Abstract
Objective
To investigate whether patients with a history of gestational diabetes mellitus (GDM) have an increased future risk for female malignancies.
Study design
A population-based study compared the incidence of long-term female malignancies (ovary, uterine, breast, and uterine cervix) in a cohort of women with and without a diagnosis of GDM. Deliveries occurred between the years 1988–2013, with a mean follow-up duration of 12 years. Women with known malignancies prior to the index pregnancy were excluded. Kaplan–Meier survival curve was used to estimate cumulative incidence of malignancies. Cox proportional hazards models were used to estimate the adjusted hazard ratios (HR) for female malignancy.
Results
During the study period, 1,04,715 deliveries met the inclusion criteria; 9.4% (n = 9893) occurred in patients with a history of GDM in at least one of their pregnancies. During the follow-up period, patients with GDM had a significantly increased risk of being diagnosed with female malignancies, including ovarian, uterine, and breast cancer. Using a Kaplan–Meier survival curve, patients with a previous diagnosis of GDM had a significantly higher cumulative incidence of female malignancies. Using a Cox proportional hazards model, adjusted for confounders, such as parity, maternal age, and fertility treatments, a history of GDM remained independently associated with female malignancies (adjusted HR, 1.3; 95% CI 1.2–1.6; P = 0.001).
Conclusion
Patients with a history of GDM have an increased risk for future breast, ovarian, and uterine malignancies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) is defined as carbohydrate intolerance that begins or is first recognized during pregnancy. There are two subtypes: Type A1, where diet modification is sufficient to control glucose levels, and type A2, where additional therapy with insulin or other medications is required. Criteria for diagnosis of GDM are usually by Carpenter and Coustan or National Diabetes Data Group. GDM is a worldwide public health problem, and complicates about 7% of all pregnancies [1, 2]. In the last decades, there is a rising incidence of GDM, with health consequences for the mothers as well as the offspring during antepartum, intrapartum, and postpartum period. Among the leading consequences are increased risks of preeclampsia, macrosomia, polyhydramnios, stillbirth, cesarean section, and their associated morbidities [2–4]. The pathophysiology of GDM is related to hormonal changes. With the progression of gestation, insulin sensitivity steadily decreases, and as a result, glucose levels increase. Therefore, to maintain glucose control, the body must compensate with an increase in insulin secretion. GDM occurs when the body can no longer succeed to compensate [5].
In addition to the immediate gestational consequences, GDM also have significant long-term implications. GDM is one of the most significant risk factors for the development of type II diabetes mellitus (T2DM). Approximately, 50% of the women with a history of GDM in one of their pregnancies will develop T2DM, representing a sevenfold increase in the risk for T2DM overtime compared with women without a history of GDM. Furthermore, a history of GDM is also a well-known risk factor for the long-term development of metabolic syndrome and cardiovascular diseases [3, 6–10].
A link between the development of several primary malignancies and T2DM was demonstrated in several previously published studies [11–14]. Since GDM has the same characteristics as T2DM, and is a predictor for the development of T2DM, it is reasonable that pregnancy complicated with GDM may be a significant risk factor for the development of future malignancies.
Gestational diabetes mellitus is characterized by hyperinsulinemia and increase in the Insulin-like growth factor 1 (IGF-1) cascade, leading to possible unsupervised growth of cells and cancer [11–14].
Nevertheless, literature regarding this possible association is rather scarce and there are very few studies that investigated this association [5, 11, 15–18].
Compared to previously published studies, our population-based data offer the strength of a large sample size along with a relatively long follow-up period. Hence, the aim of this population-based study was to investigate whether women with the previous one or more pregnancies complicated by GDM have an increased future risk for female malignancies. And thus, we hope to further enlighten the uncertainty around this important subject.
Materials and methods
Setting
The study was conducted at the Soroka University Medical Center, the sole hospital of the Negev, the southern region of Israel, serving the entire population in this region. Thus, the study is based on a non-selective population data. The Institutional Review Board (in accordance with the Helsinki declaration) approved the study.
