Abstract
Apoptosis is a physiological process by which multicellular organisms eliminate superfluous cells. Alterations in apoptosis play a key role in tumour development. The objective was to evaluate the immunohistochemical expression of p53, p21, bax, bak, fas, bcl-2 and bcl-x proteins in 10 endometriomas, 20 benign ovarian tumours (10 mucinous, 10 serous) and 30 malignant ovarian tumours (9 mucinous, 19 serous; 2 endometrioids). p53 positive cells (mean±SD) in endometriomas, and benign and malignant tumours were 1.9±3.2, 0 and 16.2±33.0, respectively. The difference was significant between benign tumours and endometriomas (P=0.003) but not between endometriomas and malignant tumours. P21 expression in endometriomas and benign and malignant tumours was 19.5±27.8, 1.7±6.7 and 4.1±8.6, respectively. Increased p21 expression was found in endometriomas compared with benign (P=0.001) and malignant (P=0.01) tumours. Bax expression was higher in endometriomas than in benign tumours (P=0.01), but no difference was found between endometriomas and malignant tumours. No difference in bak, fas, bcl-2 or bcl-x expression was observed among the groups. In endometriomas, a negative correlation was found between p53 and fas expression (P=0.04, r=0.66). Although endometriomas have histological features of benign ovarian tumours, endometriomas share with malignant ovarian tumours certain alterations in apoptosis-related proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometriosis is defined by the presence of ectopic endometrial glands and stroma outside the uterus, and is a cause of pain and infertility. The main etiopathogenetic concepts forwarded to explain the development of endometriosis are the metaplasia and transplantation theories [33, 35]. The metaplasia theory, based on the concept that the peritoneum has the potential to differentiate into Muellerian-type epithelium, postulates that invasive mechanisms assist in the establishment of endometriotic lesions beneath the peritoneal surfaces. The transplantation theory implies that endometrial cells have the ability to detach from the endometrium, reattach after passive transport through the Fallopian tubes into the peritoneum and invade target tissues. The incidence of endometriosis in the general female population ranges from 5% to 15%, but reaches more than 50% in women with dysmenorrhea and/or infertility [34, 38, 40]. Clinically, one of the most frequent locations of endometriosis has been the ovary, with cysts called endometriomas [18]. The malignant transformation of ovarian endometriotic lesions varied from 0.6% to 1% [14].
Apoptosis, also called programmed cell death, was first discovered in 1842 by Vogt [41]. More than a century later, Kerr et al., rekindled apoptosis research [20]. This physiological process is involved in cellular homeostasis, maintaining a balance between cell proliferation and programmed cell death [16]. Apoptosis can be initiated by extracellular survival signals, mainly mediated by the bax/bcl-2 complex and also by intracellular death signals (genome instability) that increase the level of p53, and extracellular death signals involving membrane receptors such as the fas/fas-ligand system. Apoptosis-related protein expression in endometriotic lesions at different sites is controversial [19, 23, 30, 42]. Indeed, in glandular epithelial cells from ovarian endometriotic tissue, McLaren et al. [26], contrary to Harada et al. [13], reported positive bcl-2 expression. Previous studies [13, 30, 38] showed lower bcl-2 expression in endometriotic cysts than in peritoneal endometriotic samples. In contrast to bcl-2 expression, few data have been reported on the expression of apoptosis-related proteins such as bax [27] and fas [13, 42], and no data are available on p21 expression in patients with endometriosis. The aims of this study were to evaluate the expression of pro-apoptotic proteins (p53, p21, bax, bak and fas) and anti-apoptotic proteins (bcl-2 and bcl-x) in endometriomas, in comparison with benign and malignant ovarian tumours.
Materials and methods
Materials
Between 1986 and 2000, tissue samples were obtained from 60 patients undergoing surgery in the Gynaecology Department of Hôtel-Dieu Hospital, Paris; the samples consisted of 10 endometriomas, 20 benign ovarian tumours (10 mucinous, and 10 serous) and 30 malignant ovarian tumours (9 mucinous, 19 serous; 2 endometrioids).
In the endometrioma group, the mean age of the patients was 34 years (range 23–43 years). All the patients were pre-menopausal, and none had received progestin or gonadotrophin releasing hormone (GnRH) analogues for at least 3 months before surgery. They underwent surgery during the proliferative phase of the menstrual cycle in five cases and during the secretory phase in five cases. All underwent cystectomy.
