Abstract
Cutaneous field cancerization in dermatology describes the anatomic region of photodamaged skin with actinic keratoses (AKs) or cutaneous squamous cell carcinoma (cSCC) that is surrounded by cellular atypia, forming a dysplastic field. The concept of field cancerization is especially relevant in dermatology, as actinic keratoses and the surrounding dysplastic region can progress to carcinomas, necessitating the treatment of the field. Recent research has focused on field-directed therapy using topical agents. This study aims to systematically review randomized controlled trials on topical treatments for actinic keratosis field cancerization, following the PRISMA guidelines. Clinical recommendations were based on the Oxford Centre for Evidence-Based Medicine. We identified 20 original randomized controlled trials for topical cutaneous field therapy. 0.5% 5-Fluorouracil/salicylic acid and 0.5% 5-fluorouracil received a clinical recommendation grade of A, while diclofenac sodium received a clinical recommendation grade of B. Calcipotriol/5-fluorouracil, Imiquimod, sunscreen combination therapies, and tirbanibulin received a recommendation grade of C. This review provides a framework for clinicians when considering topical treatments for patients with field cancerization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cutaneous field cancerization is defined as the anatomic region of photodamaged skin with actinic keratoses (AKs) or cutaneous squamous cell carcinoma (cSCC), surrounded by multifocal cellular atypia [1]. The global market size of treatments for AKs was 6.25 billion USD in 2017 and is estimated to be 9.25 billion USD in 2030. This primarily arises from photodamage due to chronic ultraviolet (UV) radiation exposure [2]. As cells in the exposed area accrue genetic alterations and divide, a dysplastic field emerges [3]. AK lesions, also known as solar keratosis, may initially develop with a dysplastic field without invasive features; however, as these cells continue to be exposed to carcinogenic elements and accrue more genetic mutations, there is potential for the eventual manifestation of carcinoma [3,4,5]. Although AKs are visible clinically, precancerous cells within the photodamaged periphery may only be revealed through histopathology [2]. Additionally, no reliable method exists to predict the progression of AKs to cSCC [2].
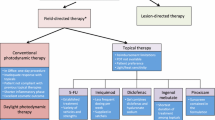
While AKs are commonly regarded as precancerous, the targeted treatment of isolated lesions does not target other nearby preneoplastic cells in its neighboring cancerized field [4, 6]. Therefore, a definitive approach is needed to treat all AKs and their surrounding dysplastic field, limiting the utility of cryotherapy, which is targeted therapy not beneficial for field therapy. Cutaneous field therapy refers to the treatment approach aimed at treating an entire field of skin, rather than individual lesions, to minimize the risk of new precancerous or cancerous lesions from developing [7]. While photodynamic therapy (PDT), a second-line non-topical therapy for cutaneous field cancerization, is safe and effective, PDT is not readily available to many patients in rural areas and was only available in 41.6% of metropolitan counties in 2017 [8]. Also, an analysis of the cost-effectiveness of different topical agents vs PDT for the treatment of AKs revealed PDT had a higher cost than topical agents, which increases healthcare spending and insurance companies may require patients to try topical agents first [9]. The limited availability of PDT necessitates the evaluation of topical agents for cutaneous field cancerization as topical agents are readily available across the United States.
The first line topical treatment for cutaneous field therapy is 5% 5-fluorouracil (5-FU), a highly effective and established treatment that has been extensively reviewed in the past. Second-line topical treatments include imiquimod and diclofenac sodium [10, 11]. Second-line treatment previously included ingenol mebutate; however, there are long-term safety concerns linking ingenol mebutate with skin cancer, prompting the European Union to suspend its use in 2020, with the manufacturer discontinuing production shortly afterwards [12]. While field therapy is effective in the treatment of field cancerization, long duration treatments, such as with 5-FU, may prevent patients from adhering to treatment [13]. Patients also prefer topical treatments that require fewer applications [13]. There is tremendous interest in topicals for cutaneous field therapy. 5-FU has been shown to be superior in efficacy compared to imiquimod (IMIQ), ingenol mebutate, and methyl aminolaevulinate PDT (MAL-PDT) [14]. The purpose of this study is to provide comprehensive review of current topical treatments for cutaneous field therapy and offer clinical recommendations based on efficacy and safety data. In addition, we aim to assess our clinical recommendations with those established by Jansen et al., while adding any additional topical treatment options [14].
