Abstract
Purpose
Histological confirmation of endometrial cancer by dilatation/curettage (D/C) in women with postmenopausal bleeding (PMB) can be challenging due to anesthesiological and/or surgical risks. Thus, less invasive methods for diagnostics are required to identify patients with minimal risk for endometrial cancer (EC) to avoid unnecessary surgical intervention. The objective of this single-center cohort study was to assess the diagnostic validity of transvaginal ultrasound (TVUS) measurements of endometrial thickness (ET) in patients with PMB for the detection of EC.
Methods
A retrospective analysis of data from patients presenting between January 2005 and August 2014 at the Department of Obstetrics and Gynecology, University Hospital Ulm, Germany, with PMB and subsequent D/C was performed. Complete data with TVUS documentation of ET and histological results of tissue samples were available from 254 patients. In addition, data on age, body mass index (BMI), ASA-score, diabetes, hypertension, and hematological laboratory values (for a smaller subsample) were recorded. To identify independent risk factors, a multivariate logistic regression with endometrial cancer as binary response variable (yes/no) was performed. Diagnostic efficacy data for different ET cutoff points (≤1 to ≤26 mm) were obtained by a receiver operator characteristic (ROC) curve analysis.
Results
The multivariate logistic regression revealed a significant independent predictive value for age and ET. However, none of the analyzed ET cutoff points showed optimal diagnostic validity, as all cutoff points with sensitivity rates above 90% (≤1 to ≤5 mm) had false positive rates of 70% and higher.
Conclusions
There is no ET cutoff point that provides good diagnostic accuracy and/or reliably excludes the presence of endometrial cancer in patients with PMB. Thus, our data analysis supports the actual German approach of histological evaluation of any PMB to confirm or exclude EC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Postmenopausal bleeding (PMB) is a frequent event in postmenopausal women and represents up to 10% of all visits in private gynecological practice [1]. The incidence of PMB in all postmenopausal patients is approximately about 10% [2, 3], mostly caused by benign findings such as endometrial hyperplasia or atrophy or benign polyps. However, PMB is also highly suspicious of being a sign for the presence of endometrial cancer (EC) or premalignant lesions, as nearly every EC patient reports PMB at some point and around 5–12% of PMB results from EC [4]. In contrast to ovarian cancer, which mostly presents late in higher tumor stages, PMB as an early symptom of EC leads to its detection in earlier stages with subsequently better outcomes. Identified risk factors for EC are estrogen excess (for example caused by hormone replacement therapy, HRT), tamoxifen therapy, obesity, hypertension, diabetes mellitus, nulliparity or genetic disorders such as HNPCC (Lynch Syndrome) [5,6,7,8,9].
For definite clarification of the underlying reason of PMB, histological examination of endometrial tissue is required. In Germany, patients usually undergo surgery with dilatation and curettage (D/C) with additional hysteroscopy. However, in addition to the negative impact on pathogenesis of EC, risk factors such as metabolic syndrome, higher age or obesity also lead to increased surgical risks [10]. Especially in patients with severe obesity, ambulatory surgery as well as anesthesiological management is much more complicated due to comorbidities [11,12,13,14,15] and sometimes severe obesity even represents a limiting factor for performing surgery. A less invasive procedure without the need of anesthesia is sampling of endometrial tissue by endometrium biopsy (e.g., using a pipelle biopsy), but herewith only a small tissue sample is collected (less than 50% of the endometrium) and cancer might be missed. Therefore, this method should be reserved for patients with indications for a widespread disease and not be considered for women with a suspected more localized endometrial abnormality (e.g., endometrial polyp) [16, 17].
Given that only 5–12% of PMB is caused by EC, the majority of patients undergo surgery unnecessarily. For clinical routine, it is necessary to identify high-risk patients that definitely should undergo surgery even in cases of higher morbidity. On the other hand, identification of low-risk patients by non-invasive methods could help avoiding unnecessary surgical procedures and associated increased morbidity and mortality. Especially in patients with severe comorbidities, the need for surgical clarification has to be balanced against risks of surgery and anesthesia, and unavailing procedures should be avoided. Based on the evidence that EC becomes more frequent with increasing endometrial thickness (ET) [18,19,20], several professional guidelines recommend transvaginal ultrasound (TVUS) measurements of ET as a non-invasive first-line investigation to predict EC risk. However, there is no consent with regard to the cutoff values for ET to be used for the indication of surgical intervention in PMB, as reflected by different ET cutoff values recommended by various professional groups [21] or in the literature [22, 23].
