Abstract
Purpose
To examine the maxillary length of euploid and aneuploid fetuses in the second and third trimester.
Methods
Retrospective study utilizing stored 2D images of second and third trimester fetal profiles obtained at the University of Tuebingen, Germany. The length of the maxilla was measured as a straight line between the anterior ventral and posterior ventral edges of the maxilla.
Results
The study population consisted of 347 euploid fetuses and 122, 36, 5, 8, and 4 fetuses with trisomy 21, 18, and 13, Turner syndrome, and triploidy. In the euploid and aneuploid group, mean gestational age was 22.3 and 22.7 weeks, respectively. The maxilla length in euploid fetuses was significantly dependent on gestational age and it was significantly shorter in fetuses with trisomy 21, 18, and 13, and triploidy but not in those with Turner syndrome. In 75.4 and 14.8%, and 11 fetuses with trisomy 21, the maxilla was below the mean, the 5th and 1st centile of the euploid population.
Conclusions
In fetuses with trisomy 21, 18, and 13 and triploidy, the maxilla is significantly shorter, but the difference is only settled, so that it is unlikely that the maxilla length will play a role in second and third screening for aneuploidy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prenatal screening for aneuploidy is most effective in the first trimester. However, ultrasound examinations later in pregnancy are likely to continue to play an important role in screening and detection of fetal aneuploidy [1]. Ultrasound findings that can be used to adjust the risk of aneuploidy can be broadly divided into two categories: fetal anomalies and soft markers. In order for these to be useful in screening, their prevalence must be different in the euploid and aneuploid populations [2].
In a meta-analysis of Agathokeous et al., the authors highlighted the relevance of an intracardiac echogenic focus, ventriculomegaly, an increased nuchal fold, echogenic bowel, hydronephrosis, a short femur and humerus, an abnormal right subclavian artery, and an absent or hypoplastic nasal bone in screening for trisomy 21. The presence or absence of each of these findings is incorporated into the screening protocol by transforming it into a likelihood ratio, which is then used to adjust the á priori risk. These can be used either to increase or decrease the risk, and if none of the markers are present, the risk can be reduced approximately eight times [3].
Recent research has focused on the fetal profile in screening for aneuploidy. It has been shown that in addition to the absence or presence of nasal bone, several other markers, such as the nasal bone length or the prenasal thickness, are abnormal in affected fetuses [4,5,6]. We have recently reported on the prefrontal space ratio which combines two facial characteristics of aneuploid fetuses: the dorsal displacement of the maxilla and the thickening of the prenasal skin [5]. The prefrontal space ratio was abnormal in about 80% of fetuses with trisomy 21 [5,6,7]. Others have measured the ratio between the prenasal thickness and the nasal bone length. This combination of markers was found to be abnormal in about 85% of the cases with trisomy 21 [6, 8].
Several radiological studies reported on oligodontia in individuals with trisomy 21. It has been demonstrated that the third molar tooth is missing in about three quarters of the adults with trisomy 21 with the maxilla being more often affected than the mandible [9,10,11]. One speculation is that the slow rate of cell growth in individuals with trisomy 21 may be responsible for underdevelopment of the maxilla.
Cicero et al. measured the length of the maxilla between 11 and 14 weeks’ gestation and evaluated its utility in the first trimester screening for aneuploidy [12]. Even though the authors did find that the maxillary measurements in fetuses with trisomy 21 were shorter than in euploid fetuses, the difference was relatively small and, therefore, difficult to employ as a screening marker.
In our study, we set out to compare the maxillary length of euploid and aneuploid fetuses in the second and third trimesters.
Methods
This was a retrospective study utilizing stored 2D images of second and third trimester fetal profiles. The prenatal ultrasound examinations used in this study were performed at the Department of Prenatal Medicine at University of Tuebingen, Germany, between 2004 and 2016.
We searched our database for pregnancies with trisomy 21, 18, and 13, triploidy, and Turner syndrome and preferentially selected images of the fetal profile that were taken between 19 and 22 weeks’ gestation. In those cases where there was no image available within this time period, we used an image from the first examination that was taken after 15 weeks’ gestation. We randomly selected two euploid fetuses that were matched for gestational age with the aneuploid cases and where the image of the profile met criteria. Each fetus was only included once in this study. Some cases had been used in our previous studies dealing with the fetal profile [5, 13, 14]. Care was taken to only include images with a true midsagittal profile. Fetuses with a facial cleft or cases where the maxilla was not clearly visible were excluded from the further analysis.
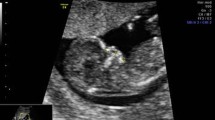
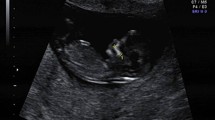
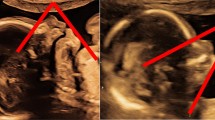
The length of the maxilla was measured as a straight line between the anterior ventral edge of the maxilla to the posterior ventral edge. Figures 1 and 2 demonstrate the measurement of the maxilla in a euploid fetus and in a fetus with trisomy 21.
