Abstract
Purpose
Although development of immune checkpoint inhibitors has revolutionized the treatment of metastatic melanoma, more than a half of treated patients experience disease progression during therapy. Cases of spontaneous vitiligo-like leukoderma have been described in melanoma patients and have been associated with a favorable outcome. This vitiligo-like leukoderma can also appear in melanoma patients undergoing immune therapies such as immune checkpoint inhibitors. However, no consensus exists about the relationship between vitiligo-like leukoderma onset and improved overall survival. Our study investigates the possible association between the onset of vitiligo-like leukoderma during immune checkpoint inhibitor treatment and a better prognosis.
Methods
A non-concurrent cohort study was conducted by identifying retrospectively 280 patients who had inoperable or metastatic melanoma and had undergone immune therapy with checkpoint inhibitors in any line of treatment. Toxicities developed during therapy were evaluated.
Results
Among the 280 study participants, 50% developed at least one type of toxicity, and vitiligo-like leukoderma was observed in 43 patients (15.4%). In the multivariate Cox model, a protective effect for mortality was observed for patients with vitiligo-like leukoderma development (HR : 0.23; 95% CI 0.11–0.44, p < 0.0001). In a sub-group analysis comprising only cutaneous melanoma in first line of treatment (N = 153), occurrence of vitiligo-like leukoderma was also an independent predictor factor for duration of clinical benefits measured by time to the next treatment (HR: 0.17; 95% CI 0.06–0.44).
Conclusion
Our findings indicate that onset of vitiligo-like leukoderma during melanoma treatment could be a marker of favorable outcome in patients treated with immune checkpoint inhibitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treatment of metastatic melanoma has undergone a remarkable evolution in the last few years, leading to the development of innovative therapeutic strategies (Maverakis et al. 2015). Target inhibitors of the mitogen-activated protein kinase pathway, both inhibitors of mutated BRAF and of the mitogen-activated protein kinase (MEK) kinase, on one hand, and the immune checkpoint inhibitors (ICI), either anti-cytotoxic T-lymphocyte antigen (CTLA)-4 (ipilimumab) or anti-programmed cell death protein 1 (PD-1) (pembrolizumab or nivolumab), on the other hand, increased the median progression-free survival (PFS) and overall survival (OS) with a portion of long-survivors (Larkin et al. 2015; Dreno et al. 2018; Robert et al. 2019a, b). Despite these encouraging results, more than half of the patients experienced disease progression during treatment. Therefore, it is essential to understand the clinical and physio-pathological features of the patients who achieve the best outcome to define the most appropriate therapeutic strategies. The main risk factor for the development of cutaneous melanoma is exposure to solar or artificial ultraviolet rays which leads to the typical mutational signature with a C > T nucleotide transition in dipyrimidine sites (León-Letelier et al. 2019). Then, other mutations occur during tumor progression, making melanoma the cancer type with the highest tumor mutational burden (TMB) (Büttner et al. 2019). Tumors with high TMB have a great probability of creating epitopes that can be presented by dendritic cells, leading to an efficient activation of immune cells (Chabanon et al. 2016). Despite this, the host immune system rarely succeeds in spontaneous melanoma clearance. However, presence of a high TMB confers a great advantage in the ICI treatment (Goodman et al. 2017), as it provides a range of antigens against which immune cells, once re-activated by ICI, can act.
In melanoma patients, spontaneous occurrence of cutaneous depigmentation or leukoderma has been reported (Failla et al. 2019). It is a clinical manifestation remarkably similar to vitiligo, an autoimmune disease characterized by the presence of autoimmune destruction of cutaneous melanocytes (Mason and Gawkrodger 2005). Antigens recognized by cytotoxic T lymphocytes nearby the leukoderma can be expressed both by normal melanocytes and by melanoma cells, thus explaining the coexistence of the neoplasm and the autoimmune response that determines skin depigmentation. Antigens recognized by T cells are generally characteristic of the melanogenesis process, such as gp100, MART-1, the tyrosinase enzyme, or the tyrosine-specific transport protein ½ (Teulings et al. 2014; Rodrigues et al. 2017). Recognition of these antigens by T lymphocytes seldom leads to regression of the cancerous lesions (Parmiani 2001).
