Abstract
Purpose
The prognostic value and clinicopathological significance of CD44 in ovarian cancer (OC) remain unclear. This meta-analysis, therefore, aims to evaluate the correlation between CD44 expression and OC.
Methods
Studies published until March 2016 were searched in PubMed, EMBASE, and ISI Web of Knowledge databases. The odds ratio (OR) and the hazard ratio (HR) with 95 % confidence interval (CI) were used to assess the effects.
Results
Twenty-four studies that include 2267 OC patients were identified for the final analysis. Sixteen studies investigated the expression difference of CD44 standard (CD44s) in 1848 patients. Results showed that high CD44s expression is associated with chemoresistance (OR 5.94, 95 % CI 1.91–18.47) and short disease-free survival (DFS) time (HR 2.57, 95 % CI 1.34–4.91). In addition, CD44s expression is not associated with tumor differentiation grade, residual mass, lymphoid nodal metastasis, and overall survival (OS). Ten studies investigated the expression difference of CD44v6 in 724 patients. Results showed that the CD44v6 expression is not correlated with FIGO stage, tumor differentiation grade, lymphoid nodal metastasis, and OS.
Conclusion
High CD44s expression possibly indicates poor prognosis in OC patients given that high CD44s expression initiates chemotherapy resistance, although this expression pattern is not an independent predictive factor for OS. Meanwhile, high CD44s expression may be related to poor DFS of OC, but this relationship must be further confirmed. In addition, the result in which CD44v6 is not associated with OS of OC patients should be interpreted with caution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer (OC) is the most lethal gynecological malignancy and the fifth leading cause of cancer deaths in women [1]. Given the lack of specific symptoms and methods for early screening, more than 70 % of patients were diagnosed with advanced stage and their 5-year survival rate was only 25 % [2]. Several independent prognostic factors, such as disease stage, age, and residual tumor bulk, were identified in the previous investigations, and these factors are useful in choosing the optimal treatment [3, 4]. However, prognoses of patients showing similar condition and receiving similar treatments obviously vary [5]. In addition, the potential factors and the underlying mechanism remain unclear [6]. Continuing exploration for new molecular biological prognostic indicators in OC patients is, therefore, necessary.
The cancer stem cell (CSC) hypothesis has been recently formulated to explain tumor occurrence and recurrence [7]. Increasing evidence has indicated that CSCs possess self-renewal ability and are responsible for tumor progression, metastasis, and therapeutic resistance [8, 9]. CD44, being an important CSC marker, is a highly heterogeneous transmembrane glycoprotein with different isoforms [10]. The CD44 gene is located on the short arm of chromosome 11 and contains 20 exons, 10 of which are non-variable exons and expressed in standard form. Alternative splicing of the remaining 10 exons produces multiple variant isoforms (CD44v1–v10) [11]. The CD44 standard (CD44s) and CD44 variants possibly play essential roles in tumor occurrence, progression, and metastasis [12, 13], and they demonstrate prognostic value in various cancers [14–17].
Until now, the correlation between CD44 expression and OC has been widely studied, but the results are controversial. Several studies have suggested that the high expression of CD44 is related to improved survival rate, whereas some studies have indicated that high CD44 expression is associated with unfavorable prognosis. In addition, some studies did not reveal any relationship between CD44 and outcome of OC. These discrepancies are possibly caused by small sample size and multiple factors. Therefore, to achieve a better understanding of the relationship between CD44 expression and OC, we performed a meta-analysis to further evaluate the prognostic value of CD44 and to analyze its correlation with other clinicopathological factors of OC.
Materials and methods
Literature search
We systematically searched the relevant studies published before March 2016 in PubMed, EMBASE, and ISI Web of Knowledge databases. No restrictions on population or sample size were set in this meta-analysis. Studies were searched using the combinations of the following keywords: “CD44,” “ovarian or ovary,” “cancer, carcinoma, tumor, or neoplasms.” Additional relevant literatures were obtained from the reference lists of the prospective articles.