For the assessment of the intraoperator reproducibility, one operator (DG) measured the maxillary length twice in 50 cases. The remaining ones were measured once. The investigator was blinded towards his own results and towards the karyotype.
Statistical analysis
Intraobserver repeatability was examined using 95% limits of agreement. The normal range in euploid fetuses was computed based on gestational age by applying univariate regression analysis. Maxilla length measurements were transformed into z scores on the basis of the linear relationship with GA. The distributions in fetuses with trisomy 21, 18, and 13 were compared with the distribution in euploid groups using Student’s t test after having verified that the distributions were normal by the Kolmogorov–Smirnov test.
For the assessment of the performance in screening for trisomy 21, we used an ROC curve analysis and compared the area under the curve of each of the examined models. We examined models based on maternal age with and without the addition of the maxilla length.
Differences were considered to be significant if the p value of less than 0.05 or there was no overlap between the respective 95% confidence intervals. The analysis was performed with Microsoft Excel for Mac 2011 (Redmond/WA, USA) and IBM SPPS23 (Armonk/NY, USA).
The study was approved by the local ethical committee (No 425/2016BO2).
Results
The study population consisted of 394 euploid and 197 aneuploid fetuses. Sixty-one (10.3%) cases were excluded from the further analysis as it was not possible to visualize the whole maxilla properly and another eight cases were excluded due to a facial cleft. Thus, there were 522 fetuses in this study: 347 euploid ones and 122, 36, 5, 8, and 4 fetuses with trisomy 21, 18, and 13, Turner syndrome and triploidy, respectively.
In the euploid group, mean maternal and gestational age was 32.0 (range 17.3–47.6) years and 22.3 (range 15.0–40.3) weeks’ gestation, and in the aneuploid group, it was 35.3 (range 18.2–46.5) years and 22.7 (range 15.0–40.3) weeks, respectively. In 241 (46.2%) cases, the gestational age was between 19 and 22 weeks. In 158 (30.3%) and 123 (23.6%) cases, the gestational age was 23 weeks and more or 18 weeks and less, respectively.
The difference between the first and second measurements of the maxilla length was −0.012 cm, and in 95% of the cases, the differences between both measurements were within −0.12 and 0.10 cm.
Mean maxillary length in the euploid population was 1.84 cm and ranged from 0.92 to 2.91 cm (Fig. 3). The maxillary length was significantly dependent on gestational age (Maxilla length = 0.903 + 0.042 × gestational age, p < 0.0001, r = 0.610). After transformation to z values, mean maxillary length was 0.0 (SD 1.0) (Table 1).
Maxillary length measurements of the chromosomal abnormal fetuses are summarized in Table 2 and Fig. 4. The maxillary length was significantly shorter in fetuses with trisomy 21 (p = 0.001), trisomy 18 (p = 0.004), trisomy 13 (p < 0.0001), and triploidy (p < 0.001), but not in those with Turner syndrome (p = 0.122), respectively.
In 18 (14.8%) and 11 (9.0%) fetuses with trisomy 21, the maxilla was below the 5th and 1st centile of the euploid population. In trisomy 18, there were 2 (5.6%) fetuses with a maxilla length below the 5th and 1st centile, respectively. The distribution of the other chromosomal abnormalities is summarized in Table 2 and Fig. 5.
Figure 6 shows the ROC curves in screening for trisomy 21 based on maternal age alone, and with the addition of the maxilla length. The area under the curve indicates that the addition of the maxilla length does not result in a significant improvement of the screening performance compared to maternal age alone [AUC MA 0.703 (95% CI 0.646–0.760), MA + maxilla length 0.729 (0.675–0.782)].
Discussion
This study demonstrates that fetuses with trisomy 21, 18, and 13, and triploidy have a significantly shorter maxilla in comparison to their euploid counterparts. However, the difference is relatively small, so it is unlikely that the maxillary length measurement will play a major role in second and third screening for aneuploidy.
To the best of our knowledge, there are no previous studies focusing on the maxillary length in aneuploid pregnancies in the second and third trimesters of pregnancy. Hermann et al. used 3D ultrasound volumes to examine the length of the maxilla in euploid pregnancies between 11 and 26 weeks’ gestation [15]. In summary, they also found a linear relationship with gestational age, but their measurements were slightly larger than in our 2D study. Cicero et al. examined 839 euploid fetuses and 88 with trisomy 21 at 11–13 weeks’ gestation, and found that in 83% of the affected cases, the maxilla length was below the mean of the normal fetuses. In 24% of the cases, the measurements were below the 5th centile, respectively [12]. Daklis et al. also used 3D volumes and examined the maxillary depth (distance between the alveolus of the maxilla in the midline anteriorly and the midpoint of the line joining the rami posteriorly) at 11–13 weeks to assess the midfacial hypoplasia of trisomy 21 fetuses. The measurements were below the 5th centile in 10% of the affected cases [16]. In this respect, both studies are consistent with our results.