Development of vitiligo-like leukoderma (VLL) has been described as a side-effect of immunomodulating treatments in cutaneous melanoma, starting with vaccine and interleukin (IL)-2 therapies up to modern treatments with ICI (Teulings et al. 2015). Incidence of VLL in patients treated with anti-CTLA-4 or anti-PD-1 was reported in a very variable range, from 3.4 and 28.0%, with an onset occurring on average at 30 weeks from inception of treatment (Belum et al. 2016; Hwang et al. 2019). Indeed, ICI therapy creates an imbalance in immune tolerance that may lead to uncontrolled immune reactions that manifest clinically as autoimmune events (Simeone et al. 2019). Besides VLL, other autoimmune reactions against normal melanocytes have been also observed in patients treated with ICI. These reactions target hair pigmentation (Dimitriou et al. 2020) or the retinal pigment epithelium, inducing a syndrome resembling the Vogt–Koyanagi–Harada disease (Gambichler et al. 2020). However, these reactions are rarer than VLL and only a few cases have been reported.
Recent systematic reviews focused on the evaluation of the possible link between immune-related adverse events (irAE) and clinical benefit in patients treated with ICI, analyzing retrospective studies and highlighting the association between autoimmune cutaneous toxicity and favorable outcome (Hua et al. 2016; Nakamura et al. 2017; Cortellini et al. 2019; Zitvogel et al. 2021). Regarding melanoma, a limited number of studies reported an association between development of irAE and better prognosis. This association was further confirmed only for autoimmune cutaneous effects such as VLL (Hua et al. 2016; Nakamura et al. 2017). However, the number of patients included in these studies was small, so that no conclusive evidence could be provided. The aim of our study was to investigate whether an association between the onset of VLL during immune checkpoint inhibitor treatment and a better prognosis exists.
Materials and methods
Patients
All patients at IDI-IRCCS, aged ≥ 18 years, who had inoperable or metastatic melanoma, according to the American Joint Committee on Cancer melanoma staging and classification (Gershenwald and Scolyer 2018) and who had undergone ICI therapy (anti-PD-1 or anti-CTLA-4) in any line of treatment in the last 10 years, from September 2010 to December 2019, were identified and included in the study. The protocol was approved by the IDI-IRCCS Institutional Ethical Committee (n. 510/3, 2018). Demographic and clinical information was retrieved from medical records. Histological type, tumor thickness, ulceration, and regression were recorded, according to published guidelines (Clark et al. 1969, 1989; Breslow 1970; Busam and Barnhill 2004). The variables considered were: age at diagnosis of primary melanoma, gender, site of the primary melanoma, Breslow thickness, presence of ulceration, lymph-node status, presence of regression, presence of satellitosis, age at start of ICI therapy, melanoma staging at the start of treatment, number of sites affected by secondary lesions, serum lactate dehydrogenase (LDH), BRAF mutation, specific ICI drug used, line of treatment, use of adjuvant treatment (i.e., interferon-α, ICI), and type of toxicity developed during ICI therapy. Toxicities were evaluated through clinical examination, laboratory, and radiological assessments, using common toxicity grade criteria (Cancer Institute 2017). Patients affected by vitiligo before initiation of ICI therapy were excluded from the study.
Evaluation
The primary objective was to evaluate the association between VLL onset and the risk of death. Secondary objectives were to evaluate the association between VLL development and time to next treatment (TTNT) and the best overall response in ICI as first-line treatment. TTNT was used as an endpoint, since it reflects not only the duration of treatment efficacy, but it also incorporates the patient tolerance to the therapy. Tumor response was assessed using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (Eisenhauer et al. 2009). The Lazio Region Internet Portal of Health was accessed to obtain official, standardized information on the vital status of all patients. For the overall survival analysis, we calculated the duration of follow-up for each subject as the number of days from the start of the first ICI treatment to the date of death or to 31 December 2019, whichever came first. Time-to-next treatment was calculated as the number of days from the start of the first ICI treatment to the date of the decision to change treatment because of progression, exhaustion of the clinical benefit, and/or toxicity. For the best overall response, each patient was assigned to one of the following categories: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD).