In this retrospective cohort study, we analyzed the diagnostic value of ET assessed by TVUS for prediction of histologically confirmed EC in patients with PMB. In addition, we investigated the association of EC with other risk factors and variables. Diagnostic accuracy was evaluated in detail for different ET cutoff points to investigate whether there is an optimal ET threshold value for the detection of EC.
Patients and methods
Data
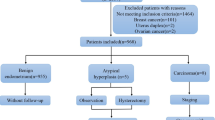
Data from all female patients presenting with PMB between January 2005 and August 2014 at the Department of Obstetrics and Gynecology, University Hospital Ulm, Germany, were evaluated retrospectively. Patients were included if TVUS examination for ET and histopathological findings were fully documented. Patients with already known malignancies, anamnestic premenopausal status, positive Human Chorionic Gonadotropin (HCG) levels or perimenopausal menorrhagia due to uterus myomatosus were excluded. In addition, patients that were referred to our department after D/C was performed in an outpatient unit were excluded, as the measured endometrial thickness is most likely biased by the preceding operation. Complete data sets with information on age (at the time of histological diagnosis), BMI, ASA-Score, diabetes and hypertension were available for 254 patients.
Diabetes and hypertension were documented in cases of already diagnosed diseases (no first diagnosis during presentation for PMB). Data on menopausal status as well as weight and height were collected at first presentation, and BMI was calculated by the formula weight (kg)/height (m)2. ASA (American Society of Anesthesiologists) physical status classification was used to assess fitness of patients before surgery. Anesthesiologists classified patients preoperatively according to the ASA score system with score 1 (normal healthy person), score 2 (mild systemic disease), score 3 (severe systemic disease), or score 4 (severe systemic disease that is a constant threat to life). As only two patients were classified with score 4, scores 3 and 4 were combined for all subsequent analyses.
For a subsample of 151 patients, the laboratory parameters hemoglobin (g/dl), hematocrit (%), erythrocytes (/pl), leucocytes (/nl), and thrombocytes (/nl) as determined at the date of hospitalization were available for analysis; in addition, blood glucose (mg/dl) was determined for 137 of these patients.
Preoperative TVUS examination was performed according to the general ultrasound guidelines using a GE Healthcare “GE Voluson 730 Expert” ultrasound machine. All patients underwent D/C with additional hysteroscopy under general anesthesia by trained physicians. Pathological investigations of all endometrial samples were performed by the Institute of Pathology, University Hospital Ulm.
Statistical analysis
Categorical variables were described using absolute and relative frequencies and continuous variables were presented using medians and ranges. Associations between the presence of histologically confirmed EC and the categorical variables diabetes, hypertension and ASA score were performed with Chi-square tests, while comparisons between patients with or without endometrial malignancy with regard to the continuous variables ET, age, BMI, and laboratory blood parameters were analyzed using the non-parametric Mann–Whitney U test. The comparisons between patients with or without EC are illustrated using Box-and-Whisker plots, where the box represents the interquartile range (IQR) and the horizontal line inside the box indicates the median. If there were no outliers, the ends of the whiskers denote minimum and maximum of the data. Outliers more than 1.5 IQR but less than 3 IQR below the lower or above the upper quartile are represented by open circles, and extreme outliers (more than 3 IQR below the lower or above the upper quartile) are indicated by stars. A multivariate logistic regression analysis with endometrial cancer as binary response variable (yes/no) was conducted to identify significant independent predictors for the presence of EC. Receiver operator characteristic (ROC) curve analysis was performed to evaluate diagnostic accuracy of ET as predictor for the presence of EC at different cutoff points. All statistical tests were two-sided and P values below 0.05 were considered statistically significant; statistical analyses were performed with the software IBM® SPSS® Statistics version 22 (IBM, Armonk NY, USA).
Results
Patient characteristics
The median patient’s age at time of diagnosis was 64 years (ranging from 40 to 92 years). Median BMI was 28.0 kg/m2 (range 16.9–103.6 kg/m2), 37.8% (n = 96) of patients were obese (BMI ≥ 30.0 kg/m2), and 7.9% (n = 20) were morbidly obese (BMI ≥ 40.0 kg/m2). 17.7% of the patients (n = 45) suffered from diabetes, and 57.1% (n = 145) from hypertension; overall, 8.3% of women (n = 21) had a metabolic syndrome defined as BMI ≥ 30 kg/m2 combined with diabetes and hypertension. 6.7% (n = 17) were classified as ASA 1, 37.4% (n = 95) as ASA 2 and 55.9% (n = 142) as ASA 3 or 4. The median values for all lab parameters were well within the normal ranges, except for slightly elevated blood glucose with a median value of 100 mg/dl (fastening <90 mg/dl). Further details of patient characteristics at baseline are shown in Table 1.