Study population
The study population was composed of consecutive patients who delivered between the years 1988–2013; follow-up period was until 2013. Patients with pregestational diabetes mellitus, known genetic predisposition for malignancy, or known female malignancies before or during the index pregnancy were excluded from the study.
Study design
A population-based retrospective cohort study was conducted. The exposure was a diagnosis of GDM. Patients who for the entire period of follow-up were not diagnosed with GDM comprised the comparison group. A retrospective follow-up of hospitalizations due to female malignancies up to 26 years after the index birth was performed. The first hospitalization for female malignancies (including ovarian cancer, uterine cancer, cervical cancer, and breast cancer) at the Soroka University Medical Center was considered an event. The exact ICD codes for each type of the female malignancies are presented in the Appendix.
Data were collected from two databases that were cross-linked and merged: the computerized perinatal database and the computerized hospitalization database of the Soroka University Medical Center. The perinatal database consists of information recorded directly after delivery by an obstetrician. Skilled medical secretaries routinely review the information prior to entering it into the database. Coding was performed after assessing medical prenatal care records together with the routine hospital documents. The hospitalization database includes demographic information and ICD 9 codes for all medical diagnoses made during hospitalizations.
Statistical analysis
Statistical analysis was performed using the SPSS package 17 edition (SPSS Inc, Chicago, IL). Statistical significance was calculated using the Chi-square test for differences in qualitative variables and the Student t test for differences in continuous variables. Kaplan–Meier survival curve was used to compare cumulative incidence of hospitalizations related to female malignancies. Cox proportional hazards models were used to estimate the adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for long-term risk for female malignancies. P < 0.05 was considered statistically significant.
Results
During the study period, there were 1,04,715 women who delivered at the Soroka University Medical Center, of which 9893 (9.4%) were patients with a history of diagnosis of GDM.
Table 1 summarizes the characteristics of patients with and without a diagnosis of GDM.
Women in the GDM group were significantly older at the index birth than women in the comparison group and had a higher parity rate than the comparison group. They also were more likely to be diagnosed with pregestational obesity and had higher rate of preeclampsia than women without GDM.
Table 2 describes the incidence of malignancies during the follow-up period in patients with and without a history of GDM. Except for uterine cervix neoplasm, a significantly higher incidence of ovarian cancer (OR 2, 95% CI 1.03–4.04, P = 0.037), uterine cancer (OR 2.1, 95% CI 1.01–4.05, P = 0.022), and breast cancer (OR 2, 95% CI 1.59–2.51, P = 0.001) was observed in patients with GDM. No association was noted between the number of times that GDM was diagnosed to the relative risk for malignancies (data not shown in the table).
After controlling for potential confounders, such as fertility treatment, maternal age, and parity, GDM remained independently associated with an increased risk of subsequent hospitalizations due to female malignancies (Table 3).
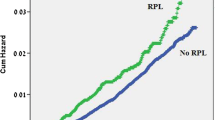
Figure 1 presents a Kaplan–Meier survival curve for the cumulative incidence of female malignancies-related hospitalizations in both study groups (with or without a history of gestational diabetes mellitus). Women with a history of GDM had a significantly higher risk of female malignancies hospitalizations over the whole follow-up period.
Discussion
The aim of this study was to discover whether women with a history of one pregnancy or more with a diagnosis of GDM have higher future long-term risk for the development of female malignancies. We found a significant relationship between a history of GDM and future risk for developing ovarian cancer, endometrium, and breast cancer.