Statistical analysis
To compare demographic, histological, clinical characteristics, and overall objective response between patients who have developed VLL to those who have not developed this toxicity, the Fisher’s exact test was used for the categorical variables, while the Mann–Whitney U test was used for the continuous variables. The Fisher’s exact test was preferred, because for some variables, the expected counts were less than 5, and because of unbalanced data (group with leukoderma n = 43, group without leukoderma, n = 237) (Corcoran et al. 2005).
For the survival analysis, the Kaplan–Meier method and the log-rank test were used to compare the survival curves between different groups. Time zero corresponds to the start of the first ICI treatment. The Cox proportional-hazard model was used to investigate the association between VLL development and the risk of mortality, and it was also used to examine prognostic factors for TTNT.
For statistical analysis, the STATA software, release 15 (Stata Corp LLC, College Station, TX) was used.
Results
Two hundred and eighty patients were included in the study. The mean age of the patients at primary melanoma diagnosis was 62.9 years (standard deviation, SD = 14.9), with a median age of 65 years (interquartile range, IQR = 54–74). Most patients were males (60.7% versus 39.3%). Median follow-up time from melanoma diagnosis was 3.4 years (IQR = 1.8–6.2) for a total of 1286 person-years of follow-up. There were 144 deaths; 5- and 10-year overall survival were 56.2% and 30.8%, respectively (Table 1).
Regarding the primary melanoma, 77.9% was of skin onset (of which 44.0% of nodular type, 43.1% of superficial diffusion, and 12.8% of other histological type); 9.3% was mucosal; 3.2% was uveal and 4.3% was of unknown origin (occult melanoma). For 5.4%, no data on primary melanoma were available. The most frequent location of the primary melanoma was the trunk (35.7%), followed by lower extremities (21.1%), head and neck (18.6%), and the upper extremities (15.0%). Breslow thickness was 3.9 mm (SD = 3.2), with a median of 3 mm (IQR = 2–5). Ulceration was present in 59.6% of cases, absent in the 29.8%, unknown for a 10.6%. Regression was present only in 4.1% of cases and the presence of satellitosis in the 10.6% of cases. One hundred and two (102, 46.8%) patients had undergone lymphadenectomy, of which 59.8% were positive and 40.2% were negative for lymph-node metastasis (Table 1).
As shown in Table 2, patients at the beginning of treatment with ICI had a mean age of 66.1 years (SD = 14.1), with a median age of 68 years (IQR = 58–77). Out of the 280 patients, 84 (30.0%) were in stage M1, 51 (18.2%) in stage M1b, 97 (34.6%) in stage M1c, and 47 (16.8%) in stage M1d. Most of the patients showed the involvement of two metastatic organ sites (35.5%), followed by one (34.8%), and by three or more (29.7%). Serum LDH value was normal in 45.0% of patients and high in 26.8%. A BRAF mutation was present in 29.6% of patients (with V600E mutation in 86.7% of cases, V600K in 12.0% of cases, one case with K601E mutation), and was not present in 66.4% of patients; for 3.9% of patients, these data were not available.