Inclusion and exclusion criteria
Studies were included in this meta-analysis based on the following criteria: (1) English or Chinese articles; (2) OC was diagnosed through pathological examination; (3) full text is available and data on clinical features and/or prognosis of OC are sufficient; and (4) CD44 expression was detected by immunohistochemistry (IHC) assay. Studies were excluded based on the following criteria: (1) letters, reviews, or case reports without original data and (2) studies using cell lines, ascites cells, and animal models. For studies published by the same authors, only those that include the largest sample sizes or the most recently published were selected to avoid data overlap.
Data extraction
Two investigators (LZ and CG) independently extracted useful data from each study. Discrepancies were resolved through discussion. The following information was extracted from each included study: name of the first author, publication year, country, ethnicity, number of patients, age, tumor pathological type, antibody and dilution, cut-off score, staining pattern, International Federation of Gynecology and Obstetrics (FIGO) stage, tumor differentiation grade, lymphoid nodal metastasis, residual tumor mass, chemotherapy sensitivity, follow-up period, and survival data. The primary endpoint was overall survival (OS). Studies using disease-free survival (DFS) were also analyzed.
Statistical analysis
All analyses were performed using Stata 12.0 (Stata Corporation, College Station, TX, USA). The odds ratio (OR) and 95 % confidence interval (CI) were used to assess the correlation between CD44 expression and clinicopathological parameters, including FIGO stage (I–II versus III–IV), tumor differentiation grade (G1–G2 versus G3), lymphoid nodal metastasis (positive versus negative), residual tumor mass (≤1 cm versus >1 cm), and chemotherapy sensitivity (resistant versus sensitive). The hazard ratio (HR) and 95 % CI of OS and DFS were extracted from eligible studies. Some studies did not clearly provide these data, so we estimated these data using the method reported by Tierney et al. [18]. By convention, HR and 95 % CI not overlapped with one would be considered statistically significant. Heterogeneity among studies was assessed by Q test, and its effect was quantified by the I 2 test. When heterogeneity existed (P < 0.05 or I 2 > 50 %), the random-effects model was used; otherwise, the fixed-effects model was employed. Sensitivity analysis was used to assess the quality and stability of the results. Begg’s and Egger’s tests were conducted to evaluate potential publication bias, with P < 0.05 being considered statistically significant.
In addition, because the cut-off score varied in different studies, we defined the high CD44 expression group in accordance with the original article. Considering that CD44v3, CD44v7–8, CD44v9, and CD44v10 were estimated in less than three studies and that the parameters were not overlapped, we only analyzed two CD44 isoforms, namely, CD44s and CD44v6.
Result
Characteristics of the included studies
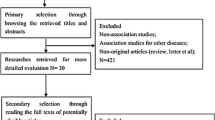
As shown in Fig. 1, a total of 651 citations were identified in the initial search. After removing the duplicates, 288 articles were left. After screening the title and abstract, we screened the full texts of the remaining 59 articles. Twelve investigations were excluded for insufficient information. Eight articles were excluded for abstract only. Six articles were excluded, because they used cell-line or animal or ascites models, and five articles were excluded, because they used immunofluorescence or RT-PCR assays. Four papers were removed as they are case reports or reviews. Finally, 24 studies [19–42] that include 2267 patients with a median of 68 patients (11–483) were included in this meta-analysis (Table 1). The publication year of the included studies ranged from 1997 to 2016. These studies were conducted in Japan, France, United States, Germany, Canada, Finland, China, Greece, Korea, Denmark, and Ukraine. The subjects of 11 studies were of Asian descent, whereas the subjects of the remaining 13 studies were non-Asians. All studies used IHC staining to detect CD44 expression.
Sixteen studies with 1848 patients used anti-CD44s antibody, and the median percentage of CD44s positive expression was 50 % (20.6–93.2 %). Among them, eight studies reported FIGO stage; eight studies reported tumor differentiation grade; five, chemotherapy sensitivity; three, residual mass; two, lymphoid nodal metastasis; and two, recurrence. Survival data were reported in 11 studies (OS in nine studies, DFS in two studies, progression-free survival (PFS) in one study, and recurrence-free survival (RFS) in one study).
Ten studies that include 724 patients used anti-CD44v6 antibody, and the median percentage of positive CD44v6 expression was 33 % (0–75.3 %). Among them, seven studies reported FIGO stage, five, tumor differentiation grade, two, lymphoid nodal metastasis, and six, OS to assess the prognostic value of CD44v6.
