Abstract
Purpose
CD44 expression in both the early and metastatic phases of many epithelial and non-epithelial cancers is strongly prognostic. The objective of the study is to evaluate whether there is any relationship between the expression of CDD44v6 and endometrial cancer (EC) staging and prognosis.
Methods
This retrospective study included 60 EC patients for whom surgical staging was performed between 2000 and 2006. Twenty-eight randomly selected patients with normal endometria served as the control group. We immunohistochemically evaluated membranous and cytoplasmic CD44v6 staining in tissue paraffin blocks. The results were graded as positive or negative.
Results
Membranous staining in both advanced and early stage EC patients was significantly higher than that in the control group (p = 0.002). The extent of either membranous or cytoplasmic staining in both advanced- and early stage patients did not differ significantly by age, tumor grade, stage, extent of myometrial invasion, lymph node involvement, cytology, adnexal involvement, or omental spreading. In advanced-stage patients, neither papillary serous not clear cell cancers exhibited cytoplasmic staining.
Conclusions
CD44v6 membranous staining can be useful for differentiating malignant from benign endometrial tissue. However, staining is not associated with EC staging or prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
CD44 (~80 kDa), a lymphocyte receptor, is a ubiquitous, multistructured, multifunctional, cell-surface adhesion molecule encoded on chromosome 11. CD44 is expressed in various endometrial compartments of many epithelial cells [1]. The CD44 expression pattern varies during the menstrual cycle; the expression of CD44 and its variants increases significantly during the secretory phase [2,3,4].
CD44 overexpression in both the early and metastatic phases of many epithelial and non-epithelial cancers is strongly prognostic [5]. Compared with normal tissue, CD44 expression is increased in non-Hodgkin’s lymphoma and breast, colon, and gastric cancers, but it is decreased in prostate and head-and-neck cancers [6,7,8,9,10]. The CD44 variant 8–10 is known as epithelial CD44 and is preferentially expressed in epithelial cells. CD44 variant 6 (CD44v6) is the major CD44 isoform regulating tumor invasion, progression, and metastasis [11]. The expression levels of CD44 in gynecological malignancies remain controversial. Several studies have shown that CD44v6 expression was decreased in premalignant lesions of the cervix and in cervical and ovarian cancer, with shedding into the peritoneal cavity [12,13,14,15,16,17]. However, Fujita et al. reported that reduced or absent CD44 expression in endometrial cancer (EC) tissue may be associated with a strong tendency toward metastasis [18].
In our study, we aimed to evaluate whether there is any relationship between the expression of CDD44v6 and EC staging and prognosis.
Materials and methods
This retrospective study (approved by the Institutional Ethics Committee of Ankara University) involved 60 EC patients for whom surgical staging was performed between 2000 and 2006 in the Oncology Clinics of Ankara University, Ankara, Turkey. The control group consisted of 28 randomly chosen patients who underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy for gynecological reasons other than malignancy. Demographic characteristics were extracted from medical records. All patients underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy, and all EC patients underwent peritoneal washing, pelvic and para-aortic lymph node dissection, and omentectomy. The clinicopathological features of all cancer patients were noted; these included the International Federation of Gynecology and Obstetrics (FIGO) stage, tumor grade, depth of myometrial invasion, cervical, serosal, or adnexal involvement, presence of distant metastases, lymphovascular space involvement, and lymph node status. Histological diagnoses were based on the FIGO (1998) criteria, and 52 endometrioid, 5 papillary serous, 2 clear cell, and 1 adenosquamous EC were identified.
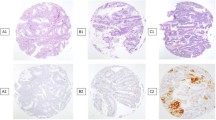
Hysterectomy specimens from 88 cases (60 ECs, 14 secretory endometria, and 14 proliferative endometria) were re-evaluated. A single pathologist selected representative EC and control samples from which 3-µm-thick paraffin-embedded specimens were prepared for CD44 immunostaining. A 1.5-mm-diameter manual tissue-array device was used to prepare microarrays. Representative tumor areas exhibiting characteristic histomorphologies were selected from “recipient” paraffin blocks. Five tissue cylinders were punched out from each “donor” paraffin block. An antibody against CD44v6 [anti-CD44 v6 (clone VFF-7); NeoMarkers, Fremont, CA, USA] was incubated with the tissue microarray sections as the initial step of the standard streptavidin–biotin peroxidase technique. Counterstaining with Meyer’s hematoxylin was performed; positive and negative controls were processed simultaneously. Both cytoplasmic staining (c-staining) and membranous staining (m-staining) were evaluated. The results were graded positive or negative, as described by Kainz et al. [19] (Fig. 1a–c).