In the benign tumour group, the patients' mean age was 45 years (range 21–75 years). Eight women were post-menopausal (40%), and twelve (60%) were pre-menopausal. No medical treatment was given before surgery, but three post-menopausal women were on hormone replacement therapy (HRT). All the pre-menopausal patients underwent cystectomy during the proliferative phase. One post-menopausal woman underwent bilateral salpingo-oophorectomy.
In the malignant tumour group, the patients' mean age was 55 years (range 26–84 years). Twenty patients (66.6%) were post-menopausal, and ten (33.3%) were pre-menopausal. Five post-menopausal women (25%) were on HRT. The disease was staged according to the FIGO classification (Federation Internationale de Gynecologie et Obstetrique) [17]. Thirteen patients had stage-I, two stage-II, fourteen stage-III, and one stage-IV disease. No neo-adjuvant chemotherapy was used before surgery; the latter consisted of hysterectomy with bilateral salpingo-oophorectomy and omentectomy in all cases, and pelvic and para-aortic lymphadenectomy in 7 cases. Surgery was followed by chemotherapy.
The diagnosis was confirmed histologically on each tissue sample by reviewing hematoxylin/eosin-stained slides. None of the endometriomas contained clear nuclear atypia.
Methods
Antibodies
To evaluate pro-apoptotic protein expression, we used an anti-human-p53 mouse monoclonal antibody (Dako, Glostrup, Denmark), an anti-human-p21 mouse monoclonal antibody (Dako), an anti-human-bax mouse monoclonal antibody (Immunotech, Marseille, France), an anti-human-bak rabbit polyclonal antibody (Santa-Cruz Biotechnology, Santa-Cruz, CA, USA), and an anti-human-fas (CD 95) mouse monoclonal antibody (Immunotech). To evaluate anti-apoptotic protein expression, we used an anti-human-bcl-2 rabbit polyclonal antibody (Santa-Cruz Biotechnology), and an anti-human-bcl-x mouse monoclonal antibody (Transduction Laboratories, Lexington, KY, USA).
Immunohistochemical technique
In all cases, 5-µm-thick, paraffin-embedded sections of formalin-fixed tissues were used. Sections were deparaffinated in xylene and rehydrated through a graded series of ethanol solutions, followed by microwave antigen retrieval for 20 min in 0.01 M sodium citrate buffer. Endogenous peroxidase activity was inhibited with 0.3% hydrogen peroxide for 15 min. After washing in Tris buffer saline-Tween casein (TBS-TC), sections were incubated for 1 h with the primary antibody. The antibodies to p53, p21, bak, fas and bcl-2 were used at a dilution of 1/50. Anti-bax was diluted 1/200, and anti-bcl-x was diluted 1/75. After washing in TBS-TC, the sections were incubated with an anti-mouse or anti-rabbit peroxidase conjugate (Valbiotech, Paris, France) for 40 min. Binding was visualised by incubating sections with DAB (3,3′diaminobenzidine) (Dako) or AEC (3-amino-9-ethylcarbazol) (Beckman Coulter, Brea, CA, USA) and lightly counterstaining with hematoxylin.
Positive controls for p53, p21, bax, bak, fas, bcl-2 and bcl-x were sections obtained from colon carcinoma, tonsil, normal breast epithelium, normal breast epithelium, rhadomyosarcoma, lymph node and aortic endothelium, respectively. For negative controls, the primary antibody was replaced by the relevant IgG subtype (monoclonal antibodies) or with non-immune serum (polyclonal antibodies).
Analysis of immunohistochemical results
For qualitative analysis, samples were considered negative when no labelled cell was observed on the tissue section, and positive in all other cases.
For semi-quantitative analysis, positive cells were counted among 500 cells in the most characteristic areas, and the percentage of labelled cells was calculated by two independent observers in five consecutive high-power fields (0.1734 mm2 per field) per section. Variations between the two observers were below 5%.
Statistical analysis
We used the Chi-square test for categorical variables, and the Kruskall-Wallis and Mann-Whitney tests for continuous variables. The Spearman correlation test was used to identify correlations between two quantitative variables. P values below 0.05 were considered significant.
Results
Qualitative expression of apoptosis-related proteins in endometriomas and benign and malignant ovarian tumours
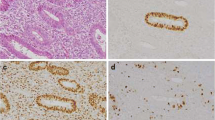
P53 immunostaining was restricted to the nuclei (Fig. 1a). P53 expression differed among the three groups of tumours (P=0.01). The paired differences were significant between endometriomas and benign tumours (P=0.01), between benign and malignant tumours (P<0.001), but not between endometriomas and malignant ovarian tumours (Table 1).
