Abstract
Inherited ichthyoses are a group of etiologically heterogeneous diseases that affect the function of the skin and that are classified as syndromic and non-syndromic entities. Irrespective of the type, all these disorders are generally produced by mutations in genes involved in a variety of cellular functions in the skin. These mutations lead to disruption of the stratum corneum and impairment of the skin barrier, producing clinical features such as hyperkeratosis, skin scaling, erythema, fissures, pruritus, inflammation, and skin pain. Despite advances in the knowledge of the pathogenesis of ichthyoses, there is, to our knowledge, no definitive cure for skin manifestations, and current treatments consist of moisturizers, emollients, and keratolytic agents. In this respect, the development of new formulations based on nanotechnology could be useful to enhance their therapeutic effectiveness. In this article, we provide a comprehensive description of pharmacological treatments for cutaneous manifestations in patients with inherited ichthyosis and discuss novel approaches with therapeutic potential for this purpose. Moreover, we offer an overview of toxicity concerns related to these treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inherited ichthyoses comprise a group of etiologically heterogeneous disorders that affect the function of the skin with variable clinical harshness. These are generally classified into two large types: syndromic ichthyoses and non-syndromic forms [1]. Syndromic ichthyoses, in addition to affecting the skin, cause other disorders, such as endocrine, muscular, neurological, and hair or nail abnormalities. These types of ichthyoses have relatively low prevalence in general population, and representative syndromes described in the literature include Netherton syndrome and Sjögren–Larsson syndrome [2]. On the other hand, non-syndromic entities are more frequent and produce clinical features solely observed in the skin. The major non-syndromic ichthyoses include recessive X-linked ichthyosis (RXLI), ichthyosis vulgaris (IV), keratinopathic ichthyosis (KPI), and autosomal recessive congenital ichthyosis (ARCI) [3].
Irrespective of the type, ichthyoses are commonly caused by mutations in genes relevant for diverse cellular functions in the skin, such as lipid biosynthesis, adhesion, normal desquamation, and DNA repair [1]. The main functional consequences of these genetic variants include the disruption of stratum corneum, impairment of skin-barrier integrity, and an increase in transepidermal water loss (TEWL), leading to clinical features such as hyperkeratosis, skin scaling, erythema, fissures, pruritus, inflammation, and skin pain.
Although there has been increasing progress in the knowledge of the pathophysiology of ichthyoses, currently there are no available specific therapies for the skin symptoms of these ichthyoses; however, supportive therapies consisting of topical formulations and systemic compounds have shown beneficial effects in clinical practice [4]. Topical treatments mainly consist of emollients, moisturizers, and keratolytic agents, while systemic treatments are primarily based on retinoids. In addition, some recent clinical trials have reported promising results with other compounds [5], and advances in the development of nanocarriers for topical use could help to improve their therapeutic efficacy.
In this article, we provide a comprehensive description of pharmacological treatments for cutaneous manifestations in patients with inherited ichthyosis and discuss novel approaches with therapeutic potential for this purpose. Moreover, we offer an overview of toxicity concerns related to these treatments.
Pathophysiology of ichthyosis
The skin exerts a pivotal function as a permeability barrier that prevents TEWL and protects against all kinds of noxious exogenous factors. The stratum corneum is the pivotal component of this barrier; it is constituted of corneocytes and lipids derived from keratinocytes, which are linked by corneodesmosomes [6]. In this respect, the normal function of this epidermal coat is mainly sustained by a constant renovation of keratinocytes, which undergo differentiation when they migrate from the stratum basale to the stratum corneum. This process of differentiation is characterized in the suprabasal layers by the synthesis and modification of proteins, lipids, and other components essential for the maintenance and function of the lipid layer [6]. These components include keratins, which are responsible for the integrity of keratinocytes, as well as cholesterol, phospholipids, glycosylceramides, peptides, enzymes, and free fatty acids, which are packed into lamellar bodies in the stratum spinosum. The lamellar bodies are biological structures that store the components and enzymes necessary for constituting the lipid layer; these components are released into the intercellular space, at the passage from the stratum granulosum into the stratum corneum, where they form lamellar structures that constitute the hydrophobic coat of the skin [6]. The final result of this differentiation program is the formation of a layer composed of corneocytes and inter-keratinocyte lipids in the stratum corneum. Thus, mutations in genes coding for proteins indispensable for the establishment of this barrier negatively affect the normal keratinization process and stratum corneum homeostasis, leading to the impairment of skin barrier integrity and producing ichthyosis (Fig. 1).