CD44 expression and clinicopathological parameters of OC
Table 2 shows the correlation results between CD44s and clinicopathological parameters. Overall analyses demonstrated that CD44s expression is associated with chemotherapy sensitivity (OR 5.94, 95 % CI 1.91–18.47, P = 0.00, I 2 = 77.2 %, Fig. 2) and postoperative recurrence (OR 0.21, 95 % CI 0.13–0.35, P = 0.00, I 2 = 39.0 %, Supplementary Fig. 1). The association between CD44s expression and chemotherapy sensitivity of OC was also statistically significant in the following subgroups: Asian (OR 8.08, 95 % CI 4.26–15.32, P = 0.00, I 2 = 0.0 %), membrane and cytoplasm staining (OR 7.61, 95 % CI 3.26–17.74, P = 0.00, I 2 = 88.6 %), and large sample size (OR 10.64, 95 % CI 6.13–18.47, P = 0.00, I 2 = 0.0 %). No associations were observed between CD44s expression and FIGO stage (OR 1.05, 95 % CI 0.37–2.94, P = 0.93, I 2 = 90.8 %, Supplementary Fig. 1), tumor differentiation grade (OR 0.62, 95 % CI 0.23–1.67, P = 0.35, I 2 = 87.4 %, Supplementary Fig. 1), residual mass (OR 0.58, 95 % CI 0.14–2.53, P = 0.47, I 2 = 83.9 %, Supplementary Fig. 1), and lymphoid nodal metastasis (OR 2.11, 95 % CI 0.99–4.48, P = 0.01, I 2 = 0.0 %, Supplementary Fig. 1). In the subgroup analyses, studies in Asian group (OR 0.44, 95 % CI 0.23–0.85, P = 0.01, I 2 = 0.0 %) and membrane and cytoplasm staining group (OR 0.31, 95 % CI 0.12–0.84, P = 0.012) indicated that high CD44s expression is associated with advanced FIGO stage.
Correlation results between CD44v6 and clinicopathological parameters are shown in Table 3. Neither the overall analysis nor the subgroup analysis showed a statistically significant association between CD44v6 expression and FIGO stage (OR 0.84, 95 % CI 0.52–1.36, P = 0.47, I 2 = 48.9 %, Supplementary Fig. 2), tumor differentiation grade (OR 0.45, 95 % CI 0.16–1.28, P = 0.14, I 2 = 53.1 %, Supplementary Fig. 2), and lymphoid nodal metastasis (OR 1.50, 95 % CI 0.06–32.79, P = 0.80, I 2 = 72.2 %, Supplementary Fig. 2).
CD44 expression and prognosis of OC
Table 4 summarizes the main data on survival. The combined HR suggests that CD44s expression does not affect the OS of patients (HR 0.83, 95 % CI 0.46–1.49, I 2 = 73.0 %, Supplementary Fig. 3), but it is possibly correlated with short DFS time (HR 2.57, 95 % CI 1.34–4.91, I 2 = 0.0 %, Fig. 3). In addition, the overall analysis did not reveal association between CD44v6 expression and OS (HR 1.33, 95 % CI 0.66–2.70, I 2 = 73.0 %, Supplementary Fig. 3). When stratified according to geographic area, staining pattern, sample size, and analysis type, statistically significant associations were observed in the Asian group (HR 1.61, 95 % CI 1.07–2.41, I 2 = 0.0 %), membrane alone group (HR 1.75, 95 % CI 1.21–2.51, I 2 = 0.0 %), and small sample size (<68) group (HR 2.35, 95 % CI 1.42–3.89, I 2 = 0.0 %).
Publication bias
To identify potential publication bias, we performed the Begg’s and Egger’s tests. No evidence of publication bias was observed in the pooled studies (Fig. 4, Supplementary Table).
Sensitivity analysis
In sensitivity analysis, we sequentially removed the eligible studies to assess the influence of each individual study on the pooled ORs or HRs. Most results were not qualitatively changed after omitting any single study at a given time, except the result of OS and CD44v6 expression. After removing the study conducted by Rodriguez–Rodriguez et al. [26], we again pooled the remaining five studies and observed the statistically significant effect of CD44v6 expression on OS of OC patients. In addition, the heterogeneity dramatically decreased from 79.6 to 0 % (Fig. 5). The association between CD44v6 and OS remained stable after excluding the study of Rodriguez–Rodriguez et al. and any other study from the sensitivity analysis.