Immunoperoxidase staining with 3-amino-9-ethylcarbazol (a–e) or diaminobenzidine (f) as chromogen, and nuclear counterstaining with Harris hematoxylin. Representative examples of p53 (a), p21 (b), bax (c), bak (d), fas (e) and bcl-2 (f) staining in endometriomas. p53 and p21 immunostaining was restricted to the nuclei. Bax, bak and bcl-2 immunostaining was detected in the cytoplasm. Fas labeling was observed in both the outer membrane and the cytoplasm
P21 immunostaining was also restricted to the nuclei (Fig. 1b). P21 expression differed among the three groups of tumours (P=0.003). The difference was significant between endometriomas and benign tumours (P=0.003) and between endometriomas and malignant ovarian tumours (P=0.04).
Bax immunostaining (Fig. 1c) and bak immunostaining (Fig. 1d) was restricted to the cytoplasmic compartment. Fas immunostaining was observed on the outer cell membrane and in the cytoplasmic compartment (Fig. 1e). Bcl-2 and bcl-x immunostaining was restricted to the cytoplasmic compartment (Fig. 1f). No difference in bax, bak, fas, bcl-2 or bcl-x expression was observed among the three groups of tumours.
In endometriomas, no qualitative difference in immunostaining was found for p53, p21, bax, bak, fas, bcl-2 or bcl-x according to the phase of the menstrual cycle. In benign and malignant tumours, no difference in qualitative immunostaining was observed according to menopausal status.
Semi-quantitative expression of apoptosis-related proteins in endometriomas and benign and malignant ovarian tumours
A difference in p53 immunostaining was observed among the three groups of tumours (P=0.02). The difference was significant between endometriomas and benign tumours (P=0.003), and between benign and malignant tumours (P=0.008), but not between endometriomas and malignant tumours (Table 2).
A difference in p21 immunostaining was also found among the three groups of tumours (P=0.002). P21 expression was higher in endometriomas than in benign (P=0.001) or malignant ovarian tumours (P=0.01), but was not different between benign and malignant tumours.
A difference in Bax immunostaining was observed among the three groups of tumours (P=0.04). Bax expression was higher in endometriomas than in benign tumours (P=0.01), but no difference was noted between endometriomas and malignant ovarian tumours.
No difference in bak, fas, bcl-2 or bcl-x immunostaining was found among the three groups of ovarian tumours.
In endometriomas, no difference in semi-quantitative apoptosis-related protein expression was found according to the phase of the menstrual cycle. In benign and malignant tumours, no difference was observed according to menopausal status.
Correlations between the expression of individual apoptosis-related proteins in endometriomas
A negative correlation was found between p53 and fas expression (P=0.04, r=0.66). A trend towards a positive correlation was observed between p53 and p21 expression (P=0.06, r=0.62). No correlation was found between the other apoptosis-related proteins.
Discussion
Qualitative and semi-quantitative alterations in apoptosis-related protein expressions were observed in endometriomas relative to benign and/or malignant ovarian tumours. Although none of the endometriotic ovarian cysts presented features of nuclear atypia, four of the ten tissue samples showed positive nuclear staining for p53. These results contrast with those from previous reports showing no p53 expression in endometriomas without atypia, with atypia, or with reactive atypia, and positive staining in endometrioid carcinomas [3, 36]. Nevertheless, our results are in line with those from Nakayama et al. [29] showing focal p53 expression in endometriotic epithelial cells without nuclear atypia. Using fragment length polymorphism scanning and polymerase chain reaction DNA sequencing, these authors [29] found no mutations in p53 exons 5–8. No loss of heterozygosity or microsatellite instability was detected either, suggesting that p53 expression by endometriotic cells could be due to overexpression of wild-type p53 protein [29]. Recently, Chang et al. [7] have reported that endometriosis is associated with p53 polymorphism and that heterozygotes and proline homozygotes have a higher risk for endometriosis.
P53 expression was increased in endometriomas compared with benign tumours, but no difference was observed between endometriomas and malignant ovarian lesions. Few data have been reported on p53 expression in endometriomas compared with that of benign or malignant ovarian tumours. In contrast to Nehzat et al. [31], in our experience, a positive p53 staining was found in 40% benign cystic endometriotic lesions. Moreover, these authors have shown that p53 expression was only found in benign endometriotic lesions next to endometrioid or clear cell carcinoma, suggesting a potential continuum from endometriosis to atypical endometriosis to carcinoma. The discrepancy of our results with those reported by Nezhat et al. [31] could be in relation to the absence of a cut-off value taken in our study. However, this p53 overexpression found in our experience is reminiscent of previously reported data on cadherins, CD44 protein and ICAM-1, demonstrating that endometriomas share biological features with borderline and/or malignant ovarian tumours [8, 9].