Methods
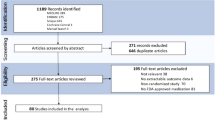
We systematically searched PubMed, Embase, and Cochrane on August 31, 2023 for topical treatments for field therapy, per the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. Search terms field cancerization, field carcinogenesis, field change cancerization or cancer field effect were combined with topical (Fig. 1). All articles resulting from 1973 to 2023 were independently reviewed by PP and JW. PP and JW scanned bibliographies of the included articles for additional relevant reports. Included articles were randomized controlled trials (RCTs) utilizing topical agents for AK cutaneous field therapy, with comparison arms including a vehicle or 5% 5-FU. Research that included non-topical agents (such as oral medications, bleaching agents, chemical peels, drugs administered intralesionally, laser treatments, and light-based therapies), whether used independently or in combination, were not considered. The studies that utilized proprietary formulations were not included, to better evaluate the isolated effects of the treatment. Studies that did not have preexisting AKs in a field as part of the inclusion criteria were not included. Reviews, conference abstracts, non-human studies, non-randomized controlled trials, non-English articles, basic science, case reports, and case series were excluded. Clinical recommendations were made per the Oxford Centre for Evidence-Based Medicine guidelines (Table 1) [15].
Results
Our systematic search yielded 1275 articles. After screening titles, abstracts, and full text articles, we identified 20 articles that met our inclusion criteria (Fig. 1). Investigated treatments included 5-FU/10% salicylic acid or 0.5% 5-FU (4), diclofenac sodium (3), 0.005% calcipotriol/0.5% 5-FU (1), imiquimod (8), sunscreen combination therapy (3), and 1% tirbanibulin (1). No studies utilizing 5% 5-FU for cutaneous field therapy was directly compared with placebo, but our review evaluated 0.5% 5-FU. Table 2 summarizes the included studies, highlighting evidence grades, designs, treatment parameters, results, and adverse effects.
0.5% 5-Fluorouracil
5-FU is a cytotoxic medication that can treat AKs by inhibiting cellular thymidylate synthase, leading to disruption of DNA replication [16]. 5% 5-FU is a well-established treatment for cutaneous field cancerization. Recently, many clinical trials have studied various doses and formulations of 5-FU [17,18,19,20]. In one 20-week RCT involving 166 patients with AKs, subjects received 0.5% 5-FU and 10% salicylic acid (SA) or vehicle cream once daily for 12 weeks. At 12 weeks, 49.5% of 0.5% 5-FU/10% SA patients achieved complete clearance of their AKs (AKCLEAR100), while 18.2% of the vehicle group had AKCLEAR100 [17].
In another RCT, 470 patients received 0.5% 5-FU/10% SA once daily for 12 weeks, 3% diclofenac sodium/HA twice daily for 12 weeks, or placebo. After 20 weeks, AKCLEAR100 was 55.45%, 32.0%, and 15.1%, respectively. Application site-reactions were greater in participants receiving 5-FU than diclofenac sodium [18].
Another RCT involving 207 patients compared the efficacy of 0.5% 5-FU with vehicle cream by adjusting the duration of treatment. The patients received 0.5% 5-FU or vehicle cream once daily for 1, 2, or 4 weeks. AKCLEAR100 was 14.9%, 37.0% and 57.8% in 1-, 2-, and 4-week treatment groups 4 weeks after treatment was completed, while 0% of those who received vehicle cream achieved AKCLEAR100 at all time points [19].
