11658-013-0109-9/s11658-013-0109-9.xml
Camps J, Wangsa D, Falke M, Brown M, Case CM, Erdos MR et al (2014) Loss of lamin B1 results in prolongation of S phase and decondensation of chromosome territories. FASEB J. 28(8):3423–3434
Freund A, Laberge R-M, Demaria M, Campisi J (2012) Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell 23(11):2066–2075. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3364172&tool=pmcentrez&rendertype=abstract
Freund A, Orjalo AV, Desprez PY, Campisi J (2010) Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med 16(5):238–246. https://doi.org/10.1016/j.molmed.2010.03.003
Scaffidi P, Misteli T (2005) Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome. Nat Med 11(4):440–445. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1351119&tool=pmcentrez&rendertype=abstract
Dreesen O, Chojnowski A, Ong PF, Zhao TY, Common JE, Lunny D et al (2013) Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J Cell Biol 200(5):605–617
Shimi T, Butin-israeli V, Adam SA, Hamanaka RB, Goldman AE, Lucas CA et al (2011) The role of nuclear lamin B1 in cell proliferation and senescence the role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev 25:2579–2593
Shah PP, Donahue G, Otte GL, Capell BC, Nelson DM, Cao K et al (2013) Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev 27(16):1787–1799
Dutta EH, Behnia F, Boldogh I, Saade GR, Taylor BD, Kacerovský M et al (2015) Oxidative stress damage-associated molecular signaling pathways differentiate spontaneous preterm birth and preterm premature rupture of the membranes. Mol Hum Reprod 22(2):143–157
Pirdel L, Pirdel M (2014) Role of iron overload-induced macrophage apoptosis in the pathogenesis of peritoneal endometriosis. Reproduction 147:199
Mier-Cabrera J, Jiménez-Zamudio L, García-Latorre E, Cruz-Orozco O, Hernández-Guerrero C (2011) Quantitative and qualitative peritoneal immune profiles, T-cell apoptosis and oxidative stress-associated characteristics in women with minimal and mild endometriosis. BJOG An Int J Obstet Gynaecol 118(1):6–16
Harlev A, Gupta S, Agarwal A (2015) Targeting oxidative stress to treat endometriosis. Expert Opin Ther Targets 19:1447–1464. https://doi.org/10.1517/14728222.2015.1077226
Augoulea A, Alexandrou A, Creatsa M et al (2012) Pathogenesis of endometriosis: the role of genetics, inflammation and oxidative stress. Arch Gynecol Obstet 286:99–103. https://doi.org/10.1007/s00404-012-2357-8
Huhtinen K, Ståhle M, Perheentupa A, Poutanen M (2012) Estrogen biosynthesis and signaling in endometriosis. Mol Cell Endocrinol 358:146–154. https://doi.org/10.1016/j.mce.2011.08.022
Piccinato CA, Neme RM, Torres N et al (2016) Increased expression of CYP1A1 and CYP1B1 in ovarian/peritoneal endometriotic lesions. Reproduction 151:683–692. https://doi.org/10.1530/REP-15-0581
Foster JS, Henley DC, Bukovsky A et al (2001) Multifaceted regulation of cell cycle progression by estrogen: regulation of Cdk inhibitors and Cdc25A independent of cyclin D1-Cdk4 function. Mol Cell Biol 21:794–810. https://doi.org/10.1128/MCB.21.3.794-810.2001
Moritani S, Ichihara S, Hasegawa M et al (2012) Stromal p16 expression differentiates endometrial polyp from endometrial hyperplasia. Virchows Arch 461:141–148. https://doi.org/10.1007/s00428-012-1276-1
Acknowledgement
We would like to thank Aline da Silva and Helen Mendes for technical assistance during sample collection and processing.
Author information
Authors and Affiliations
Contributions
H Malvezzi: Protocol/project development; Data collection or management; Data analysis; Manuscript writing/editing, BG Viana: Data collection or management, C Dobo: Data collection or management, RZ Filippi: Data collection or management, S Podgaec: Protocol/project development, Data collection or management; Data analysis; Manuscript writing/editing, CA Piccinato: Protocol/project development; Data collection or management; Data analysis; Manuscript writing/editing.
Corresponding author
Ethics declarations
Conflicts of interest
Helena Malvezzi declares that she has no conflict of interest. Bruno Gallani Viana declares that he has no conflict of interest. Cristine Dobo declares that she has no conflict of interest. Renee Zon Filippi declares that she has no conflict of interest. Sérgio Podgaec declares that he has no conflict of interest. Carla Azevedo Piccinato declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Committee on Human Research of the Hospital Albert Einstein (Number 56229916.9.0000.0071; São Paulo, Brazil), which is part of the Ethics Committee of the Brazilian Ministry of Health (CONEP) on July 13th, 2016.
Informed consent
Informed consent was obtained from all individual participants included in the study. All patients provided informed consent after the nature of the study was fully explained, and institutional review board approval.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Malvezzi, H., Viana, B.G., Dobo, C. et al. Depleted lamin B1: a possible marker of the involvement of senescence in endometriosis?. Arch Gynecol Obstet 297, 977–984 (2018). https://doi.org/10.1007/s00404-018-4691-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4691-y