In a recent paper from Cossellu et al., the authors examined the spheno-frontal distance in 30 fetuses with trisomy 21 and compared the results to 80 euploid fetuses using stored 3D volumes for both groups [17]. They found that in almost all of the affected cases, the spheno-frontal distance was below the 5th centile. One could hypothesize that both the spheno-frontal distance and the maxillary length are surrogate parameters for midfacial hypoplasia, which is a common finding in fetuses with trisomy 21. In this respect, it is surprising that our results differ substantially from the ones shown by Cosellu et al. However, in a recent paper from our group, we were not able to reproduce the good results previously shown by Cosellu et al. [14]. In our study, the spheno-frontal distance in fetuses with trisomy 21 was below the 5th centile in only about 25%. This result is in line with the results from this study dealing with maxillary length.
Numerous studies have been published that look at the effectiveness of prenatal ultrasound markers and fetal anomalies in screening for aneuploidy. In a meta-analysis of Agathokleous et al., the authors have summarized 48 of such studies and estimated the likelihood ratio for each of the commonly used markers [3]. The authors found that an echogenic focus in the left ventricle, ventriculomegaly, increased nuchal fold, echogenic bowel, mild hydronephrosis, short humerus and femur, aberrant right subclavian artery, and an abnormal nose bone was found in about 7.5–60% of the affected cases and in 0.2–6.4% of the euploid fetuses, respectively. Their meta-analysis suggested that the only markers that remain significant even as an isolated finding are ventriculomegaly, increased nuchal fold, an aberrant right subclavian artery, and an abnormal nasal bone with a combined positive likelihood ratio of 3.8–6.6.
More recent studies have focused on the fetal profile in screening for trisomy 21. Almost all research groups focused on the combination of facial markers that may reflect typical features of affected fetuses. Maymon et al. combined the prenasal thickness with the nasal bone length and detected 70% of fetuses with trisomy 21 [18]. Similarly, Vos et al. used the prenasal thickness-to-nose bone ratio and detected 86% of the affected fetuses for a false positive rate of 5% [6, 8]. Others have proposed to use the prefrontal space ratio, a marker that combines prenasal thickness and midfacial hypoplasia in fetuses with trisomy 21. The detection rates were about 80% for a false positive rate of 5% [5,6,7, 19]. Sonek et al. introduced the frontomaxillary angle, an angle between the top edge of the upper palate and the skin over the forehead. They reported a detection rate of almost 90% for a false positive rate of about 3% [4]. Unfortunately, other studies failed to reproduce these excellent results [20].
Compared to screening for aneuploidy with cell-free fetal DNA (cfDNA) with its detection and false positive rates of about 90–99% and 0.1%, the relevance of the ultrasound markers could be questioned [21, 22]. However, at the present time, screening using cfDNA remains too expensive to be universally offered, so that in the foreseeable future, ultrasound markers will still play an important role in second and third trimester screening for chromosomal abnormalities.
Our study has some limitations. First, the study was performed retrospectively using stored 2D images, and second, the data were collected in a single center only. As the images were not initially taken for the purpose of the measurement of the maxilla, it was difficult to identify the posterior end of the maxilla in some cases. However, the reproducibility analysis indicates that a difference between two measurements was within an acceptable range.
In summary, the maxillary length in aneuploid fetuses is shorter than in their euploid counterparts. However, prenatal screening for trisomy 21 that combines maxillary length and maternal age is not significantly better than screening by maternal age alone. Therefore, it is unlikely that maxillary length will play an important role in screening for trisomy 21.