As shown in Table 3, two hundred patients (71.4%) received ICI therapy as a first-line treatment, 76 (27.1%) as a second-line treatment, and only 4 patients (1.4%) as third- or fourth-line treatment. Among the patients who received ICI as a first-line therapy, 103 patients (51.5%) received nivolumab, 47 pembrolizumab (23.5%), and 50 ipilimumab (25.0%). Out of 280 patients, 248 (88.6%) did not receive any adjuvant therapy before ICI treatment. Among the 280 patients studied, 140 (50.0%) developed at least one type of toxicity. The following toxicities were observed: gastro-intestinal (19.3%); endocrinological (15.4%); VLL (15.4%); skin toxicity excluding VLL (11.4%); pulmonary (4.3%); skeletal muscle (2.5%); neurological (1.4%); cardiovascular (0.7%); hematological (0.7%); ocular (0.7%) and renal toxicity (0.4%). It is important to note that, among the 43 patients who developed VLL, 25 patients (58.1%) did not have any other toxicity. The median time of VLL onset after the start of ipilimumab, pembrolizumab, and nivolumab as a first-line treatment was 7 months (IQR = 3–14), 6 months (IQR = 4–8), and 10 months (4–17) respectively. No difference was found between ICI treatment types and onset of VLL (p = 0.713). From the start of ICI therapy, the median follow-up time was 9.9 months (ranging from 6 days to 81 months) for a total of 4651 months of follow-up.
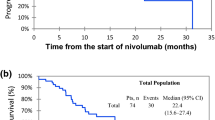
A significant survival advantage in patients who developed VLL during ICI treatment compared to those who did not develop such a toxicity was observed (log-rank test, p < 0.0001; Fig. 1A). Five-year overall survival for persons who developed VLL was 75.1% versus 24.2% for those who did not develop it. This effect was only observed for VLL and not for other toxicities, regardless of the toxicity grade. A significant poorer survival was found for patients with high LDH compared to those with normal serum LDH levels (log-rank test, p < 0.0001; Fig. 1B); with metastatic disease M1c and M1d compared to M1a–M1b (log-rank test, p < 0.0001; Fig. 2A); and in patients who did not undergo previous adjuvant treatment compared to those who did (log-rank test, p = 0.047; Fig. 2B).
Table 4 shows the results of the multivariate analysis for mortality. After controlling for sex, age, staging, LDH levels, use of adjuvant therapy, and first-line use of ICIs, VLL development was associated with reduced mortality (hazard ratio, HR: 0.32; 95% CI 0.22–0.48, p < 0.0001). The protective effect was even greater if the analysis was restricted to patients with cutaneous melanoma who underwent treatment with ICI in first line (HR: 0.24; 95% CI 0.12–0.45, p < 0.0001). For patients in which ICI was the first-line therapy and VLL was the only toxicity observed, mortality risk was 6 times lower than in patients with no toxicity (HR: 0.15; 95% CI 0.05–0.43, p < 0.0001). High serum LDH levels (HR: 2.33; 95% CI 1.51–3.60, p < 0.0001), staging (M1d versus M1a-1b) (HR: 2.25; 95% CI 1.39–3.63, p = 0.001), and use of ICIs not as a first-line treatment (HR: 1.47; 95% CI 1.02–2.13, p = 0.040), were all independently associated with an increased risk of mortality in the multivariate model, while the use of adjuvant therapy was associated with a protective effect (HR: 0.54; 95% CI 0.29–0.99, p = 0.047).
A sub-group analysis was conducted only for patients with ICIs as a first-line therapy (N = 153). The median TTNT was 4.9 months. The occurrence of VLL was also an independent predictor factor for duration of clinical benefits measured by TTNT (only VLL), HR: 0.17; 95% CI 0.06–0.44, p < 0.0001; VLL and other toxicities HR: 0.35; 95% CI 0.21–0.59, p< 0.0001. Both high LDH serum levels and advance staging were associated with shorter TTNT (Table 5).
All other factors considered in the study, such as Breslow thickness, ulceration, site of primary melanoma, presence of regression, presence of satellitosis, lymphadenectomy, BRAF status, and types of ICI used in first line (ipilimumab, pembrolizumab, or nivolumab), were not associated with mortality.
Demographic and clinical characteristics of patients who developed VLL and of the ones who do not developed it were examined (Table 6). No difference between groups were found for age, types of melanoma, anatomic site of the primary melanoma, BRAF status, use of adjuvant therapy, and type of ICI. However, statistically significant differences were observed for sex (p = 0.043) and LDH levels (p = 0.021). Most patients who developed VLL were females (53.5% versus 36.7%) and had normal serum LDH levels (80.0% versus 59.0%). For patients with ICI as first-line treatment, the best objective response was also analyzed. The rates of clinical complete responses (CR) (35.3% versus 9.1%) and partial responses (PR) (38.2% versus 28.0%) were higher in patients who developed VLL than in patients who did not have such a toxicity, while progressive disease (PD) was significantly lower in patients with VLL (5.9% versus 40.9%, p < 0.0001).