The median ET was 10 mm with a range from 1 to 49 mm. The majority of patients had an ET >4 mm (83.1%; n = 211), about half of the patients had an ET >10 mm (49.6%; n = 126), and 28.3% (n = 72) of the women with PMB showed an ET of more than 15 mm. Figure 1 illustrates the frequency distribution of ET according to different size classes.
Endometrial cancer was diagnosed in 31.9% (n = 81) of our 254 patients.
Associations with endometrial cancer
In our patient cohort, thickness of the endometrium was significantly higher in women with EC than without (P < 0.001; Fig. 2a), with a median ET of 14.3 mm (range 1–49 mm) for patients with EC and 9.0 mm (range 1–47 mm) for patients with no malignancy. Women with EC were significantly older than women with no malignancy (median age 69 vs. 61 years, P < 0.001; Fig. 2b). However, we found no significant difference between women with and without EC with regard to BMI (median BMI 28.3 vs. 28.0 kg/m2, P = 0.70).
There were no significant differences between women with EC and women with no malignancy with regard to the hematological parameters hematocrit (median 39 vs 40%, P = 0.90), hemoglobin (median 13.3 vs 13.35 g/dl, P = 0.94), erythrocytes (median 4.5 vs 4.5/pl, P = 0.75), leucocytes (median 7.80 vs 6.95/nl, P = 0.19), thrombocytes (median 279 vs 268/nl, P = 0.52) or blood glucose (median 101 vs 99 mg/dl, P = 0.94).
No significant associations between EC and the risk factors diabetes (P = 0.90) or hypertension (P = 0.12) were found. In addition, there was no significant association between EC and the presence of a metabolic syndrome (P = 0.26). In contrast, EC was significantly related to the ASA score (P = 0.021), as out of the 81 patients with EC only 1.2% had ASA score 1 and 65.4% had ASA score 3 or 4, while out of the 173 patients with no malignancy 9.2% had ASA score 1 and only 51.4% had ASA score 3 or 4.
A multivariate logistic regression with EC (yes/no) as binary response variable revealed an independent significant predictive value only for ET (odds ratio for a 1 mm increase in ET 1.058, 95% CI 1.020–1.098, P = 0.003) and age (odds ratio for a 1 year increase in age 1.054, 95% CI 1.025–1.084, P < 0.001), while BMI (P = 0.72), ASA score (P = 0.26), diabetes (P = 0.16) and hypertension (P = 0.92) did not significantly contribute to the model.
Predictive value of endometrial thickness
The diagnostic key performance indicators for the prediction of EC in patients with PMB based on different cutoff points of ET measured by TVUS are shown in Table 2. Figure 3 shows the corresponding receiver operator characteristic (ROC) curve. The area under the curve (AUC) with endometrial thickness as predictive factor for diagnosis of endometrial cancer was significantly larger than random assignment (AUC 0.686, 95% CI 0.616–0.757, P < 0.001). However, the ROC curve illustrates that none of the cutoff points provided optimal diagnostic results in terms of combining the clinically required high sensitivity with acceptable specificity rates. To further illustrate these results, Fig. 4 shows the frequencies (%) of true positives, true negatives, false positives and false negatives for all cutoff points analyzed.
Discussion
In accordance with the results of other studies, increasing ET and older age were associated with a higher prevalence of EC in our cohort of women with PMB. However, other well-known risk factors such as diabetes, hypertension or obesity were not associated with the presence of EC in our collective.
The median weight of our patients was 75 kg and median BMI was 28 kg/m2. Using the classification proposed by international guidelines, 31.1% of all patients in our analyzed cohort were overweight (BMI 25.0–29.9 kg/m2) and 37.8% were obese (BMI ≥ 30 kg/m2). Several studies describe overweight and metabolic syndrome as risk factors for overall increased PMB and endometrial pathologies [24, 25]; thus, the high percentage of overweight and obese women in our cohort of patients with PMB is not unexpected. Our failure to detect a significant association between the presence of EC and obesity or the related factors diabetes and hypertension may be due to this disproportionally high percentage of overweight or obese patients in our preselected cohort. In addition, this fact could also explain the relatively high proportion of women with PMB that were diagnosed with endometrial cancer in our study. Our collective comprises an above-average number of patients with high comorbidity (especially for anesthesiologic reasons) that are referred to us because they cannot be treated safely in an outpatient setting. As many of those comorbidities (e.g., obesity, diabetes) are also risk factors for endometrial cancer, this might explain the high percentage of diagnosed cancers in our study. Furthermore, while patients with PMB but no additional risk factors usually are treated in outpatient clinics, patients that are clinically highly suspicious for endometrial cancer might be more often directly referred to our specialized Gynecologic Oncology Unit.