Despite the probable etiological link, probably due to methodological difficulties, very few studies have previously examined the relationship between GDM and future malignancies. Published data regarding this specific link are still insufficient and controversial. Sella et al. followed 11,264 women who were diagnosed with GDM between the years 1995–2009. In their study, women with history of GDM had a relative risk of 7.6 (95% CI: 1.69–29.45) for pancreatic cancer and 4.53 (95% CI: 1.81–11.31) for hematological malignancies. The risk was significant only in women with 5 or more years of follow-up. In contrary to our study, no association with any other malignancies, including gynecological malignancies or breast cancer, was demonstrated in this study [5], and a reasonable idea for the difference between the studies can be the duration of the study. While Sella et al. followed the women for 14 years, our study followed them for 26 years. With processes, such as malignancies, time can be a crucial factor. Different conclusions, which is in line with the results of our study, with stronger associations in postmenopausal breast cancer (RR 1.5, 95% CI 1.0–2.1) were demonstrated by Dawson et al. [16] as well as Perrin et al. [17] that found subclinical glucose intolerance during pregnancy to be associated with a dose-related increase in the risk of malignant neoplasm, particularly malignant neoplasm of the breast. As claimed before, with process such as malignancies, the follow-up period is crucial for detection of correlation. The fact that the last have found stronger associations for postmenopausal women demonstrates that claim as most of the women diagnosed with GDM long before the menopause. Tong et al. recently reviewed nine studies with a total of 14,608 women who were diagnosed with GDM. No significant association was found between GDM and the risk of breast cancer (OR = 1.01 95% CI 0.87–1.17) or any other female malignances. Nevertheless, authors concluded that the association between GDM and cancer is still inconclusive due to the low prevalence of cancer and long time lag between GDM and observable malignancies [15]. In our study, patients were followed for up to 26 years (mean of 11.2 years). To the best of our knowledge, this is the study with the longest follow-up period published to date, a fact which can explain the differences between the results.
Our population-based study offers the strength of a large sample size that allows such a comparison. In addition, data were obtained from computerized files and not based on self-reports that might lead to a recall bias. The data have been collected for a long time period which gives statistical power to the results. Our hospital is the only hospital serving the entire population of southern region of Israel and provides both maternity services and tertiary oncology medical services. Thus, as long as patients live in the area, they would use the hospital services. However, malignancies that were diagnosed and treated outside of the hospital could not be ascertained. It is, therefore, possible that some malignancies were missed. However, the same stands for patients with and without a history of GDM.
In conclusion, according to the results of our study, GDM is an independent risk factor for ovarian, endometrium, and breast cancer. We found no association between GDM and cervical cancer and no association between the number of times that GDM was diagnosed to the relative risk for malignancies. As the incidence of GDM rises, studies are needed to further establish the association of GDM to long-term risk for cancer, especially breast cancer. Pregnancy may serve as a window of opportunities for a future diagnosis of maternal morbidity. Informing patients regarding possible future morbidity is important for primary prevention and the early detection. In addition, it is of importance that primary care providers treating women years after the gestations will be aware of this association.