References
Sonek JD, Wagner P, Nicolaides KH (2016) Inverted Pyramid of Care. Clin Lab Med. 36(2):305–317
Cicero S (2003) Sonographic markers of fetal aneuploidy?A review. Placenta 24:S88–S98
Agathokleous M, Chaveeva P, Poon LCY, Kosinski P, Nicolaides KH (2013) Meta-analysis of second-trimester markers for trisomy 21. Ultrasound Obstet Gynecol 41(3):247–261
Sonek J, Borenstein M, Downing C, McKenna D, Neiger R, Croom C et al (2007) Frontomaxillary facial angles in screening for trisomy 21 at 14–23 weeks’ gestation. Am J Obstet Gynecol. 197(2):160.e1–160.e5
Yazdi B, Sonek J, Oettling C, Hoopmann M, Abele H, Schaelike M et al (2013) Prefrontal space ratio in second- and third-trimester screening for trisomy 21. Ultrasound Obstet Gynecol 41(3):262–266
Vos FI, De Jong-Pleij EAP, Bakker M, Tromp E, Pajkrt E, Kagan KO et al (2015) Nasal bone length, prenasal thickness, prenasal thickness-to-nasal bone length ratio and prefrontal space ratio in second- and third-trimester fetuses with Down syndrome. Ultrasound Obstet Gynecol 45(2):211–216
Chaveeva P, Agathokleous M, Poon LCY, Markova D, Nicolaides KH (2013) Second-trimester screening for trisomy-21 using prefrontal space ratio. Fetal Diagn Ther 34(1):50–55
De Jong-Pleij EAP, Vos FI, Ribbert LSM, Pistorius LR, Tromp E, Bilardo CM (2011) Prenasal thickness-to-nasal bone length ratio: a strong and simple second- and third-trimester marker for trisomy 21. Ultrasound Obstet Gynecol 39(2):185–190
Suri S, Tompson BD, Atenafu E (2011) Prevalence and patterns of permanent tooth agenesis in Down syndrome and their association with craniofacial morphology. Angle Orthod 81(2):260–269
Lomholt JF, Russell BG, Stoltze K, Kjaer I (2002) Third molar agenesis in Down syndrome. Acta Odontol Scand 60(3):151–154
Shapira J, Chaushu S, Becker A (2000) Prevalence of tooth transposition, third molar agenesis, and maxillary canine impaction in individuals with Down syndrome. Angle Orthod 70(4):290–296
Cicero S, Curcio P, Rembouskos G, Sonek J, Nicolaides KH (2004) Maxillary length at 11–14 weeks of gestation in fetuses with trisomy 21. Ultrasound Obstet Gynecol 24(1):19–22
Kagan KO, Sonek J, Berg X, Berg C, Mallmann M, Abele H et al (2015) Facial markers in second- and third-trimester fetuses with trisomy 18 or 13, triploidy or Turner syndrome. Ultrasound Obstet Gynecol 46(1):60–65
Wagner N, Wagner P, Haen S, Schmidt S, Yerlikaya G, Maden Z et al (2014) Effective management and intrauterine treatment of congenital cytomegalovirus infection: review article and case series. J Matern Fetal Neonatal Med. 27(2):209–214
Hermann NV, Darvann TA, Sundberg K, Kreiborg S, Joergensen C (2015) Maxillary length in 11- to 26-week-old normal fetuses studied by 3D ultrasound. Prenat Diagn 35(6):571–576
Dagklis T, Borenstein M, Peralta CFA, Faro C, Nicolaides KH (2006) Three-dimensional evaluation of mid-facial hypoplasia in fetuses with trisomy 21 at 11 + 0 to 13 + 6 weeks of gestation. Ultrasound Obstet Gynecol 28(3):261–265
Cossellu G, Persico N, D’Ambrosi F, Carbone F, Fabietti I, Boito S et al (2016) Sphenofrontal distance on three-dimensional ultrasound in euploid and trisomy-21 fetuses at 16–24 weeks’ gestation. Ultrasound Obstet Gynecol 48(2):177–180
Maymon R, Levinsohn-Tavor O, Cuckle H, Tovbin Y, Dreazen E, Wiener Y et al (2005) Second trimester ultrasound prenasal thickness combined with nasal bone length: a new method of Down syndrome screening. Prenat Diagn 25(10):906–911
Sonek J, Molina F, Hiett AK, Glover M, McKenna D, Nicolaides KH (2012) Prefrontal space ratio: comparison between trisomy 21 and euploid fetuses in the second trimester. Ultrasound Obstet Gynecol 40(3):293–296
Molina F, Persico N, Borenstein M, Sonek J, Nicolaides KH (2008) Frontomaxillary facial angle in trisomy 21 fetuses at 16–24 weeks of gestation. Ultrasound Obstet Gynecol 31(4):384–387
Gil MM, Quezada MS, Revello R, Akolekar R, Nicolaides KH (2015) Analysis of cell-free DNA in maternal blood in screening for fetal aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol 45(3):249–266
Kagan KO, Hoopmann M, Singer S, Schaeferhoff K, Dufke A, Mau-Holzmann UA (2016) Discordance between ultrasound and cell free DNA screening for monosomy X. Arch Gynecol Obstet 294(2):219–224
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This is a retrospective study involving ultrasound images from patients that were seen at the University of Tuebingen, Germany.
The study was approved by the local ethical committee. This is also stated in the Methods section.
Rights and permissions
About this article
Cite this article
Hoopmann, M., Sonek, J., Goldschmid, D. et al. Maxillary length in euploid and aneuploid fetuses. Arch Gynecol Obstet 295, 331–336 (2017). https://doi.org/10.1007/s00404-016-4251-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-016-4251-2