One RCT involving 24 patients directly compared the efficacy of 0.5% 5-FU and 5% 5-FU. Patients underwent a split-face treatment, applying 0.5% 5-FU once daily or 5% 5-FU twice daily to either side of the face for 4 weeks. Both treatment arms achieved 43% AKCLEAR100 4 weeks after the end of treatment. Mild localized skin reactions were reported in both treatment arms, with no significant difference. Patients reported they preferred treatment with 0.5% 5-FU rather than 5% 5-FU [20].
Grade of recommendation: a for the included 5-FU formulations for cutaneous field therapy based on 3 level 1b studies and 1 level 2b study. (See Table 2).
Diclofenac sodium
Diclofenac sodium, a nonsteroidal anti-inflammatory drug (NSAID), inhibits cyclooxygenase-2 to decrease cellular proliferation and induce apoptosis in AKs [10]. One RCT involving 96 patients analyzed the target lesion number score (TLNS) 3 months after patients were given 3% topical diclofenac sodium in 2.5% HA gel or vehicle gel. Patients who received diclofenac sodium had a significantly decreased TLNS compared to vehicle [21]. Mild applications site reactions were reported [21]. Another RCT involving 30 male patients, subjects received 3% diclofenac sodium and HA gel twice a day for 60 days or 5% 5-FU 5 days of the week for 4 weeks for field therapy. Patients who received 5-FU had a 57.13% reduction of their field cancerization, while those who received diclofenac sodium had 62.45% reduction two months after the end of treatment [22]. Field cancerization was measured by imaging AKs and the surrounding skin with reflectance confocal microscopy, which allows real-time rendering of cellular and subcellular skin comparable to histological examination. Reduction of field cancerization was assessed by measuring field cancerization at baseline and at the end of the trial [23]. No AEs were reported [22].
In another RCT, 195 patients received 3% diclofenac sodium/2.5% HA or vehicle gel twice a day for either 30 days or 60 days. AKCLEAR100 at 30 days after the final treatment was 14.3% and 33.3% in the 30- and 60-day treatment groups, respectively, with mild AEs [24]. Patients who received vehicle gel reported 4.1% and 10.2% AKCLEAR100 after 30 and 60 days, respectively [24].
Grade of recommendation: B for diclofenac sodium for cutaneous field therapy based on 1 level 1b study and 2 level 2b studies (see Table 2).
Calcipotriol/5% 5-FU
Calcipotriol, a vitamin D3 analog, has antiproliferative effects which has been used to treat psoriasis [25]. One RCT involving 131 patients who had previously received cryotherapy for cutaneous field cancerization treated subjects with 0.005% calcipotriol ointment with 5% 5-FU cream or petroleum jelly with 5% 5-FU twice a day for 4 days. After 8 weeks, subjects who received calcipotriol had an 87.8% reduction of AKs on their face, 76.4% on their scalp, 68.8% in their right upper extremity, and 79% on their left upper extremity. Patients who received petroleum jelly and 5% 5-FU had a 26.3% reduction of AKs on their face, 5.7% on their scalp, 9.6% on their right upper extremity, and 16.3% on their left upper extremity [26]. The combination of calcipotriol and 5% 5-FU demonstrated superior efficacy compared to 5% 5-FU alone; however, patients receiving calcipotriol experienced significantly more burning and skin redness [26].
Grade of recommendation: C for calcipotriol/5% 5-FU for cutaneous field therapy based on 1 level 2b study. (See Table 2).