A diagnostic tool for distinguishing patients with high from those with low risk for EC would have great benefit in clinical routine. The determination of ET by TVUS is an easy, non-invasive and inexpensive method. Several studies have demonstrated that ET in postmenopausal women is related to EC. In asymptomatic postmenopausal women, the use of endometrial thickness as a screening test for endometrial carcinoma is not recommended [26,27,28]. In women with PMB, ET less than 4 or 5 mm is associated with a very low risk of EC, but the risk rises with increasing ET, especially with an ET >20 mm [18,19,20]. Therefore, the American College of Obstetricians and Gynecologists (ACOG) recommends histological clarification in women with PMB and ET >4 mm, but suggests expecting behavior in women with PMB and ET ≤4 mm [21]. In case of endometrial heterogeneity, persistent bleeding or inappropriate visualizing, invasive diagnostics should follow. Similar recommendations were published by the Southern California Permanente Medical Group’s Abnormal Uterine Bleeding Working Group [4]. A meta-analysis with data from almost 6000 women with PMB supported this procedure, as 96% (95% CI 94–98%) of all patients with cancer had an abnormal ET defined as ≥5 mm. Calculated based on a 10% average risk for EC in case of postmenopausal bleeding, the authors estimated the risk for EC in a patient with PMB and a normal ET to be 1% [22]. In a more recent meta-analysis, Timmermans et al. [23] found a sensitivity of only 90% with an ET threshold of 5 mm (assessed by TVUS) for the detection of EC, but sensitivity increased to 97.9% (95% CI 90.1–99.6%) when using a threshold of 3 mm. Therefore, the authors recommend the use of a cutoff of 3 mm for better diagnostic accuracy [23]. A cutoff value of 3 mm was also suggested by a recent retrospective cohort study by Wong et al. [29]. In summary, TVUS has been recommended as first-line investigative tool for women with PMB by several international professional guidelines, but there is still no consensus on the ET cutoff value to be used for the decision of further invasive versus conservative diagnostic procedures.
In our study, the cutoff points of 3, 4 and 5 mm showed a sensitivity of 95.1, 91.4 and 90.1%, but the corresponding false positive rates (i.e., 1—specificity) were unacceptably high with 86.1, 79.2, and 73.4%, respectively. The cutoff point of 16 mm was associated with the largest proportion of correctly classified patients (72.4%) but sensitivity was only 42%. A low cutoff point for the identification of patients with no risk of endometrial cancer also seems not feasible, as there were patients with EC in our cohort with an ET below the cutoff even if the lowest possible cutoff point of ≤1 mm was used.
Given our results and the inhomogeneous data presented in the literature, we do not recommend an indication for D/C in patients with PMB that is solely based on TVUS-assessed ET, as sufficient diagnostic accuracy in terms of high sensitivity combined with moderate to low rates of false positives was not reached by any of the ET cutoff points investigated. Thus, we recommend histological confirmation by D/C in all patients presenting with PMB whenever possible. However, other possibilities discussed as an alternative in cases D/C is not an appropriate or available option (e.g., because of comorbidities, limited access to operation rooms, or financial considerations) are office-based endometrial biopsies or the use of micro-hysteroscopes to perform a targeted biopsy under direct visualization [30]. Specifically, in high-risk patients with thick endometrium but without option for surgical intervention through D/C an endometrial biopsy should be performed for confirmation of suspected diagnosis, as pipelle biopsy and D/C showed almost equal success rates in non-focal endometrial pathologies [16, 17, 31]. While those procedures are less expensive, do not need an operating room and carry less risk for uterine perforation, the main concern here is that sampling might only include normal endometrium and might miss pathological findings especially in small and/or localized tumors. In case of histologically confirmed malignancy and strong contraindication for surgical treatment, local radiation should be discussed as an optional therapy [32].