Discussion
CD44, a transmembrane glycoprotein, is primarily recognized as a receptor for hyaluronan (HA), which is involved in cell adhesion, extravasation, and migration [11, 43]. HA is a major component of extracellular matrix, and its binding with CD44 influences the activities of CD44 [44] and induces upregulation of adhesion molecules and thus promotes cell adhesion [45]. In endothelial cells, CD44 expression and its binding force with HA are stimulated by pro-inflammatory cytokines, which can promote the capture of hematopoietic cells and tumor cells [46]. The interactions of the cytoplasmic tail of CD44 with the actin cytoskeleton can be induced by CD44–HA binding; thus, CD44 promotes tumor cell migration [47]. In addition, although the mechanism remains unclear, CD44 and HA are both correlated with drug resistance [10]. Overall, CD44 is a potential prognostic marker for various diseases [48–50].
The clinical significance and prognostic value of CD44s expression in OC remain controversial. The present meta-analysis results, which include 1848 patients, showed that CD44s expression is not associated with tumor differentiation grade, residual mass, lymphoid nodal metastasis, and OS. However, a positive finding was observed in chemotherapy sensitivity analysis (OR 5.94, 95 % CI 1.91–18.47), suggesting that high CD44s expression possibly contributes to a high risk of chemotherapy resistance in OC patients. In accordance with the National Comprehensive Cancer Network guidelines, the chemotherapy sensitive group included patients with clinical remission over 12 months post-chemotherapy, whereas the resistant group achieved clinical remission but showed recurrence during the late stage of chemotherapy or within 12 months. These findings indicate that CD44s may worsen the prognosis by initiating chemotherapy resistance, although CD44s is not an independent predictive factor for OS. The results also suggest that high CD44s expression is a meaningful predictor of poor DFS (HR 2.57, 95 % CI 1.34–4.91). In addition, although no association was observed in the overall analysis, subgroup analyses showed that high CD44s expression is correlated with advanced FIGO stage in Asian subjects (OR 8.08, 95 % CI 4.26–15.32) and membrane and cytoplasm staining pattern (OR 7.61, 95 % CI 3.26–17.74). Considering the limited number of studies, we viewed these results as preliminary and they require validation. Furthermore, OC patients showing high CD44s expression displayed a low risk of recurrence (OR 0.20, 95 % CI 0.10–0.39), inconsistent with the result for chemotherapy sensitivity and DFS. To better interpret this result, we formulated two hypotheses to explain the discrepancy. First, multiple factors, such as FIGO stage and residual mass, could influence the recurrence of cancer, and these potential confounding factors are not counted before analyzed. Second, given that only two published articles with insufficient sample size were included, a small-study bias may have arisen from their results, which may explain the discrepancy.
Similarly, the previous reported relationship between CD44v6 expression and ovarian carcinoma is disputable. The present meta-analysis, which includes 724 patients, did not reveal any association between CD44v6 expression and FIGO stage, tumor stage, lymphoid nodal metastasis, and OS in the overall analyses. However, after excluding the study by Rodriguez–Rodriguez et al. [26], the result of OS was reversed and the heterogeneity was reduced from 79.6 to 0 %, suggesting that this result was not robust and that their study contributes to the heterogeneity. To explore the differences between the study by Rodriguez–Rodriguez et al. and the five other studies, we re-assessed their paper and found several possible reasons to explain why this study caused such heterogeneity First, the study by Rodriguez–Rodriguez et al. included the largest sample size in the analysis of CD44v6 OS and is the only one that has reported that CD44v6 is a good prognostic marker in OC. Second, the IHC methodology may partly explain the inconsistency that has possibly resulted from the differences such as in antibody, cut-off criteria, and staining pattern. Third, no therapy regimens, such as whether the patients received preoperative chemotherapy or not, were described in the manuscript. Given that different therapeutic regimens can modify the effects of CD44 expression [51], it is likely caused the discrepancy. Finally, their study includes a long follow-up time (1–92 months), and their result might have been affected by some losses. Therefore, we cannot draw a definite conclusion regarding the relationships between CD44v6 and OS of OC patients.