Imiquimod
Imiquimod (IMIQ) can be used for the treatment of cutaneous malignancies by binding to toll-like receptors and inducing apoptosis and the release of immunomodulatory cytokines [27]. Eight RCTs used 5% IMIQ for field therapy and compared IMIQ with vehicle cream. In a study involving 43 patients with kidney, heart, or liver transplants, patients received 5% IMIQ or vehicle cream for 3 days a week. After 16 weeks, 62.1% of IMIQ patients had AKCLEAR100 compared to 0% of vehicle cream patients [28]. Another study involving 44 patients treated solar keratoses with 5% IMIQ or vehicle cream three times a week for 3 weeks. After 14 weeks, there was 75% clearance of solar keratoses in 72% and 30% of patients who received IMIQ or vehicle cream, respectively [29].
In one RCT, 479 patients received 3.75% IMIQ, 2.5% IMIQ, or placebo for cutaneous field therapy. The treatment protocol entailed patients applying the study drug once daily for 2 weeks, followed by a 2-week break, and then another 2 weeks of daily application. At 14 weeks, 35.6%, 30.6%, and 6.3% of patients achieved AKCLEAR100 in the 3.75% IMIQ, 2.5% IMIQ, and placebo groups, respectively [30].
Two 20-week RCTs showed significant improvement of cutaneous field cancerization in patients who received 5% IMIQ once daily three times per week for four weeks, a 4-week washout period, followed by a repeat 4-week cycle if patients had no clearance of AKs compared to placebo [31, 32]. In two other well-designed 24-week RCTs, patients received 5% IMIQ daily three times per week for 16 weeks and showed significant improvements in AKCLEAR100 compared to placebo [11, 33]. One study reported patients who experienced more erythema with treatment had better clearance of AKs [11].
One RCT treated varied the frequency of IMIQ administration for field therapy in 149 patients. Patients were instructed to apply 5% IMIQ once daily, two, three, five, or seven times a week for 8 weeks. After 16 weeks, AKCLEAR100 was 3.2%, 6.9%, 3.3%, and 6.7% in treatment arms, respectively. None of the patients who received vehicle gel achieved AKCLEAR100. Adverse effects increased as the frequency of IMIQ increased, and all treatment arms had significantly more adverse effects compared to placebo [34].
Grade of recommendation: C for imiquimod for cutaneous field therapy based on 7 level 1b studies and 1 level 2b study (see Table 2).
Sunscreen combination therapies
While sunscreen is known to prevent the progression of AKs, it has also been recently been investigated as the base for combination therapy for the treatment of cutaneous field cancerization [35,36,37]. One RCT involving 28 elderly patients treated cutaneous field cancerization with 50 SPF sunscreen or 50 SPF sunscreen, 1% photolyase, and 1% endonuclease twice a day for 6 months. Photolyase and endonuclease are DNA-repair enzymes hypothesized to assist in DNA-repair due to sun damage [38]. Field cancerization was measured with fluorescence diagnostics using methylaminolevulinate. Field cancerization decreased 29% in the enzyme group and 10% in sunscreen only group [35]. Another study involving 50 patients found cutaneous field cancerization decreased 36% in patients using 50 SPF sunscreen and piroxicam 0.8% and 11% in patients using 50 SPF sunscreen after 12 weeks [36].
One partially blinded RCT treated patients with 99 SPF sunscreen, 99 SPF sunscreen and topical antioxidants, 99 SPF sunscreen and photolyase, or 99 SPF sunscreen, photolyase, and topical antioxidants. Total AK clearance on the forearms improved significantly from baseline in all treatment groups by the end of the trial. There was no significant difference in AK clearance between treatment groups and only the antioxidant group had significantly decreased total AKs compared to sunscreen only [37].
Grade of recommendation: C for sunscreen combination therapies for cutaneous field therapy based on 3 level 2b studies (see Table 2).
Tirbanibulin
Tirbanibulin is a novel topical treatment of AKs that inhibits tubulin polymerization and disrupts microtubule formation [39]. In one RCT involving 702 patients, 53.7% of patients who received 1% tirbanibulin once a day for 5 days achieved AKCLEAR100 after 57 days, while 8.6% of placebo patients had AKCLEAR100. Among the patients who had AKCLEAR100 with 1% tirbanibulin treatment, 47% of patients had recurrent lesions, and 42% had new lesions after one year. Both treatment arms reported similar instances of mild localized skin reactions, that mostly resolved by the end of the study [40].