References
American Diabetes Association (2004) Gestational diabetes mellitus. Diabetes Care 27(Suppl 1):S88–S90
Cheng YW, Block-Kurbisch I, Caughey AB (2009) Carpenter-Coustan criteria compared with the national diabetes data group thresholds for gestational diabetes mellitus. Obstet Gynecol 114(2 Pt 1):326–332
Malcolm J (2012) Through the looking glass: gestational diabetes as a predictor of maternal and offspring long-term health. Diabetes Metab Res Rev 28(4):307–311
Kjos SL, Buchanan TA (1999) Gestational diabetes mellitus. N Engl J Med 341(23):1749–1756
Sella T, Chodick G, Barchana M, Heymann AD, Porath A, Kokia E et al (2011) Gestational diabetes and risk of incident primary cancer: a large historical cohort study in Israel. Cancer Causes Control 22(11):1513–1520
Bellamy L, Casas JP, Hingorani AD, Williams D (2009) Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 373(9677):1773–1779
Xu Y, Shen S, Sun L, Yang H, Jin B, Cao X (2014) Metabolic syndrome risk after gestational diabetes: a systematic review and meta-analysis. PLoS One 9(1):e87863. doi:10.1371/journal.pone.0087863
Kessous R, Shoham-Vardi I, Pariente G, Sherf M, Sheiner E (2013) An association between gestational diabetes mellitus and long-term maternal cardiovascular morbidity. Heart 99(15):1118–1121
Shek NW, Ngai CS, Lee CP, Chan JY, Lao TT (2014) Lifestyle modifications in the development of diabetes mellitus and metabolic syndrome in Chinese women who had gestational diabetes mellitus: a randomized interventional trial. Arch Gynecol Obstet 289(2):319–327
Kerimoğlu OS, Yalvaç S, Karcaaltıncaba D, Kandemir O, Altınbaş SK, Dede H (2010) Early post-partum diabetes mellitus screening rates in patients with history of gestational diabetes. Arch Gynecol Obstet 282(6):613–616
Chodick G, Zucker I (2011) Diabetes, gestational diabetes and the risk of cancer in women: epidemiologic evidence and possible biologic mechanisms. Womens Health (Lond Engl) 7(2):227–237
Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA et al (2010) Diabetes and cancer: a consensus report. CA Cancer J Clin 60(4):207–221
Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S (2006) Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med 166(17):1871–1877
Friberg E, Orsini N, Mantzoros CS, Wolk A (2007) Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia 50(7):1365–1374
Tong GX, Cheng J, Chai J, Geng QQ, Chen PL, Shen XR et al (2014) Association between gestational diabetes mellitus and subsequent risk of cancer: a systematic review of epidemiological studies. Asian Pac J Cancer Prev 15(10):4265–4269
Dawson SI (2004) Long-Term risk of malignant neoplasm associated with Gestational glucose intolerance. Cancer 100(1):149–155
Perrin MC, Terry MB, Kleinhaus K, Deutsch L, Yanetz R, Tiram E, Calderon-Margalit R, Friedlander Y, Paltiel O, Harlap S (2008) Gestational diabetes and the risk of breast cancer among women in the Jerusalem Perinatal Study. Breast Cancer Res Treat 108(1):129–135
Bejaimal SA, Wu CF, Lowe J, Feig DS, Shah BR, Lipscombe LL (2016) Short-term risk of cancer among women with previous gestational diabetes: a population-based study. Diabet Med 33(1):39–46
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Funding
This study was not funded by any grant.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Appendix