To our best knowledge, this meta-analysis is the first to systematically analyze the association between CD44 expression and OC. However, some limitations should be mentioned. First, a relatively large heterogeneity was found in the majority of the analyses. To identify the source of heterogeneity, subgroup analyses according to geographic area of the subjects, staining pattern, sample size, and analysis type were conducted, but none of them could completely explain it. In fact, many other factors, such as age, histological types, cut-off points, antibodies, dilution ratios, surgical interventions, chemotherapy regimens, and study design, may contribute to the heterogeneity. Given that no unified standard exists for some factors and that detailed data are lacking, conducting further analysis of these variables would be difficult. Second, as we mentioned above, the association between OS and CD44v6 expression is unstable. Third, the present meta-analysis did not analyze the relationship between OC and other CD44 variants, such as CD44v3, CD44v5, CD44v7–8, and CD44v9 because of insufficient information. Fourth, several clinicopathological and survival parameters, including distant metastasis, ascites status, microvascular invasion, tumor size, CA125 level, PFS, and RFS were not analyzed because of the lack of overlapped data. Finally, some selection bias may have been caused by the inclusion of studies published only in English and Chinese. Some potential meaningful data published in other languages were not retrieved. Therefore, future large-scale and well-designed studies are needed to validate our results and to further analyze the associations between different CD44 variants and OC.
In conclusion, this meta-analysis found that high expression of CD44s predicts poor prognosis by initiating chemotherapy resistance of OC, although this expression pattern is not an independent predictive factor for OS. Moreover, high CD44s expression is possibly related to poor DFS in OC patients, although this conclusion requires confirmation. The result that CD44v6 expression is possibly not associated with OS in OC should be interpreted with caution. Further studies must validate and confirm these associations to achieve a more comprehensive understanding of their possible roles in OC.
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66(1):7–30. doi:10.3322/caac.21332
Kipps E, Tan DS, Kaye SB (2013) Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat Rev Cancer 13(4):273–282. doi:10.1038/nrc3432
Winter WE 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, Markman M, Armstrong DK, Muggia F, McGuire WP, Gynecologic Oncology Group S (2007) Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol Off J Am Soc Clin Oncol 25(24):3621–3627. doi:10.1200/JCO.2006.10.2517
Winter WE 3rd, Maxwell GL, Tian C, Sundborg MJ, Rose GS, Rose PG, Rubin SC, Muggia F, McGuire WP, Gynecologic Oncology G (2008) Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol Off J Am Soc Clin Oncol 26(1):83–89. doi:10.1200/JCO.2007.13.1953
Bogush TA, Popova AS, Dudko EA, Bogush EA, Tyulyandina AS, Tyulyandin SA, Davydov MI (2015) ERCC1 as a marker of ovarian cancer resistance to platinum drugs. Antibiot Khimioterapiia Antibiot Chemoterapy [sic]/Minist Med Mikrobiol Prom SSSR 60(3–4):42–50
Kim TH, Lee HH, Hwang JY, Kim JH, Jang WC, Lee A (2014) Genetic alteration in ovarian cancer. Arch Gynecol Obstet 290(5):827–830. doi:10.1007/s00404-014-3392-4
Rahman M, Deleyrolle L, Vedam-Mai V, Azari H, Abd-El-Barr M, Reynolds BA (2011) The cancer stem cell hypothesis: failures and pitfalls. Neurosurgery 68(2):531–545. doi:10.1227/NEU.0b013e3181ff9eb5 (discussion 545)