Discussion
In recent years, treatment of metastatic melanoma has undergone a sudden and encouraging evolution. Search for effective therapies to improve the prognosis of patients with inoperable melanoma has led to the development of innovative drugs, including CTLA-4 and PD-1/PD-ligand-1 (PD-L1) inhibitors, used alone or in combination. Despite these reassuring results, many patients have primary resistance to such treatments, obtaining limited benefit from these therapeutic strategies. Understanding clinical aspects related to treatment response would allow the selection of patients who would truly benefit from these therapies. The possible link between the onset of adverse events and the response to ICI treatment is one of the subjects of greatest interest in oncology, due to the hypothesis that greater toxicity would also indicate greater anti-neoplastic effect.
One of the side-effect signs that appears to be related to a better response to ICI treatment in cutaneous melanoma is VLL development. Indeed, some studies suggest the existence of a link between development of VLL and a better outcome in patients treated with ICI (Hua et al. 2016; Nakamura et al. 2017). However, these studies were conducted in a small number of patients and no multi-variate analysis was performed to control for other known prognostic factors of progression (e.g., LDH and metastatic stage). Thus, no firm conclusions could be drawn (Hua et al. 2016; Nakamura et al. 2017).
To further verify the existence of this association, we performed a retrospective analysis on 280 metastatic melanoma patients who had undergone at least one ICI treatment. Our findings confirm, after controlling for confounding factors, a significant association between VLL development and a better prognosis in terms of overall survival and TTNT that agrees with other studies published elsewhere (Hua et al. 2016; Nakamura et al. 2017). The effect was even stronger for patients in which no other toxicity, other than VLL. The latter finding could be related to a probable greater use of high-dose corticosteroids in patients who developed other autoimmune toxicities with consequent partial anti-inflammatory and immunosuppressive effects, as well as to the higher probability of discontinuing treatment in the presence of other toxicities. Indeed, VLL development itself is devoid of clinical significance, except for its psychological impact that could alter patient quality of life.
In our study, 43 out of 280 patients (15.4%), developed VLL which is lower in comparison with other studies (Hua et al. 2016; Nakamura et al. 2017). In our study, the median time of VLL onset after the start of ipilimumab, pembrolizumab, and nivolumab as a first-line treatment was 7 months (IQR = 3–14), 6 months (IQR = 4–8), and 10 months (4–17), respectively, which is in agreement with a previous study (Babai et al. 2020).
Our data show that complete objective response was reached by 35.3% of the patients who developed VLL, while in the study by Nakamura et al. (2017), conducted on 35 unresectable stage III or IV melanoma patients treated with nivolumab, 9 patients (25.7%) developed VLL and objective response was 22.2% among patients with VLL. Hua et al. (2016) conducted a study on 67 unresectable stage III or IV melanoma patients treated with pembrolizumab. During the treatment with pembrolizumab, VLL. appeared in 17 (25%) patients and complete objective response was 18% among patients who developed VLL. The median overall survival was 18.2 months in the group of patients that developed VLL compared to 8.5 months in the group of non-VLL. In our study, median overall survival in the group of patients that developed VLL. was 34.0 months vs 8 months in the group of non-VLL.
It is interesting to note that VLL, or at least nevus lightening, was reported in melanoma patients treated with BRAF/MEK inhibitors (Zhao et al. 2018). Since a role for BRAF/MEK inhibition in the activation of the immune system was hypothesized (Smalley 2020), there is the possibility that a higher infiltration of activated T cells in the melanoma lesion would lead, in genetically predisposed patients treated with this target therapy, to the development of VLL. In either case, VLL development could be considered as a sign of active immune responses in the treated patients. Nevertheless, the number of reported cases is too small to associate VLL development with a positive response to treatment with BRAF/MEK inhibitors or with a better overall survival.