Grade of recommendation: C for tirbanibulin for cutaneous field therapy based on 1 level 1b study. (See Table 2).
Clinical recommendations
We strongly recommend 0.5% 5-FU/10% SA once daily for 12 weeks or 0.5% 5-FU once daily for 4 weeks as topical agents for cutaneous field therapy. 85% of patients that applied 0.5% 5-FU on one side of the face and 5% 5-FU on the other reported they preferred treatment with 0.5% 5-FU, endorsing less irritation and easier application [20]. 5% 5-FU is the most efficacious and cost-effective treatment for cutaneous field cancerization with comparison to Ingenol Mebutate, IMIQ, and MAL-PDT [14]. Life-threatening reactions associated with topical 5-FU have been reported in patients deficient in dihydropyrimidine dehydrogenase (DPD), the enzyme responsible for the catabolism of 5-FU [41, 42]. While such cases are uncommon, DPD deficiency is present in 3–5% of the population [43]. A lower dose of 5-FU may demonstrate fewer side effects, but clinicians should discuss side effects with patients and avoid prescribing in patients with DPD deficiency. Due to superior efficacy, mild side effect profile, and low-risk for severe adverse reactions, 0.5% 5-FU/10% SA and 0.5% 5-FU receives the strongest recommendation.
We recommend 3% diclofenac sodium in 2.5% hyaluronic acid gel twice a day for 60 days as a topical agent for cutaneous field therapy. The included studies utilizing diclofenac sodium reported mild side effects and high efficacy [21, 22, 24]. Patients report preference of topical treatments that require few administrations and due to the frequent applications of diclofenac sodium, a stronger recommendation cannot be made [13].
Calcipotriol and 5% 5-FU may be used as a topical agent for cutaneous field therapy. The study investigating this combination compared the efficacy of calcipotriol and 5% 5-FU with petroleum jelly and 5% 5-FU [26]. It is unclear whether petroleum jelly affected the absorption of 5-FU. The study reported more side effects and greater efficacy associated with calcipotriol [26]. Clinicians should discuss the side effect profile associated with calcipotriol and 5% 5-FU therapy with patients and monitor side effects. Further RCTs must be conducted before a stronger recommendation can be made, however, due to the same active ingredient as 5% 5-FU, clinicians should only consider calcipotriol and 5% 5-FU as topical treatment for patients without DPD deficiency.
Imiquimod (5%) three times a week for 4 weeks may serve as a topical agent for cutaneous field therapy. All the included RCTs reported LSRs, while two of the trials reported systemic AEs such as headache, fatigue, nausea, and leukopenia [11, 28,29,30,31,32,33,34]. Clinicians should discuss adverse effects associated with IMIQ with patients and can adjust dosing and frequency. Given the varying efficacy and safety profile, further RCTs comparing IMIQ to other treatments must be conducted before a stronger recommendation can be given.
Sunscreen combination therapy may serve as a treatment option for cutaneous field therapy. Sunscreen with DNA repair enzymes or piroxicam can provide benefit in treating field cancerization; however, the benefit is miniscule and not comparable to the efficacy of 5% 5-FU [35,36,37]. Sunscreen combination therapies may be used as initial treatment for mild cases or as an adjuvant therapy with 5% 5-FU. Sunscreen has recently been investigated for its endocrine-disrupting properties; however, AEs are extremely rare and still up for debate [44]. Furthermore, the limited patient populations and inconsistent drug formulations in the included studies restrict the robustness of their conclusions. While the protective benefits of sunscreen are well established, its role in field cancerization treatment is yet to be solidly established, meriting only a weak recommendation at this stage.