Jordan CT, Guzman ML, Noble M (2006) Cancer stem cells. N Engl J Med 355(12):1253–1261. doi:10.1056/NEJMra061808
Nguyen LV, Vanner R, Dirks P, Eaves CJ (2012) Cancer stem cells: an evolving concept. Nat Rev Cancer 12(2):133–143. doi:10.1038/nrc3184
Zoller M (2011) CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer 11(4):254–267. doi:10.1038/nrc3023
Naor D, Sionov RV, Ish-Shalom D (1997) CD44: structure, function, and association with the malignant process. Adv Cancer Res 71:241–319
Volz Y, Koschut D, Matzke-Ogi A, Dietz MS, Karathanasis C, Richert L, Wagner MG, Mely Y, Heilemann M, Niemann HH, Orian-Rousseau V (2015) Direct binding of hepatocyte growth factor and vascular endothelial growth factor to CD44v6. Biosci Rep 35(4):e00236. doi:10.1042/BSR20150093
Preca BT, Bajdak K, Mock K, Sundararajan V, Pfannstiel J, Maurer J, Wellner U, Hopt UT, Brummer T, Brabletz S, Brabletz T, Stemmler MP (2015) A self-enforcing CD44s/ZEB1 feedback loop maintains EMT and stemness properties in cancer cells. Int J Cancer J Int Cancer 137(11):2566–2577. doi:10.1002/ijc.29642
Wu Y, Li Z, Zhang C, Yu K, Teng Z, Zheng G, Wang S, Liu Y, Cui L, Yu X (2015) CD44 family proteins in gastric cancer: a meta-analysis and narrative review. Int J Clin Exp Med 8(3):3595–3606
Li X, Ma X, Chen L, Gu L, Zhang Y, Zhang F, Ouyang Y, Gao Y, Huang Q, Zhang X (2015) Prognostic value of CD44 expression in renal cell carcinoma: a systematic review and meta-analysis. Sci Rep 5:13157. doi:10.1038/srep13157
Hu B, Luo W, Hu RT, Zhou Y, Qin SY, Jiang HX (2015) Meta-analysis of prognostic and clinical significance of CD44v6 in esophageal cancer. Medicine 94(31):e1238. doi:10.1097/MD.0000000000001238
Wojciechowski M, Krawczyk T, Smigielski J, Malinowski A (2015) CD44 expression in curettage and postoperative specimens of endometrial cancer. Arch Gynecol Obstet 291(2):383–390. doi:10.1007/s00404-014-3407-1
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8:16. doi:10.1186/1745-6215-8-16
Yorishima T, Nagai N, Ohama K (1997) Expression of CD44 alternative splicing variants in primary and lymph node metastatic lesions of gynecological cancer. Hiroshima J Med Sci 46(1):21–29
Darai E, Walker-Combrouze F, Fauconnier A, Madelenat P, Potet F, Scoazec JY (1998) Analysis of CD44 expression in serous and mucinous borderline tumours of the ovary: comparison with cystadenomas and overt carcinomas. Histopathology 32(2):151–159
Kayastha S, Freedman AN, Piver MS, Mukkamalla J, Romero-Guittierez M, Werness BA (1999) Expression of the hyaluronan receptor, CD44S, in epithelial ovarian cancer is an independent predictor of survival. Clin Cancer Res Off J Am Assoc Cancer Res 5(5):1073–1076
Saegusa M, Machida D, Hashimura M, Okayasu I (1999) CD44 expression in benign, premalignant, and malignant ovarian neoplasms: relation to tumour development and progression. J Pathol 189(3):326–337. doi:10.1002/(SICI)1096-9896(199911)189:3<326:AID-PATH425>3.0.CO;2-6
Schroder W, Rudlowski C, Biesterfeld S, Knobloch C, Hauptmann S, Rath W (1999) Expression of CD44(v5-10) splicing variants in primary ovarian cancer and lymph node metastases. Anticancer Res 19(5B):3901–3906
Afify AM, Ferguson AW, Davila RM, Werness BA (2001) Expression of CD44S and CD44v5 is more common in stage III than in stage I serous ovarian carcinomas. Appl Immunohistochem Mol Morphol AIMM/Off Publ Soc Appl Immunohistochem 9(4):309–314
Ross JS, Sheehan CE, Williams SS, Malfetano JH, Szyfelbein WM, Kallakury BV (2001) Decreased CD44 standard form expression correlates with prognostic variables in ovarian carcinomas. Am J Clin Pathol 116(1):122–128. doi:10.1309/KUK0-1M3D-LGNE-THXR