LDH within normal limits, M1a and/or M1b disease, and a previous adjuvant treatment, remained independent factors associated with a better prognosis in terms of survival, in line with the literature data (Keung et al. 2020). The analysis of patients' clinical characteristics showed an association with gender, disease burden, metastatic sites, and the likelihood of VLL onset. Patients of female sex, with metastatic disease involving only skin, lymph nodes or lungs, and/or with serum LDH within the normal limits, were more likely to develop VLL. This evidence could be explained by the fact that a greater aggressiveness of tumor disease, and the presence of visceral and/or encephalic metastases, could underlie a lower ability to develop an effective immune reaction. It is known that some metastatic niches, such as those in the liver and brain, have a greater ability to evade the immune response due to a more immunosuppressive environment (Tumeh et al. 2017; Di Giacomo et al. 2019). Regarding gender, female sex is known to be associated with the ability to develop a more efficient immune response (Klein and Flanagan 2016). A higher probability of longer progression-free survival in female patients was also reported in a recent multicenter study that analyzed clinical and biological features of melanoma patients who developed VLL after ICI treatment (Guida et al. 2021). Differently from our present study, the recent paper from Guida et al. (2021) did not match the VLL patient subset with one without VLL and could not obtain conclusive data about significance of VLL development in the entire population.
It is important to note that this is the largest observational study conducted so far that shows an independent association between VLL development and better prognosis measured by objective overall survival, overall response, and TTNT.
In conclusion, our study confirms, on a large patient cohort of advanced cutaneous melanoma, that VLL development as an ICI toxicity is an independent marker of a more favorable prognosis. Therefore, VLL onset should be considered as a sign of positive response to ICI treatment. Future studies of the biological mechanisms and of the actual causal chain leading to VLL development would clarify the different degrees of response to ICI treatment, possibly leading to an optimization of the therapeutic strategies.
Availability of data and materials
All relevant data are within the papers.
Abbreviations
- MEK:
-
Mitogen-activated protein kinase
- ICI:
-
Immune checkpoint inhibitors
- CTLA-4:
-
Cytotoxic T-lymphocyte antigen-4
- PD-1:
-
Programmed cell death protein 1
- PFS:
-
Progression free survival
- OS:
-
Overall survival
- TMB:
-
Tumor mutational burden
- VLL:
-
Vitiligo-like leukoderma
- IL:
-
Interleukin
- irAE:
-
Immune-related adverse events
- LDH:
-
Lactate dehydrogenase
- TTNT:
-
Time-to-next treatment
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- CR:
-
Complete response
- PR:
-
Partial response
- SD:
-
Stable disease
- PD:
-
Progressive disease
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- HR :
-
Hazard ratio
- PD-L1:
-
PD-ligand-1
References
Babai S, Voisin AL, Bertin C et al (2020) Occurrences and outcomes of immune checkpoint inhibitors-induced vitiligo in cancer patients: a retrospective cohort study. Drug Saf 43:111–117. https://doi.org/10.1007/s40264-019-00875-6
Belum VR, Benhuri B, Postow MA et al (2016) Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer 60:12–25. https://doi.org/10.1016/j.ejca.2016.02.010
Barnhill RL, Piepkorn M, Busam KJ (2004) Melanocytes. In: Pathology of melanocytic nevi and malignant melanoma Springer: New York, pp 1–10
Breslow A (1970) Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg 172:902–908. https://doi.org/10.1097/00000658-197011000-00017
Büttner R, Longshore JW, López-Ríos F et al (2019) Implementing TMB measurement in clinical practice: considerations on assay requirements. ESMO Open 4:e000442