Tirbanibulin (1%) once a day for 5 days may serve as a topical agent for cutaneous field therapy. The included RCT reported mild LSR associated with treatment; however, patients had high relapse of lesions [40]. RCTs with comparisons to standard treatments and more long-term safety data must be conducted before a higher recommendation can be made.
Conclusion
As it is not possible to identify AKs that may transform into SCC; it is important to treat all AKs and the surrounding field [3]. We performed a systematic review on the topical treatments for cutaneous field therapy. We strongly recommend 0.5% 5-FU/SA and 0.5% 5-FU as topical treatment options for cutaneous field therapy. We recommend diclofenac sodium as a topical treatment option for cutaneous field therapy. Calcipotriol/5% 5-FU, IMIQ, sunscreen combination therapy, and 1% tirbanibulin may be considered as treatment options, but risks and benefits should be considered prior to prescribing. More research must be conducted for long-term efficacy and potential adverse events.
Our findings are in line with previous research that identified 5-FU as the most effective treatment option for cutaneous field cancerization [14]. Imiquimod serves as an alternative therapy to 5-FU with a lower recommendation, which is supported by our results [14]. This review supports previous research while also incorporating alternative topical treatment options.
The limitations of this systematic review include that some RCTs have small patient populations (n < 50) and varied drug treatment formulations and clinical parameters. Differences in treatment formulations and dosing may have a significant impact on efficacy, limiting the universality of the findings. Also, many of the studies determined efficacy by AKCLEAR100, which can favor studies that treated a smaller number of AKs. The number of AKs treated, listed in Table 2, may impact the reliability of efficacy measurements. Non-English articles that may have contributed to this study were not included. A strength of this study is that we included mostly double-blind, placebo controlled RCTs. Also, this study utilized PRISMA guidelines and highlighted key parameters to provide evidence-based recommendations, delivering clinically relevant information to clinicians.
Many of the studies determined efficacy of treatment by clinical assessment of AK lesions. Only two studies included utilized more objective, accurate methods of determining the extent of field cancerization and sub-clinical lesions such as cross polarized light photography or fluorescence diagnosis [35, 36, 45]. A novel scoring system specifically for extensive field cancerization has recently been developed and is more accurate than the Actinic Keratosis Field Assessment Scale (AK-FAS) [46]. Future studies should utilize these updated methods to better assess outcomes and provide more robust recommendations. Future RCTs examining effects of topical medication on cutaneous field cancerization should also focus on varied patient populations, including patients with darker skin and patients who are immunocompromised.
Data availability
The data that support the evidence presented in this article is accessible from public databases including PubMed, Embase, and Cochrane.
References
Willenbrink TJ et al (2020) Field cancerization: definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol 83(3):709–717
Huang A et al (2019) Updates on treatment approaches for cutaneous field cancerization. Curr Dermatol Rep 8(3):122–132
Lanoue J, Chen C, Goldenberg G (2016) Actinic keratosis as a marker of field cancerization in excision specimens of cutaneous malignancies. Cutis 97(6):415–420
Christensen SR (2018) Recent advances in field cancerization and management of multiple cutaneous squamous cell carcinomas. F1000Res. https://doi.org/10.12688/f1000research.12837.1
Rogers HW et al (2015) Incidence estimate of nonmelanoma skin cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol 151(10):1081–1086
Figueras Nart I et al (2018) Defining the actinic keratosis field: a literature review and discussion. J Eur Acad Dermatol Venereol 32(4):544–563
Torezan LA, Festa-Neto C (2013) Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol 88(5):775–786
Cheraghlou S, Feng H, Cohen JM (2020) Trends in the access and cost of photodynamic therapy among Medicare beneficiaries in the United States, 2012–2017. JAMA Dermatol 156(9):1021–1022
Jansen MHE et al (2020) A trial-based cost-effectiveness analysis of topical 5-fluorouracil vs. imiquimod vs. ingenol mebutate vs. methyl aminolaevulinate conventional photodynamic therapy for the treatment of actinic keratosis in the head and neck area performed in the Netherlands. Br J Dermatol 183(4):738–744
Nelson CG (2011) Diclofenac gel in the treatment of actinic keratoses. Ther Clin Risk Manag 7:207–211
Korman N et al (2005) Dosing with 5% imiquimod cream 3 times per week for the treatment of actinic keratosis: results of two phase 3, randomized, double-blind, parallel-group, vehicle-controlled trials. Arch Dermatol 141(4):467–473
Heron CE, Feldman SR (2021) Ingenol mebutate and the treatment of actinic keratosis. J Drugs Dermatol 20(1):102–104
Rosso JD et al (2021) Advances and considerations in the management of actinic keratosis: an expert consensus panel report. J Drugs Dermatol 20(8):888–893
Jansen MHE et al (2019) Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med 380(10):935–946
Oxford Centre for Evidence-Based Medicine: levels of evidence (March 2009). Centre for Evidence Based Medicine 2009. cited 7 Aug 2023.