In conclusion, based on our retrospective cohort analysis, only older age and increased ET are significant and independent risk factors for the presence of EC in women with PMB. We could not find any association between EC and obesity, related conditions such as diabetes and hypertension, or hematological laboratory parameters. However, ET assessed by TVUS has only limited value as diagnostic tool to predict the presence of EC in women with PMB, because there is no cutoff value that combines the required high sensitivity with clinically acceptable low false positive rates. In addition, EC was found even in patients with ET ≤1 mm. Thus, in accordance with actual German guidelines, we recommend histological confirmation by D/C in all patients presenting with PMB. TVUS should be used as a preoperative diagnostic tool that might provide the surgeon with additional information important for the choice of surgical procedures, or as an alternative to endometrial sampling in postmenopausal women who cannot undergo further surgery.
References
Moodley M, Roberts C (2004) Clinical pathway for the evaluation of postmenopausal bleeding with an emphasis on endometrial cancer detection. J Obstet Gynaecol 24:736–741. doi:10.1080/014436104100009394
Astrup K, de Olivarius NF (2004) Frequency of spontaneously occurring postmenopausal bleeding in the general population. Acta Obstet Gynecol Scand 83:203–207
Smith-Bindman R, Weiss E, Feldstein V (2004) How thick is too thick? When endometrial thickness should prompt biopsy in postmenopausal women without vaginal bleeding. Ultrasound Obstet Gynecol 24:558–565. doi:10.1002/uog.1704
Munro MG, Southern California Permanente Medical Group’s Abnormal Uterine Bleeding Working Group (2014) Investigation of women with postmenopausal uterine bleeding: clinical practice recommendations. Perm J 18:55–70. doi:10.7812/TPP/13-072
Denschlag D, Ulrich U, Emons G (2010) The diagnosis and treatment of endometrial cancer: progress and controversies. Dtsch Ärzteblatt Int 108:571–577. doi:10.3238/arztebl.2011.0571
Colombo N, Creutzberg C, Amant F et al (2016) ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer 26:2–30. doi:10.1097/IGC.0000000000000609
Liao C, Zhang D, Mungo C et al (2014) Is diabetes mellitus associated with increased incidence and disease-specific mortality in endometrial cancer? A systematic review and meta-analysis of cohort studies. Gynecol Oncol 135:163–171. doi:10.1016/j.ygyno.2014.07.095
Schouten LJ, Goldbohm RA, van den Brandt PA (2004) Anthropometry, physical activity, and endometrial cancer risk: results from the Netherlands Cohort Study. J Natl Cancer Inst 96:1635–1638. doi:10.1093/jnci/djh291
Secord AA, Hasselblad V, Von Gruenigen VE et al (2016) Body mass index and mortality in endometrial cancer: a systematic review and meta-analysis. Gynecol Oncol 140:184–190. doi:10.1016/j.ygyno.2015.10.020
Orekoya O, Samson ME, Trivedi T et al (2016) The impact of obesity on surgical outcome in endometrial cancer patients: a systematic review. J Gynecol Surg 32:149–157. doi:10.1089/gyn.2015.0114
Tung A (2010) Anaesthetic considerations with the metabolic syndrome. Br J Anaesth 105(Suppl):i24–i33. doi:10.1093/bja/aeq293
Joshi GP, Ahmad S, Riad W et al (2013) Selection of obese patients undergoing ambulatory surgery: a systematic review of the literature. Anesth Analg 117:1082–1091. doi:10.1213/ANE.0b013e3182a823f4
Bouwman F, Smits A, Lopes A et al (2015) The impact of BMI on surgical complications and outcomes in endometrial cancer surgery—an institutional study and systematic review of the literature. Gynecol Oncol 139:369–376
Cullen A, Ferguson A (2012) Perioperative management of the severely obese patient: a selective pathophysiological review. Can J Anaesth (J Can d’anesthésie) 59:974–996. doi:10.1007/s12630-012-9760-2
Hodgson LE, Murphy PB, Hart N (2015) Respiratory management of the obese patient undergoing surgery. J Thorac Dis 7:943–952. doi:10.3978/j.issn.2072-1439.2015.03.08
Demirkiran F, Yavuz E, Erenel H et al (2012) Which is the best technique for endometrial sampling? Aspiration (pipelle) versus dilatation and curettage (D&C). Arch Gynecol Obstet 286:1277–1282. doi:10.1007/s00404-012-2438-8