Cancer Institute N (2017) Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm
Chabanon RM, Pedrero M, Lefebvre C et al (2016) Mutational landscape and sensitivity to immune checkpoint blockers. Clin Cancer Res 22:4309–4321
Clark WH, From L, Bernardino EA, Mihm MC (1969) The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res 29:705–727
Clark WH, Elder DE, Guerry D et al (1989) Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst 81:1893–1904. https://doi.org/10.1093/jnci/81.24.1893
Corcoran CD, Senchaudhuri P, Mehta CR, Patel NR (2005) Exact inference for categorical data. Encycl Biostat. https://doi.org/10.1002/0470011815.b2a10019
Cortellini A, Buti S, Agostinelli V, Bersanelli M (2019) A systematic review on the emerging association between the occurrence of immune-related adverse events and clinical outcomes with checkpoint inhibitors in advanced cancer patients. Semin Oncol 46:362–371
Di Giacomo AM, Valente M, Cerase A et al (2019) Immunotherapy of brain metastases: breaking a “dogma.” J Exp Clin Cancer Res 38:419
Dimitriou F, Mangana J, Dummer R (2020) Hair depigmentation and hair loss in advanced melanoma treated with combined immunotherapy and targeted therapy. Acta Derm Venereol 100:1–2. https://doi.org/10.2340/00015555-3355
Dreno B, Ascierto PA, McArthur GA et al (2018) Efficacy and safety of cobimetinib (C) combined with vemurafenib (V) in patients (pts) with BRAF V600 mutation–positive metastatic melanoma: analysis from the 4-year extended follow-up of the phase 3 coBRIM study. J Clin Oncol 36:9522–9522. https://doi.org/10.1200/jco.2018.36.15_suppl.9522
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Failla CM, Carbone ML, Fortes C et al (2019) Melanoma and vitiligo: in good company. Int J Mol Sci. https://doi.org/10.3390/ijms20225731
Gambichler T, Seifert C, Lehmann M et al (2020) Concurrent Vogt-Koyanagi-Harada disease and impressive response to immune checkpoint blockade in metastatic melanoma. Immunotherapy 12:439–444. https://doi.org/10.2217/imt-2019-0206
Gershenwald JE, Scolyer RA (2018) Melanoma Staging: American Joint Committee on Cancer (AJCC) 8th Edition and Beyond. Ann Surg Oncol 25:2105–2110
Goodman AM, Kato S, Bazhenova L et al (2017) Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 16:2598–2608. https://doi.org/10.1158/1535-7163.MCT-17-0386
Guida M, Strippoli S, Maule M et al (2021) Immune checkpoint inhibitor associated vitiligo and its impact on survival in patients with metastatic melanoma: an Italian Melanoma Intergroup study. ESMO Open 6:100064. https://doi.org/10.1016/j.esmoop.2021.100064
Hua C, Boussemart L, Mateus C et al (2016) Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 152:45–51. https://doi.org/10.1001/jamadermatol.2015.2707
Hwang SJE, Park JJW, Wakade D et al (2019) Cutaneous adverse events of anti-programmed death 1 antibodies combined with anti-cytotoxic T-lymphocyte-Associated protein 4 therapy use in patients with metastatic melanoma. Melanoma Res 29:172–177. https://doi.org/10.1097/CMR.0000000000000518
Keung EZ, & Gershenwald JE, (2020) Clinicopathological Features, Staging, and Current Approaches to Treatment in High-Risk Resectable Melanoma, J. Natl. Cancer Inst 112(9):875–885. https://doi.org/10.1093/jnci/djaa012
Klein SL, Flanagan KL (2016) Sex differences in immune responses. Nat Rev Immunol 16:626–638
Larkin J, Chiarion-Sileni V, Gonzalez R et al (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:23–34. https://doi.org/10.1056/nejmoa1504030
León-Letelier RA, Bonifaz LC, Fuentes-Pananá EM (2019) OMIC signatures to understand cancer immunosurveillance and immunoediting: melanoma and immune cells interplay in immunotherapy. J Leukoc Biol 105:915–933
Mason CP, Gawkrodger DJ (2005) Vitiligo presentation in adults. Clin Exp Dermatol 30:344–345. https://doi.org/10.1111/j.1365-2230.2005.01779.x