We also observed p21 overexpression in endometriomas compared with benign and malignant ovarian tumours. Furthermore, despite the small number of endometriotic cysts included in this study, a trend towards a positive correlation was found between p53 and p21 expression. The p21 overexpression in endometriotic cysts partly contrasts with the lack of any association found by Hsieh et al. [15] between the p21 gene codon 31 arginine/serine polymorphism and endometriosis.
From the pathological point of view, overexpression of both p21 and p53 in endometriomas is in keeping with previous data [11], underlining the interaction of these apoptotic proteins. Indeed, the p53-inducible gene p21 encodes an inhibitor of cyclin-dependent kinases involved in G1 arrest [2, 5, 10]. Moreover, these latter studies also showed that p21 was not necessarily required for p53-mediated apoptosis. Recently, in univariate analysis of a series of borderline ovarian tumours, Werness et al. [43] showed the prognostic relevance of p53 expression in the absence of p21 expression.
We observed increased bax protein expression in endometriomas compared with benign ovarian tumours. However, no difference in bax expression was observed between endometriomas and malignant ovarian tumours, probably owing to marked overlap of the relevant values. Few data have been reported on bax expression in endometriotic lesions. Goumenou et al. [12] reported similar bax expression in adenomyotic and endometriotic samples, and no variation according to the phase of the menstrual cycle. Furthermore, previous studies have shown no difference in endometrial bax expression between women with and without endometriosis [27] or between eutopic and ectopic endometrium [26], whereas bax expression was higher in endometrioid carcinomas than in normal and hyperplastic endometrium [22]. We found no correlation between p53 and bax expression. However, previous studies have shown that bax induction by p53 is necessary to inhibit tumour growth [44] and that the contribution of bax to p53-mediated apoptosis is cell-type dependent [21, 25].
The fas/fas-ligand system is a major pathway for the induction of apoptosis in cells and tissues [28]. It has been suggested that cellular fas ligand expression protects the host tissue from attack by T cells, which are killed through fas ligation. In the present study, despite higher fas expression in endometriomas than in benign tumours, the difference did not reach significance. However, a negative correlation was found between p53 and fas expression. This is in keeping with data showing that transcription of the death receptor CD95/fas/Apo1 is dependent on p53, through a p53-response element located within the first intron of the CD95 gene [32].
The B-cell lymphoma/leukaemia-2 gene (bcl-2) is a proto-oncogene that prevents apoptosis [39]. Using reverse-transcriptase polymerase chain reaction and Western blotting [24] or immunohistochemistry [6], a higher bcl-2 expression has been reported in normal compared with neoplastic ovarian tissues. We found no difference in bcl-2 expression among the three groups of tumours. This contrasts with a study from Ben-Hur et al. [4], Chan et al. [6] and Zusman et al. [45], showing a decrease in bcl-2 expression from benign to borderline and malignant ovarian tumours. However, bcl-2 expression is cell- and tissue dependent, and both p53 and bcl-2 expression are reduced in serous ovarian carcinomas [1]. Indeed, recently, Nezhat and Kalir [30] have demonstrated a qualitative underexpresssion of bcl-2 in cystic compared with non-cystic endometriotic lesions suggesting a differential expression pattern of bcl-2 according to endometriotic lesions. Moreover, Nezhat et al. [31] reported a less frequent bcl-2 expression in benign endometriotic cysts than endometrioid, clear cell and serous papillary carcinomas. These results suggest that bcl-2 pattern expression differs according to histological type of epithelial ovarian tumours.
We found no variation in apoptosis-related protein expression according to the phase of the menstrual cycle. This is in line with previous reports on bcl-2 [12, 13, 42], fas [42] and bax expression [12]. Watanabe et al. [42] suggested that the absence of cyclic changes in apoptosis-related proteins in ectopic endometrium points to a difference in the mechanisms of proliferation or differentiation between eutopic and ectopic endometrium. Furthermore, we found no difference in apoptosis-related proteins according to menopausal status in women with benign and malignant ovarian tumours.