However, although typically all ichthyoses cause abnormalities in the skin, depending on the specific mutation involved, there are some differences in the molecular processes of epidermal injury [4].
In this regard, genes involved in syndromic forms include the serine protease inhibitor of Kazal-type 5 (SPINK5) gene and the fatty aldehyde dehydrogenase (FALDH) gene [7, 8]. The SPINK5 gene encodes for the lympho-epithelial Kazal-type-related inhibitor (LEKTI), and mutations in this gene give rise to the Netherton syndrome, which is characterized by cutaneous and non-cutaneous features, including thinning of the stratum corneum and the granular layer, eczematous atopic dermatitis, urticaria, angioedema, food allergies, malnutrition, growth retardation, pancreatitis, pulmonary hypertension, systemic infections, and hypothyroidism [7, 9]. SPINK5 gene mutations lead to the dysfunction of LEKTI and the increased proteolytic activity of kallikreins, producing defective epidermal cell–cell adhesion, skin inflammation and affecting skin-barrier formation and physiological desquamation processes [9]. Likewise, it has been shown that FALDH gene mutations are associated with the Sjögren–Larsson syndrome [8]. Patients with this syndromic ichthyosis present congenital ichthyosiform exanthema, gait disorder, spastic paralysis of the limbs, and mental weakness. The FALDH gene encodes an enzyme located in the microsomes, which is required for oxidizing long-chain fatty aldehyde into fatty acids. Mutations in this gene produce decreased activity of the FALDH enzyme; thus, the pathogenesis of the Sjögren–Larsson syndrome could be related to the tissue accumulation of fatty aldehyde, fatty alcohol, and other lipid metabolism products [2, 8].
On the other hand, the transglutaminase-1 (TGM1) gene, the ceramide synthase-3 (CERS3) gene, and the ATP-binding cassette subfamily A member 12 (ABCA12) gene are genes linked to non-syndromic ichthyosis. The TGM1 gene encodes for an enzyme that participates in the formation of the cornified cell envelope, which is a structure that surrounds the corneocytes and aids in providing stability and strength to the epidermis. It has been suggested that mutations in the TGM1 gene produce low enzymatic activity of the protein encoded, affecting the assembly of the cornified cell envelope and leading to dysregulation of the epidermal-barrier function [10]. Similarly, the protein product of the CERS3 gene is a ceramide synthase enzyme that regulates the synthesis of ultra-long-chain ceramides, which helps to create a protective barrier in the epidermis. Pathogenic variants in the CERS3 gene inactivate the enzyme product and produce a specific loss of long-chain ceramides in differentiating keratinocytes, which would lead to the impairment of epidermal differentiation and the harmful alteration of lipid homeostasis of the permeability barrier [11]. Furthermore, the ABCA12 gene encodes a protein involved in lipid transport to the stratum corneum via lamellar bodies. Therefore, mutations in the ABCA12 gene may lead to defects in lipid transfer to the upper intercellular layers and to the lack of the proteolytic enzymes needed for corneocytes desquamation, producing abnormalities in the architecture of the stratum corneum [12].
In summary, genetic mutations in genes coding for proteins indispensable for the establishment of the permeability barrier negatively affect the normal keratinization process and stratum corneum homeostasis, leading to the impairment of skin integrity and producing the clinical symptoms of inherited ichthyoses.
Topical treatments
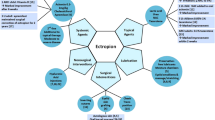
The treatment of the cutaneous manifestations of ichthyoses depends on the type and severity of symptoms of each patient. This is one of the reasons for which there is no definitive treatment for this condition. However, the majority of patients require increasing skin hydration and lubrication, to improve desquamation, to reduce TEWL and, in general, to restore skin function. For this purpose, diverse topical keratolytics and emollients have been used. For instance, sodium chloride, urea, and glycerol have been successfully applied for improving hydration [5, 13, 14], whereas salicylic acid, alpha-hydroxy acids (AHA) such as lactic and glycolic acid, and urea (> 5%) have been widely explored as keratolytic agents [5, 15]. In addition, retinoids have been used as treatment due to their capability to normalize the abnormal growth and proliferation of keratinocytes [16, 17]. Moreover, occlusive agents such as lanolin or petrolatum have been applied to block or reduce the TEWL. The most common agents employed as treatment will be briefly described here (see Fig. 2).