Appendix
Dig | ICD code | diagnosis |
|---|---|---|
OVARY | ||
7300 | V1043 | PERSONAL HISTORY OF MALIGNANT NEOPLASM OF OVARY |
21899 | 183 | MALIGNANT NEOPLASM OF OVARY AND OTHER UTERINE ADNEXA |
21900 | 1830 | MALIGNANT NEOPLASM OF OVARY |
21901 | 1832 | MALIGNANT NEOPLASM OF OVARY |
21905 | 1838 | MALIGNANT NEOPLASM OF OTHER SPECIFIED SITES OF UTERINE ADNEXA |
21906 | 1839 | MALIGNANT NEOPLASM OF UTERINE ADNEXA, UNSPECIFIED |
21758 | 1588 | MALIGNANT NEOPLASM OF SPECIFIED PARTS OF PERITONEUM |
21759 | 1589 | MALIGNANT NEOPLASM OF PERITONEUM, UNSPECIFIED |
UTERUS | ||
22575 | 2332 | CARCINOMA IN SITU OF OTHER AND UNSPECIFIED PARTS OF UTERUS |
21895 | 182 | MALIGNANT NEOPLASM OF BODY OF UTERUS |
21896 | 1820 | MALIGNANT NEOPLASM OF CORPUS UTERI, EXCEPT ISTHMUS |
21897 | 1821 | MALIGNANT NEOPLASM OF ISTHMUS |
21898 | 1828 | MALIGNANT NEOPLASM OF OTHER SPECIFIED SITES OF BODY OF UTERUS |
21888 | 179 | MALIGNANT NEOPLASM OF UTERUS, PART UNSPECIFIED |
7299 | V1042 | PERSONAL HISTORY OF MALIGNANT NEOPLASM OF OTHER PARTS OF UTERUS |
CERVICAL CA | ||
22575 | 2331 | CARCINOMA IN SITU OF CERVIX UTERI |
7928 | V1041 | PERSONAL HISTORY OF MALIGNANT NEOPLASM OF CERVIX UTERI |
21889 | 180 | MALIGNANT NEOPLASM OF CERVIX UTERI |
21890 | 1800 | MALIGNANT NEOPLASM OF ENDOCERVIX |
21891 | 1801 | MALIGNANT NEOPLASM OF EXOCERVIX |
21892 | 1808 | MALIGNANT NEOPLASM OF OTHER SPECIFIED SITES OF CERVIX |
BREAST CA | ||
7295 | V103 | PERSONAL HISTORY OF MALIGNANT NEOPLASM OF BREAST |
22574 | 2330 | CARCINOMA IN SITU OF BREAST |
22630 | 2383 | NEOPLASM OF UNCERTAIN BEHAVIOR OF BREAST |
22642 | 2393 | NEOPLASM OF UNSPECIFIED NATURE OF BREAST |
21857 | 174 | MALIGNANT NEOPLASM OF FEMALE BREAST |
21858 | 1740 | MALIGNANT NEOPLASM OF NIPPLE AND AREOLA OF FEMALE BREAST |
21859 | 1741 | MALIGNANT NEOPLASM OF CENTRAL PORTION OF FEMALE BREAST |
21860 | 1742 | MALIGNANT NEOPLASM OF UPPER-INNER QUADRANT OF FEMALE BREAST |
21861 | 1743 | MALIGNANT NEOPLASM OF LOWER-INNER QUADRANT OF FEMALE BREAST |
21862 | 1744 | MALIGNANT NEOPLASM OF UPPER-OUTER QUADRANT OF FEMALE BREAST |
21863 | 1745 | MALIGNANT NEOPLASM OF LOWER-OUTER QUADRANT OF FEMALE BREAST |
21864 | 1746 | MALIGNANT NEOPLASM OF AXILLARY TAIL OF FEMALE BREAST |
21865 | 1747 | MALIGNANT NEOPLASM OF OTHER SPECIFIED SITES OF FEMALE BREAST |
21866 | 1748 | MALIGNANT NEOPLASM OF BREAST (FEMALE), UNSPECIFIED |
SURGERY PROCEDUREFOR BREAST CA | ||
1182-1183 | Z8521 | LOCAL EXCISION OF LESION OF BREAST |
1184 | Z85210 | LOCAL EXC.BREAST LESION + REG. LYMPH NODE EXC. |
1185 | Z85211 | LOCAL EXC. BREAST LESION + RAD. CERVICAL NODE EXC. |
1186 | Z8522 | RESECTION OF QUADRANT OF BREAST |
1187 | Z85220 | QUADRANT RESECT.BREAST+RAD.CERVICAL NODE EXC. |
1188 | Z85221 | QUADRANT RESECT.BREAST`REGIONAL LYMPH NODE EXC. |
1189 | Z8523 | SUBTOTAL MASTECTOMY |
1199 | Z854 | MASTECTOMY |
1200 | Z8541 | UNILATERAL SIMPLE MASTECTOMY |
1201 | Z8542 | BILATERAL SIMPLE MASTECTOMY |
1202 | Z8543 | UNILATERAL EXTENDED SIMPLE MASTECTOMY |
1203 | Z85430 | RADICAL MODIFIED MASTECTOMY |
1204 | Z8544 | BILATERAL EXTENDED SIMPLE MASTECTOMY |
1205 | Z8545 | UNILATERAL RADICAL MASTECTOMY |
1206 | Z8546 | BILATERAL RADICAL MASTECTOMY |
1207 | Z8547 | UNILATERAL EXTENDED RADICAL MASTECTOMY |
1208 | Z8548 | BILATERAL EXTENDED RADICAL MASTECTOMY |
Rights and permissions
About this article
Cite this article
Fuchs, O., Sheiner, E., Meirovitz, M. et al. The association between a history of gestational diabetes mellitus and future risk for female malignancies. Arch Gynecol Obstet 295, 731–736 (2017). https://doi.org/10.1007/s00404-016-4275-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-016-4275-7