Ghafouri-Fard S et al (2021) 5-Fluorouracil: a narrative review on the role of regulatory mechanisms in driving resistance to this chemotherapeutic agent. Front Oncol 11:658636
Stockfleth E et al (2017) Efficacy and safety of 5-fluorouracil 0.5%/salicylic acid 10% in the field-directed treatment of actinic keratosis: a phase III, randomized, double-blind, vehicle-controlled trial. Dermatol Ther (Heidelb) 7(1):81–96
Stockfleth E et al (2011) Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol 165(5):1101–1108
Jorizzo J et al (2002) Randomized trial evaluating a new 0.5% fluorouracil formulation demonstrates efficacy after 1-, 2-, or 4-week treatment in patients with actinic keratosis. Cutis 70(6):335–339
Loven K et al (2002) Evaluation of the efficacy and tolerability of 0.5% fluorouracil cream and 5% fluorouracil cream applied to each side of the face in patients with actinic keratosis. Clin Ther 24(6):990–1000
Wolf JE Jr et al (2001) Topical 3.0% diclofenac in 2.5% hyaluronan gel in the treatment of actinic keratoses. Int J Dermatol 40(11):709–713
Mazzella C et al (2018) Management of clinical and subclinical actinic keratoses with histological and immunohistochemical assessments by confocal microscopy. Dermatol Ther 31(5):e12672
Ulrich M et al (2010) Reflectance confocal microscopy for noninvasive monitoring of therapy and detection of subclinical actinic keratoses. Dermatology 220(1):15–24
Rivers JK et al (2002) Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol 146(1):94–100
Kim GK (2010) The rationale behind topical vitamin d analogs in the treatment of psoriasis: where does topical calcitriol fit in? J Clin Aesthet Dermatol 3(8):46–53
Cunningham TJ et al (2017) Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest 127(1):106–116
Bubna AK (2015) Imiquimod—its role in the treatment of cutaneous malignancies. Indian J Pharmacol 47(4):354–359
Ulrich C et al (2007) Topical immunomodulation under systemic immunosuppression: results of a multicentre, randomized, placebo-controlled safety and efficacy study of imiquimod 5% cream for the treatment of actinic keratoses in kidney, heart, and liver transplant patients. Br J Dermatol 157(Suppl 2):25–31
Chen K et al (2003) Short-course therapy with imiquimod 5% cream for solar keratoses: a randomized controlled trial. Australas J Dermatol 44(4):250–255
Swanson N et al (2010) Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol 62(4):582–590
Jorizzo J et al (2007) Vehicle-controlled, double-blind, randomized study of imiquimod 5% cream applied 3 days per week in one or two courses of treatment for actinic keratoses on the head. J Am Acad Dermatol 57(2):265–268
Alomar A, Bichel J, McRae S (2007) Vehicle-controlled, randomized, double-blind study to assess safety and efficacy of imiquimod 5% cream applied once daily 3 days per week in one or two courses of treatment of actinic keratoses on the head. Br J Dermatol 157(1):133–141
Szeimies RM et al (2004) Imiquimod 5% cream for the treatment of actinic keratosis: results from a phase III, randomized, double-blind, vehicle-controlled, clinical trial with histology. J Am Acad Dermatol 51(4):547–555
Gebauer K, Shumack S, Cowen PS (2009) Effect of dosing frequency on the safety and efficacy of imiquimod 5% cream for treatment of actinic keratosis on the forearms and hands: a phase II, randomized placebo-controlled trial. Br J Dermatol 161(4):897–903