Rodriguez-Rodriguez L, Sancho-Torres I, Mesonero C, Gibbon DG, Shih WJ, Zotalis G (2003) The CD44 receptor is a molecular predictor of survival in ovarian cancer. Med Oncol 20(3):255–263. doi:10.1385/MO:20:3:255
Sillanpaa S, Anttila MA, Voutilainen K, Tammi RH, Tammi MI, Saarikoski SV, Kosma VM (2003) CD44 expression indicates favorable prognosis in epithelial ovarian cancer. Clinical Cancer Res Off J Am Assoc Cancer Res 9(14):5318–5324
Qu JY, Li S, Lin H, Wu JB, Wang YQ (2004) Relationship between expression of hyaluronan and pathologic features of ovarian adenocarcinoma. Ai zheng Aizheng Chin J Cancer 23(2):177–180
Zagorianakou N, Stefanou D, Makrydimas G, Zagorianakou P, Briasoulis E, Karavasilis B, Agnantis NJ (2004) CD44s expression, in benign, borderline and malignant tumors of ovarian surface epithelium. Correlation with p53, steroid receptor status, proliferative indices (PCNA, MIB1) and survival. Anticancer Res 24(3a):1665–1670
Hong SC, Song JY, Lee JK, Lee NW, Kim SH, Yeom BW, Lee KW (2006) Significance of CD44v6 expression in gynecologic malignancies. J Obstet Gynaecol Res 32(4):379–386. doi:10.1111/j.1447-0756.2006.00422.x
Steffensen KD, Alvero AB, Yang Y, Waldstrom M, Hui P, Holmberg JC, Silasi DA, Jakobsen A, Rutherford T, Mor G (2011) Prevalence of epithelial ovarian cancer stem cells correlates with recurrence in early-stage ovarian cancer. J Oncol 2011:620523. doi:10.1155/2011/620523
Zhou DX, Liu YX, Xue YH (2012) Expression of CD44v6 and Its Association with Prognosis in Epithelial Ovarian Carcinomas. Pathol Res Int 2012:908206. doi:10.1155/2012/908206
Hu Z, Gao J, Zhang D, Liu Q, Yan L, Gao L, Liu J, Liu D, Zhang S, Lin B (2013) High expression of Lewis y antigen and CD44 is correlated with resistance to chemotherapy in epithelial ovarian cancers. PLoS One 8(2):e57250. doi:10.1371/journal.pone.0057250
Shi J, Zhou Z, Di W, Li N (2013) Correlation of CD44v6 expression with ovarian cancer progression and recurrence. BMC Cancer 13:182. doi:10.1186/1471-2407-13-182
Ryabtseva OD, Lukianova NY, Shmurakov YA, Polishchuk LZ, Antipova SV (2013) Significance of adhesion molecules expression for estimation of serous ovarian cancer prognosis. Exp Oncol 35(3):211–218
Zhang J, Chang B, Liu J (2013) CD44 standard form expression is correlated with high-grade and advanced-stage ovarian carcinoma but not prognosis. Hum Pathol 44(9):1882–1889. doi:10.1016/j.humpath.2013.02.016
Wang A, Lu L, Wang Y, Sun Y, Zhang Y, Guo C, Gu Y, Liu A (2014) Expression and clinicopathologic significance of CD44v6/CD24 in ovarian serous carcinomas. Zhonghua bing li xue za zhi Chin J Pathol 43(1):20–24
Wang H, Tan M, Zhang S, Li X, Gao J, Zhang D, Hao Y, Gao S, Liu J, Lin B (2015) Expression and significance of CD44, CD47 and c-met in ovarian clear cell carcinoma. Int J Mol Sci 16(2):3391–3404. doi:10.3390/ijms16023391
Tjhay F, Motohara T, Tayama S, Narantuya D, Fujimoto K, Guo J, Sakaguchi I, Honda R, Tashiro H, Katabuchi H (2015) CD44 variant 6 is correlated with peritoneal dissemination and poor prognosis in patients with advanced epithelial ovarian cancer. Cancer Sci 106(10):1421–1428. doi:10.1111/cas.12765
Zhu LC, Gao J, Hu ZH, Schwab CL, Zhuang HY, Tan MZ, Yan LM, Liu JJ, Zhang DY, Lin B (2015) Membranous expressions of Lewis y and CAM-DR-related markers are independent factors of chemotherapy resistance and poor prognosis in epithelial ovarian cancer. Am J Cancer Res 5(2):830–843
Bonneau C, Rouzier R, Geyl C, Cortez A, Castela M, Lis R, Darai E, Touboul C (2015) Predictive markers of chemoresistance in advanced stages epithelial ovarian carcinoma. Gynecol Oncol 136(1):112–120. doi:10.1016/j.ygyno.2014.10.024