Data were compared using SPSS for Windows version 11.5 software. Continuous variables were expressed as means with standard deviations. The independent samples t test was used to compare parametric continuous variables. The Mann–Whitney U test was employed to compare non-parametric continuous variables, and the Chi-squared test or Fisher’s exact test, as appropriate, was used to compare categorical variables. The mean values of the three groups were compared by the one-way analysis of variance. Between-group comparisons were performed using Tukey’s post hoc test. A p value <0.05 was considered to reflect statistical significance.
Results
Sixty patients of mean age 60.4 ± 10.9 years yielded malignant endometrial specimens. The mean age was lower in the control group than in either early stage or advanced-stage EC women, with statistical significance (p < 0.01). Staging performed using the FIGO 1988 system identified 34 stage I patients (56.6%) (4 IA, 14 IB, and 16 IC), 4 stage II patients (6.6%) (1 IIA and 3 IIB), 18 stage III patients (30%) (4 IIIA and 14 IIIB), and 4 stage IV patients (6.6%) (all IVB). The histological diagnoses were as follows: 52 (86.6%) endometrioid, 5 (8.3%) papillary serous, 2 (3.3%) clear cell, and 1 (1.6%) adenosquamous. The mean grade of advanced-stage EC was significantly higher than that of early stage EC (2.54 ± 0.51 vs. 2.06 ± 0.60, respectively; p < 0.001). Benign endometrial tissue samples served as controls; the mean age of the donors was 43.9 ± 5.52 years. Histologically, 14 samples were proliferative and 14 secretory (Table 1).
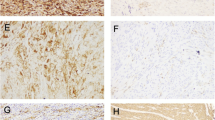
CD44v6 m-staining was detected in 50% (13/26), 35.5% (12/34), and 3.6% (1/28) of advanced- stage EC, early stage EC, and normal endometrial samples, respectively; malignant endometrial tissue stained significantly more frequently than did normal endometrial tissue (p < 0.001); however, staining did not differ by cancer stage (p = 0.252) (Fig. 2). CD46 c-staining was detected in 15.4% (4/26), 20.6% (7/34), and 42.9% (12/28) of advanced-stage EC, early stage EC, and normal endometrial samples, respectively. Malignant endometrial tissue stained significantly more frequently than did normal endometrial tissue (p = 0.046); however, staining did not differ by cancer stage (p = 0.742) (Fig. 3).
We found no significant staining difference between CD44v6+ and CD44v6− advanced-stage EC cases in terms of age, histological type, grade, extent of myometrial invasion, lymph node invasion, adnexal metastasis, omental metastasis, or intraperitoneal cytology (Tables 2, 3). C-staining was not apparent in the aggressive histological subgroups including papillary serous and clear cell cancers (Table 3). The benign endometrial specimens exhibited no significant difference in CD44v6 m-staining/c-staining between the two histological types (p = 0.127) (Table 4).
Discussion
We first showed that CD44v6 was expressed at a significantly higher frequency in only the membranous compartments of early and advanced-stage EC compared with benign endometrial tissue. We found no significant relationship between CD44v6 expression and several important prognostic factors. We found that CD44v6 staining intensity increased during the secretory phase of the menstrual cycle, but the difference was not significant.
CD44 is a cell-surface protein playing roles in cell−cell and cell−matrix interactions in both cancerous tissues and normal epithelium [1]. The normal endometrium expresses CD44 in the secretory phase but not in the proliferative phase [2, 3]. Impairment of CD44-mediated cell–cell interactions may increase the invasive and metastatic potential of cancer cells. Many recent studies have investigated CD44 expression in malignant tissues [5,6,7,8,9,10, 18]. Overexpression of CD44 variants has been reported both in EC cases and normal endometrial tissue [1]. Most studies found that CD44 was either under- or overexpressed. However, the expression pattern is affected by cell type; in general, CD44 is overexpressed in malignant endometrial cells. Earlier studies reported that CD44v6 was expressed at a significantly higher level in EC than in the normal endometrium [20,21,22,23,24]. In the present study, we found that EC cells expressed CD44v6 more intensely in only the membranous cell compartments of patients with either early or advanced-stage EC, compared with the normal endometrium.