Maverakis E, Cornelius LA, Bowen GM et al (2015) Metastatic melanoma—a review of current and future treatment options. Acta Derm Venereol 95:516–524
Nakamura Y, Tanaka R, Asami Y et al (2017) Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol 44:117–122. https://doi.org/10.1111/1346-8138.13520
Parmiani G (2001) Melanoma antigens and their recognition by T cells. Keio J Med 50:86–90
Robert C, Grob JJ, Stroyakovskiy D et al (2019a) Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 381:626–636. https://doi.org/10.1056/nejmoa1904059
Robert C, Ribas A, Schachter J et al (2019b) Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 20:1239–1251. https://doi.org/10.1016/S1470-2045(19)30388-2
Rodrigues M, Ezzedine K, Hamzavi I et al (2017) New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol 77:1–13. https://doi.org/10.1016/j.jaad.2016.10.048
Simeone E, Grimaldi AM, Festino L et al (2019) Immunotherapy in metastatic melanoma: a novel scenario of new toxicities and their management. Melanoma Manag 6:MMT30. https://doi.org/10.2217/mmt-2019-0005
Smalley KSM (2020) Two worlds collide: Unraveling the role of the immune system in Braf–Mek inhibitor responses. Cancer Discov 10:176–178. https://doi.org/10.1158/2159-8290.CD-19-1441
Teulings HE, Willemsen KJ, Glykofridis I et al (2014) The antibody response against MART-1 differs in patients with melanoma-associated leucoderma and vitiligo. Pigment Cell Melanoma Res 27:1086–1096. https://doi.org/10.1111/pcmr.12294
Teulings HE, Limpens J, Jansen SN et al (2015) Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol 33:773–781. https://doi.org/10.1200/JCO.2014.57.4756
Tumeh PC, Hellmann MD, Hamid O et al (2017) Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res 5:417–424. https://doi.org/10.1158/2326-6066.CIR-16-0325
Zhao CY, Chou S, Liu RC, Fernandez-Peñas P (2018) Naevus lightening in melanoma patients under BRAF/MEK inhibitor combination therapy versus checkpoint immunotherapy: a histological and immunohistochemistry analysis. Pigment Cell Melanoma Res 31:341–344. https://doi.org/10.1111/pcmr.12669
Zitvogel L, Perreault C, Finn OJ, Kroemer G (2021) Beneficial autoimmunity improves cancer prognosis. Nat Rev Clin Oncol 18:591–602. https://doi.org/10.1038/s41571-021-00508-x
Acknowledgements
This project was funded, in part, with grants from the “Progetto Ricerca Corrente”, RC20_4.1, of the Italian Ministry of Health. MLC is the recipient of a Young Investigator Fellowship from Fondazione Umberto Veronesi. Authors would like to acknowledge the efforts and care of the Oncology Department nurse team, and the contribution and compliance of the patients.
Funding
This project was funded, in part, with grants from the “Progetto Ricerca Corrente”, RC20_4.1, of the Italian Ministry of Health. MLC is the recipient of a Young Investigator Fellowship from Fondazione Umberto Veronesi.
Author information
Authors and Affiliations
Contributions
Conceptualization: SD, FDG, CMF, and CF; data collection and management: SV, FRDP, SM, MLC, RM, and FMM; formal analysis: SV, FRDP, SM, and MLC; methodology: SV, FRDP, SM, MLC, and CF; validation: SM, CF; writing original draft: SV, FRDP, MLC, CMF, and CF; writing review and editing: DA, RM, FMM, SD, PM, FDG, CMF, and CF; funding acquisition: CMF, CF, DA, FDG, and PM.
Corresponding author
Ethics declarations
Conflicts of interest
No competing interests.
Ethics approval and consent to participate
The protocol was approved by the IDI-IRCCS Institutional Ethical Committee (n. 510/3, 2018).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Verkhovskaia, S., Di Pietro, F.R., Mastroeni, S. et al. Vitiligo-like leukoderma as an indicator of clinical response to immune checkpoint inhibitors in late-stage melanoma patients. J Cancer Res Clin Oncol 148, 2529–2538 (2022). https://doi.org/10.1007/s00432-021-03811-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03811-3