Elzarkaa AA, Sabaa BE, Abdelkhalik D, Mansour H, Melis M, Shaalan W, Farouk M, Malik E, Soliman AA (2016) Clinical relevance of CD44 surface expression in advanced stage serous epithelial ovarian cancer: a prospective study. J Cancer Res Clin Oncol. doi:10.1007/s00432-016-2116-5
Nagano O, Saya H (2004) Mechanism and biological significance of CD44 cleavage. Cancer Sci 95(12):930–935
Toole BP (2004) Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 4(7):528–539. doi:10.1038/nrc1391
Lundell BI, McCarthy JB, Kovach NL, Verfaillie CM (1997) Activation of beta1 integrins on CML progenitors reveals cooperation between beta1 integrins and CD44 in the regulation of adhesion and proliferation. Leukemia 11(6):822–829
Lesley J, English NM, Gal I, Mikecz K, Day AJ, Hyman R (2002) Hyaluronan binding properties of a CD44 chimera containing the link module of TSG-6. J Biol Chem 277(29):26600–26608. doi:10.1074/jbc.M201068200
Fehon RG, McClatchey AI, Bretscher A (2010) Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol 11(4):276–287. doi:10.1038/nrm2866
Mashayekhi F, Aryaee H, Mirzajani E, Yasin AA, Fathi A (2015) Soluble CD44 concentration in the serum and peritoneal fluid samples of patients with different stages of endometriosis. Arch Gynecol Obstet 292(3):641–645. doi:10.1007/s00404-015-3654-9
Xu H, Tian Y, Yuan X, Liu Y, Wu H, Liu Q, Wu GS, Wu K (2016) Enrichment of CD44 in basal-type breast cancer correlates with EMT, cancer stem cell gene profile, and prognosis. OncoTargets Ther 9:431–444. doi:10.2147/OTT.S97192
Liu Y, Wu Y, Gu S, Sun Z, Rui Y, Wang J, Lu Y, Li H, Xu K, Sheng P (2014) Prognostic role of CD44 expression in osteosarcoma: evidence from six studies. Diagn Pathol 9:140. doi:10.1186/1746-1596-9-140
Morrison R, Schleicher SM, Sun Y, Niermann KJ, Kim S, Spratt DE, Chung CH, Lu B (2011) Targeting the mechanisms of resistance to chemotherapy and radiotherapy with the cancer stem cell hypothesis. J Oncol 2011:941876. doi:10.1155/2011/941876
Acknowledgments
This study was funded by the National Natural Science Foundation of China (Grant Number: 81571411).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
L. Zhao and C. Gu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
404_2016_4137_MOESM2_ESM.tif
Supplementary material 2 (TIFF 3783 kb) Supplementary Fig. 1 Forest plots showing the association between CD44s expression and clinicopathological parameters of patients with ovarian cancer. “a” represents CD44s expression and FIGO stage; “b” represents CD44s expression and tumor differentiation grade; “c” represents CD44s expression and tumor residual mass; “d” represents CD44s expression and lymphoid nodal metastasis; “e” represents CD44s expression and recurrence
404_2016_4137_MOESM3_ESM.tif
Supplementary material 3 (TIFF 1925 kb) Supplementary Fig. 2 Forest plots showing the association between CD44v6 expression and clinicopathological parameters of patients with ovarian cancer. “a” represents CD44v6 expression and FIGO stage; “b” represents CD44v6 expression and tumor differentiation grade; “c” represents CD44v6 expression and lymphoid nodal metastasis
404_2016_4137_MOESM4_ESM.tif
Supplementary material 4 (TIFF 1811 kb) Supplementary Fig. 3 Forest plots showing the association of CD44 expression and OS of patients with ovarian cancer. “a” represents CD44s expression and OS; “b” represents CD44v6 expression and OS
Rights and permissions
About this article
Cite this article
Zhao, L., Gu, C., Huang, K. et al. The prognostic value and clinicopathological significance of CD44 expression in ovarian cancer: a meta-analysis. Arch Gynecol Obstet 294, 1019–1029 (2016). https://doi.org/10.1007/s00404-016-4137-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-016-4137-3