The main limitation of this study is the small number of endometriotic cyst samples studied. This was partly due to the inclusion of tissue samples from women without previous medical treatment, at least for 3 months before surgery, and without cytologic atypia. Despite this limitation, in accordance with previous studies [11, 12, 27, 29], our data show that endometriomas share a similar pattern of apoptosis-related protein expression with malignant ovarian lesions.
In conclusion, our results point to a lack of cyclical regulation of apoptosis-related protein expression in endometriomas. Moreover, although endometriomas have histological features of benign ovarian tumours, their pattern of apoptotic protein expression was closer to that of malignant than benign ovarian lesions. From the pathological point of view, these data could suggest a role of defective apoptosis in endometriomas.
References
Athanassiadou P, Petrakakou E, Sakelariou V, Zerva C, Liossi A, Michalas S, Athanassiades P (1998) Expression of p53, bcl-2 and heat shock protein (hsp72) in malignant and benign ovarian tumours. Eur J Cancer Prev 7:225–231
Attardi LD, Lowe SW, Brugarolas J, Jacks T (1996) Transcriptional activation by p53, but not induction of the p21 gene, is essential for oncogene-mediated apoptosis. EMBO J 15:3693–3701
Bayramoglu H, Duzcan E (2001) Atypical epithelial changes and mutant p53 gene expression in ovarian endometriosis. Pathol Oncol Res 7:33–38
Ben-Hur H, Gurevich P, Huszar M, et al (1999) Apoptosis and apoptosis-related proteins in the epithelium of human ovarian tumors: immunohistochemical and morphometric studies. Eur J Gynaecol Oncol 20:249–253
Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ (1995) Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377:552–557
Chan WY, Cheung KK, Schorge JO, et al (2000) Bcl-2 and p53 protein expression, apoptosis, and p53 mutation in human epithelial ovarian cancers. Am J Pathol 156:409–417
Chang CC, Hsieh YY, Tsai FJ, Tsai CH, Tsai HD, Lin CC (2002) The proline form of p53 codon 72 polymorphism is associated with endometriosis. Fertil Steril 77:43–45
Darai E, Bringuier AF, Walker-Combrouze F, Feldmann G, Madelenat P, Scoazec JY (1998) Soluble adhesion molecules in serum and cysts fluids from patients with cystic tumours of the ovary. Hum Reprod 13:2831–2835
Darai E, Leblanc M, Walker-Combrouze F, Bringuier AF, Madelenat P, Scoazec JY (1998) Expression of cadherins and CD44 isoforms in ovarian endometrial cysts. Hum Reprod 13:1346–1352
Deng C, Zhang P, Harper JW, Elledge SJ, Leder P (1995) Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82:675–684
Gottlieb TM, Oren M (1998) p53 and apoptosis. Semin Cancer Biol 8:359–368
Goumenou A, Panayiotides I, Matalliotakis I, Vlachonikolis I, Tzardi M, and Koumantakis E (2001) Bcl-2 and Bax expression in human endometriotic and adenomyotic tissues. Eur J Obstet Gynecol Reprod Biol 99:256–260
Harada M, Suganuma N, Furuhashi M, Nagasaka T, Nakashima N, Kikkawa F, Tomoda Y, Furui K (1996) Detection of apoptosis in human endometriotic tissues. Mol Hum Reprod 2:307–315
Heaps JM, Nieberg RK, Berek JS (1990) Malignant neoplasms arising in endometriosis. Obstet Gynecol 75:1023–1028
Hsieh YY, Tsai FJ, Chang CC, Chen WC, Tsai CH, Tsai HD, Lin CC (2001) p21 gene codon 31 arginine/serine polymorphism: non-association with endometriosis. J Clin Lab Anal 15:184–187
Hsu SY, Hsueh AJ (2000) Tissue-specific Bcl-2 protein partners in apoptosis: an ovarian paradigm. Physiol Rev 80:593–614
International Federation of Gynecology and Obstetrics (1971) Classification and staging of malignant tumours in the female pelvis. Acta Obstet Gynecol Scand 50:1–7
Jenkins S, Olive DL, Haney AF (1986) Endometriosis: pathogenetic implications of the anatomic distribution. Obstet Gynecol 67:335–338
Jones RK, Searle RF, Bulmer JN (1998) Apoptosis and bcl-2 expression in normal human endometrium, endometriosis and adenomyosis. Hum Reprod 13:3496–3502
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ (1995) Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 270:96–99