Carducci M et al (2015) Comparative effects of sunscreens alone vs sunscreens plus DNA repair enzymes in patients with actinic keratosis: clinical and molecular findings from a 6-month, randomized. Clinical Study J Drugs Dermatol 14(9):986–990
Bobyr I et al (2019) Fluorescent photodiagnostic evaluation of field cancerization treated with a medical device containing piroxicam 08% and sunscreen SPF 50+ for actinic keratosis. Photodermatol Photoimmunol Photomed 35(4):277–279
Alvares BA et al (2022) Efficacy of sunscreen with photolyase or regular sunscreen associated with topical antioxidants in treating advanced photodamage and cutaneous field cancerization: a randomized clinical trial. An Bras Dermatol 97(2):157–165
Luze H et al (2020) DNA repair enzymes in sunscreens and their impact on photoageing-A systematic review. Photodermatol Photoimmunol Photomed 36(6):424–432
Berman B, Grada A, Berman DK (2022) Profile of tirbanibulin for the treatment of actinic keratosis. J Clin Aesthet Dermatol 15(10 Suppl 1):S3–S10
Blauvelt A et al (2021) Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med 384(6):512–520
Kishi P, Price CJ (2018) Life-threatening reaction with topical 5-fluorouracil. Drug Saf Case Rep 5(1):4
Johnson MR et al (1999) Life-threatening toxicity in a dihydropyrimidine dehydrogenase-deficient patient after treatment with topical 5-fluorouracil. Clin Cancer Res 5(8):2006–2011
Papanastasopoulos P, Stebbing J (2014) Molecular basis of 5-fluorouracil-related toxicity: lessons from clinical practice. Anticancer Res 34(4):1531–1535
Suh SA-O et al (2020) The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol 59:1365–4632
Ortonne JP et al (2010) Effectiveness of cross polarized light and fluorescence diagnosis for detection of sub-clinical and clinical actinic keratosis during imiquimod treatment. Exp Dermatol 19(7):641–647
Baker C et al (2022) Method of Assessing Skin Cancerization and Keratoses(TM) (MASCK): development and photographic validation in multiple anatomical sites of a novel assessment tool intended for clinical evaluation of patients with extensive skin field cancerization. Clin Exp Dermatol 47(6):1144–1153
Acknowledgements
PP performed the literature search and wrote the main manuscript. PP and JW reviewed the studies independently. JW, DB, JM, and EA helped to review the manuscript. JJ helped to develop the concept and edit/review the final manuscript. All authors reviewed and approved the final manuscript.
Funding
No funding has been received for this article. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the U.S. Department of Veterans Affairs or the United States Government.
Author information
Authors and Affiliations
Contributions
PP performed the literature search and wrote the main manuscript. PP and JW reviewed the studies independently. JW, DB, JM, and EA helped to review the manuscript. JJ helped to develop the concept and edit/review the final manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patel, P., Wang, J., Bitterman, D. et al. Systematic review of randomized controlled trials of topicals for actinic keratosis field therapy. Arch Dermatol Res 316, 108 (2024). https://doi.org/10.1007/s00403-024-02839-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00403-024-02839-y