Guido RS, Kanbourshakir A, Rulin MC, Christopherson WA (1995) Pipelle endometrial sampling—sensitivity in the detection of endometrial cancer. J Reprod Med 40:553–555
Karlsson B, Granberg S, Wikland M et al (1995) Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding—a nordic multicenter study. Am J Obstet Gynecol 172:1488–1494. doi:10.1016/0002-9378(95)90483-2
Goldstein SR, Nachtigall M, Snyder JR, Nachtigall L (1990) Endometrial assessment by vaginal ultrasonography before endometrial sampling in patients with postmenopausal bleeding. Am J Obstet Gynecol 163:119–123
Ferrazzi E, Torri V, Trio D et al (1996) Sonographic endometrial thickness: a useful test to predict atrophy in patients with postmenopausal bleeding. An Italian multicenter study. Ultrasound Obstet Gynecol 7:315–321. doi:10.1046/j.1469-0705.1996.07050315.x
American College of Obstetricians and Gynecologists (2009) ACOG Committee Opinion No. 426: the role of transvaginal ultrasonography in the evaluation of postmenopausal bleeding. Obstet Gynecol 113:462–464. doi:10.1097/AOG.0b013e31819930cc
Smith-Bindman R, Kerlikowske K, Feldstein VA et al (1998) Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA 280:1510–1517
Timmermans A, Opmeer BC, Khan KS et al (2010) Endometrial thickness measurement for detecting endometrial cancer in women with postmenopausal bleeding: a systematic review and meta-analysis. Obstet Gynecol 116:160–167. doi:10.1097/AOG.0b013e3181e3e7e8
Özdemir S, Batmaz G, Ates S et al (2015) Relation of metabolic syndrome with endometrial pathologies in patients with abnormal uterine bleeding. Gynecol Endocrinol 31:725–729. doi:10.3109/09513590.2015.1058355
Bueloni-Dias FN, Spadoto-Dias D, Delmanto LRMG et al (2016) Metabolic syndrome as a predictor of endometrial polyps in postmenopausal women. Menopause 23:759–764. doi:10.1097/GME.0000000000000616
Breijer MC, Peeters JAH, Opmeer BC et al (2012) Capacity of endometrial thickness measurement to diagnose endometrial carcinoma in asymptomatic postmenopausal women: a systematic review and meta-analysis. Ultrasound Obstet Gynecol 40:621–629. doi:10.1002/uog.12306
Worley MJ, Dean KL, Lin SN et al (2011) The significance of a thickened endometrial echo in asymptomatic postmenopausal patients. Maturitas 68:179–181. doi:10.1016/j.maturitas.2010.10.007
Yasa C, Dural O, Bastu E et al (2016) Evaluation of the diagnostic role of transvaginal ultrasound measurements of endometrial thickness to detect endometrial malignancy in asymptomatic postmenopausal women. Arch Gynecol Obstet 294:311–316. doi:10.1007/s00404-016-4054-5
Wong AS-W, Cheung CW, Fung LW-Y et al (2016) Development and validation of prediction models for endometrial cancer in postmenopausal bleeding. Eur J Obstet Gynecol Reprod Biol 203:220–224. doi:10.1016/j.ejogrb.2016.05.004
Di Spiezio Sardo A, Bettocchi S, Spinelli M et al (2010) Review of new office-based hysteroscopic procedures 2003–2009. J Minim Invasive Gynecol 17:436–448. doi:10.1016/j.jmig.2010.03.014
Dijkhuizen FPHLJ, Mol BWJ, Brölmann HAM, Heintz APM (2000) The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: a meta-analysis. Cancer 89:1765–1772. doi:10.1002/1097-0142(20001015)89:8<1765:AID-CNCR17>3.0.CO;2-F
Rose PG, Baker S, Kern M et al (1993) Primary radiation therapy for endometrial carcinoma: a case controlled study. Int J Radiat Oncol Biol Phys 27:585–590
Author information
Authors and Affiliations
Contributions
AS: Data collection, Manuscript writing. FE: Data collection, Manuscript editing. EB: Data collection, Manuscript editing. WJ: Data collection, Manuscript editing. UF-H: Data collection, Manuscript editing. M: Data collection, Manuscript editing. NG: Data collection, Manuscript writing. TWPF: Data analysis, Manuscript writing. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Grant support
None.
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this retrospective study formal consent is not required.
Rights and permissions
About this article
Cite this article
Schramm, A., Ebner, F., Bauer, E. et al. Value of endometrial thickness assessed by transvaginal ultrasound for the prediction of endometrial cancer in patients with postmenopausal bleeding. Arch Gynecol Obstet 296, 319–326 (2017). https://doi.org/10.1007/s00404-017-4439-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-017-4439-0