Kokawa K, Shikone T, Otani T, et al (2001) Apoptosis and the expression of bax and bcl-2 in hyperplasia and adenocarcinoma of the uterine endometrium. Hum Reprod 16:2211–2218
Lebovic DI, Mueller MD, Taylor RN (2001) Immunobiology of endometriosis. Fertil Steril 75:1–10
Marone M, Scambia G, Mozzetti S, et al (1998) Bcl-2, bax, bcl-xl, and bcl-xs expression in normal and neoplastic ovarian tissues. Clin Cancer Res 4:517–524
McCurrach ME, Connor TM, Knudson CM, Korsmeyer SJ, Lowe SW (1997) Bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc Natl Acad Sci U S A 94:2345–2349
McLaren J, Prentice A, Charnok-Jones DS, Sharkey AM, Smith SK (1997) Immunolocalization of the apoptosis regulating proteins bcl-2 and bax in human endometrium and isolated peritoneal fluid macrophages in endometriosis. Hum Reprod 12:146–152
Meresman GF, Vighi S, Buquet RA, Contreras-Ortiz O, Tesone M, Rumi LS (2000) Apoptosis and expression of Bcl-2 and Bax in eutopic endometrium from women with endometriosis. Fertil Steril 74:760–766
Nagata S (1994) Fas and Fas ligand: a death factor and its receptor. Adv Immunol 57:129–144
Nakayama K, Toki T, Zhai YL, Lu X, Horiuchi A, Nikaido T, Konishi I, Fujii S (2001) Demonstration of focal p53 expression without genetic alterations in endometriotic lesions. Int J Gynecol Pathol 20:227–231
Nezhat FR, Kalir T (2002) Comparative immunohistochemical studies of endometriosis lesions and endometriotic cysts. Fertil Steril 78:820–824
Nezhat F, Cohen C, Rahaman J, Gretz H, Cole P, Kalir T (2002) Comparative immunohistochemical studies of bcl-2 and p53 proteins in benign and malignant ovarian endometriotic cysts. Cancer 94:2935–2940
Owen-Schaub LB, Zhang W, Cusack JC, et al (1995) Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol Cell Biol 15:3032–3040
Ridley JH (1968) The histogenesis of endometriosis. A review of facts and fancies. Obstet Gynecol 23:1–35
Ryan IP, Taylor RN (1997) Endometriosis and infertility: new concepts. Obstet Gynecol Surv 52:365–371
Sampson JA (1927) Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 14:422–469
Schneider J, Jimenez E, Rodriguez F, del Tanago JG (1998) c-myc, c-erb-B2, nm23 and p53 expression in human endometriosis. Oncol Rep 5:49–52
Stovall DW, Bowser LM, Archer DF, Guzick DS (1997) Endometriosis-associated pelvic pain: evidence for an association between the stage of disease and a history of chronic pelvic pain. Fertil Steril 68:13–18
Suganuma N, Harada M, Furuhashi M, Nawa A, Kikkawa F (1997) Apoptosis in human endometrial and endometriotic tissues. Horm Res 48:42–47
Vaux Dl, Cory S, Adams JM (1988) Bcl-2 gene promotes haematopoetic cell survival and co-operates with c-myc to immortalize pre-B cells. Nature 335:440–442
Vercellini P, Trespidi L, De Giorgi O (1996) Endometriosis and pelvic pain: relation to disease stage and localization. Fertil Steril 65:299–304
Vogt J (1872) Untersuchungen uber die Entwicklungsgeschichte der Geburtshelferkroete (Alytes obstetricians). Jent & Gassman, Solothurn, Switzerland
Watanabe H, Kanzaki H, Narukawa S, Inoue T, Katsuragawa H, Kaneko Y, and Mori T (1997) Bcl-2 and Fas expression in eutopic and ectopic human endometrium during the menstrual cycle in relation to endometrial cell apoptosis. Am J Obstet Gynecol 176:360–368
Werness BA, Freedman AN, Piver MS, Romero-Gutierrez M, Petrow E (1999) Prognostic significance of p53 and p21(waf1/cip1) immunoreactivity in epithelial cancers of the ovary. Gynecol Oncol 75:413–418
Yin C, Knudson CM, Korsmeyer SJ, Van Dyke T (1997) Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature 385:637–640
Zusman I, Gurevich P, Gurevich E, Ben-Hur H (2001) The immune system, apoptosis and apoptosis-related proteins in human ovarian tumors (a review). Int J Oncol 18:965–972
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fauvet, R., Poncelet, C., Hugol, D. et al. Expression of apoptosis-related proteins in endometriomas and benign and malignant ovarian tumours. Virchows Arch 443, 38–43 (2003). https://doi.org/10.1007/s00428-003-0813-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-003-0813-3