We found no significant relationship between CD44v6 expression and important prognostic factors including tumor stage, grade, extent of myometrial invasion, lymphovascular invasion, adnexal metastasis, omental metastasis, or positive intraperitoneal cytology. The previous studies found that CD44v6 overexpression was correlated with lymphovascular space invasion, a higher tumor grade, and deep myometrial invasion [20, 25, 26]. In contrast, other authors reported that loss of CD44v6 expression was correlated with poorer prognoses, higher recurrence rates, and shorter disease-free survival [13, 18, 24, 27]. Similarly, Gun et al. found no relationship between CD44v6 expression and the clinicopathological parameters of EC [21].
The cell-surface hyaluronan receptor CD44 may be downregulated in aggressive EC [13, 28]. Therefore, papillary serous and clear cell EC (aggressive cancer subtypes) might exhibit reduced cytoplasmic CD44 staining. Indeed, we could not detect such staining. However, our sample sizes were small (five patients with papillary serous EC and two patients with clear cell EC), and thus, our statistical power was low. Further work is needed.
CD44 and its variants are expressed primarily during the secretory phase of the menstrual cycle and may play functional roles in the endometrium. The absence of CD44v6 staining in the proliferative phase may suggest that expression is under hormonal control (perhaps progesterone). As reported earlier, we found that CD44v6 was expressed at lower levels in normal endometria (compared with EC tissue), but more so in the secretory phase, although statistical significance was not attained [1, 18, 29, 30].
In conclusion, CD44v6 m-staining can be used to differentiate malignant from benign endometrial tissue. However, staining was not associated with EC stage, grade, histological subtype, lymph node involvement, extent of myometrial invasion, cytology, adnexal or cervical involvement, or omental disease. The absence of CD44v6 c-staining may indicate that an aggressive subtype of EC is present. Further well-designed studies are needed.
References
Behzad F, Seif MW, Campbell S et al (1994) Expression of two isoforms of CD44 in human endometrium. Biol Reprod 51:739–747
Afify AM, Craig S, Paulino AF (2006) Temporal variation in the distribution of hyaluronic acid, CD44 s, and CD44v6 in the human endometrium across the menstrual cycle. Appl Immunohistochem Mol Morphol 14:328–333
Afify AM, Craig S, Paulino AF et al (2005) Expression of hyaluronic acid and its receptors, CD44 s and CD44v6, in normal, hyperplastic, and neoplastic endometrium. Ann Diagn Pathol 9:312–318
Saegusa M, Hashimura M, Okayasu I (1998) CD44 expression in normal, hyperplastic, and malignant endometrium. J Pathol 184:297–306
Woodman AC, Sugiyama M, Yoshida K et al (1996) Analysis of anomalous CD44 gene expression in human breast, bladder, and colon cancer and correlation of observed mRNA and protein isoforms. Am J Pathol 149:1519–1530
Koopman G, Heider KH, Horst E et al (1993) Activated human lymphocytes and aggressive non-Hodgkin’s lymphomas express a homologue of the rat metastasis-associated variant of CD44. J Exp Med 177:897–904
Rodriguez C, Monges G, Rouanet P et al (1995) CD44 expression patterns in breast and colon tumors: a PCR-based study of splice variants. Int J Cancer 64:347–354
Yamaguchi A, Saito M, Gio T et al (1995) Expression of CD44 variant exons 8–10 in gastric cancer. Jpn J Cancer Res 86:1166–1171
Kallakury BV, Yang F, Figge J et al (1996) Decreased levels of CD44 protein and mRNA in prostate carcinoma. Correlation with tumor grade and ploidy. Cancer 78:1461–1469
Assimakopoulos D, Kolettas E, Patrikakos G et al (2002) The role of CD44 in the development and prognosis of head and neck squamous cell carcinomas. Histol Histopathol 17:1269–1281
Naor D, Sionov RV, Ish-Shalom D (1997) CD44: structure, function, and association with the malignant process. Adv Cancer Res 71:241–319
Faleiro-Rodrigues C, Lopes C (2004) E-cadherin, CD44 and CD44v6 in squamous intraepithelial lesions and invasive carcinomas of the uterine cervix: an immunohistochemical study. Pathobiology 71:329–336
Saegusa M, Hashimura M, Machida D et al (1999) Down-regulation of CD44 standard and variant isoforms during the development and progression of uterine cervical tumours. J Pathol 187:173–183
Hong SC, Song JY, Lee JK et al (2006) Significance of CD44v6 expression in gynecologic malignancies. J Obstet Gynaecol Re 32:379–386
Elzarkaa AA, Sabaa BE, Abdelkhalik D et al (2016) Clinical relevance of CD44 surface expression in advanced stage serous epithelial ovarian cancer: a prospective study. J Cancer Res Clin Oncol 142:949–958
Zhao L, Gu C, Huang K, Zhang Z et al (2016) The prognostic value and clinicopathological significance of CD44 expression in ovarian cancer: a meta-analysis. Arch Gynecol Obstet 294:1019–1029
Wojciechowski M, Krawczyk T, Śmigielski J et al (2015) CD44 expression in curettage and postoperative specimens of endometrial cancer. Arch Gynecol Obstet 291:383–390
Fujita N, Yaegashi N, Ide Y et al (1994) Expression of CD44 in normal human versus tumor endometrial tissues: possible implication of reduced expression of CD44 in lymph-vascular space involvement of cancer cells. Cancer Res 54:3922–3928
Kainz C, Kohlberger P, Sliutz G et al (1995) Splice variants of CD44 in human cervical cancer stage IB to IIB. Gynecol Oncol 57:383–387
Hoshimoto K, Yamauchi N, Takazawa Y et al (2003) CD44 variant 6 in endometrioid carcinoma of the uterus: its expression in the adenocarcinoma component is an independent prognostic marker. Pathol Res Pract 199:71–77
Gun BD, Bahadir B, Bektas S et al (2012) Clinicopathological significance of fascin and CD44v6 expression in endometrioid carcinoma. Diagn Pathol 7:80
Stokes GN, Shelton JB Jr, Zahn CM et al (2002) Association of CD44 isoform immunohistochemical expression with myometrial and vascular invasion in endometrioid endometrial carcinoma. Gynecol Oncol 84:58–61
Tokumo K, Kodama J, Seki N et al (1998) CD44 exon v6 correlates with cellular differentiation but not with progression and metastasis of cervical cancer. Eur J Cancer 34:2107–2111
Ayhan A, Tok EC, Bildirici I et al (2001) Overexpression of CD44 variant 6 in human endometrial cancer and its prognostic significance. Gynecol Oncol 80:355–358
Yorishima T, Nagai N, Ohama K (1997) Expression of CD44 alternative splicing variants in primary and lymph node metastatic lesions of gynecological cancer. Hiroshima J Med Sci 46:21–29
Leblanc M, Poncelet C, Soriano D et al (2001) Alteration of CD44 and cadherins expression: possible association with augmented aggressiveness and invasiveness of endometrial carcinoma. Virchows Arch 438:78–85
Soslow RA, Shen PU, Isacson C et al (1998) The CD44v6-negative phenotype in high-grade uterine carcinomas correlates with serous histologic subtype. Mod Pathol 11:194–199
Sugino T, Gorham H, Yoshida K (1996) Progressive loss of CD44 gene expression in invasive bladder cancer. Am J Pathol 149:873–882
Albers A, Thie M, Hohn HP et al (1995) Differential expression and localization of integrins and CD44 in the membrane domains of human uterine epithelial cells during the menstrual cycle. Acta Anat 153:12–19
Poncelet C, Leblanc M, Walker-Combrouze F, Soriano D, Feldmann G, Madelenat P et al (2002) Expression of cadherins and CD44 isoforms in human endometrium and peritoneal endometriosis. Acta Obstet Gynecol Scand 81:195–203
Author contributions
HKC: protocol and project development, data collection, data analysis, and manuscript writing. MG: project development, data management, data analysis, manuscript writing, and editing. FO: project development and manuscript editing. DK: protocol development, data management, and data analysis. AE: data management and data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Funding
None.
Ethical approval
All procedures performed in human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The project was approved by the Ethical Committee of the Ankara University (Verdict No. 21/2006, June 2006).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kansu-Celik, H., Gungor, M., Ortac, F. et al. Expression of CD44 variant 6 and its prognostic value in benign and malignant endometrial tissue. Arch Gynecol Obstet 296, 313–318 (2017). https://doi.org/10.1007/s00404-017-4430-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-017-4430-9