Urea
Urea is an essential component of the natural moisturizing factor, which is also constituted of a mixture of humectants present in the stratum corneum. It has been reported that the presence of urea helps to preserve the physical properties of hydrated lipids systems under dry conditions [18]. On the other hand, it has been described that urea stimulates the synthesis of cornified envelope proteins, such as involucrin and filaggrin, the latter of which is highly involved in the pathogenesis of ichthyosis [19, 20]. Thereby, urea acts as humectant agent, increasing the water content in the stratum corneum, and reducing the proliferation of epidermal cells. In addition to a humectant effect, it has been reported that urea also presents antimicrobial and barrier-regenerating effects. Additionally, urea retains the fluidity of the stratum corneum, facilitating the penetration of other active ingredients. However, despite that urea represents an interesting option for the treatment of ichthyosis due to its excellent properties, it is not a popular choice for children due to its consistency and texture. Thus, new formulations based on urea lotion have been developed. Furthermore, in order to improve its therapeutic efficacy, urea may be combined with other ingredients such as sodium chloride, lactic acid, or retinoic acid. In this respect, Tadini et al. [21] evaluated a 10% urea-based lotion as treatment for ichthyosis twice daily for 4 weeks. The authors compared the efficiency of this treatment with that of a standard emollient cream based on glycerol paraffin. The authors reported that the urea-based lotion improved skin hydration and reduced skin defects, such as scaling, fissures, and excessive redness. In addition, the urea-based lotion presented better behavior compared with the emollient cream utilized as control. The results of these authors suggested that the urea treatment presented a moisturizing effect and increased the expression of filaggrin [22]. In the same way, Küster et al. [23] evaluated the moisturizing capacity, keratolytic activity, and acceptance of Laceran lotion with urea (10%) and urea-free Laceran as a control, in 60 children during 8 weeks. The authors’ results showed that the lotion base possesses an inferior effect as compared with that presented using the 10% urea treatment With respect to safety, distinct topical formulations, such as creams, emulsions, or foams, have been employed with the addition of urea at different concentrations without adverse results being reported [24]. However, the urea concentration in these formulations has been related to its therapeutic action. For example, topical formulations with ≤ 10% of urea presented a moisturizing effect, whereas for keratolytic action, formulations with ≥ 10% of urea were more effective.
Alpha-hydroxy acids
AHA, such as lactic acid and glycolic acid, have keratolytic activity; these compounds are used as treatment to decrease accumulated scales in patients with ichthyosis. However, these could produce irritation and erythema and their use on areas exposed to ultraviolet light is limited [25]. Lactic acid is a moisturizer agent that presents more hygroscopic action that glycerol and urea [4]. The therapeutic efficacy of lactic acid is due to its capacity to abate intracellular junctions in the stratum corneum and to promote skin-cell turnover. Usually, the maximal concentration of this component in formulations is 12%; due to that at higher concentrations, it could cause metabolic acidosis [16, 25]. Thus, treatment with lactic acid is usually combined with other agents to enhance formulation effectiveness. For example, Ganemo et al. [15] compared the efficiency of a formulation based on fatty cream (Locobase®) containing 5% lactic acid and 20% propylene glycol vs. 5% urea in Locobase®, 20% propylene glycol in Locobase®, and the combination of 5% lactic acid and 20% propylene glycol in Essex® cream. The authors studied 20 patients with lamellar ichthyosis and observed remarkable improvement of the skin barrier with the two formulations containing 5% lactic acid and 20% propylene glycol. It should be mentioned that, based on their findings, the authors recommended an initial treatment with lactic acid and propylene glycol applied twice daily to reduce hyperkeratosis and then combined with a barrier-repair cream.
On the other hand, formulations with combinations of glycolic acid and other components have been analyzed in several studies. In this regard, Khalil et al. [26] evaluated the changes in disease severity of 15 patients with congenital ichthyosis after a treatment based on glycolic acid (10–20%), cream, and a combination of lovastatin cream (2%), and cholesterol (2%), during 3 months. These authors reported a reduction in severity at 2 and 3 months, with a decrease in percentage of 33.7% and 57.5%, respectively.
Salicylic acid
Salicylic acid is a beta-hydroxy acid (BHA) with keratolytic and comedolytic effects as a result of its lipophilic structure. It has been employed in several skin products, such as cosmetics and products for treating dry skin, acne, and psoriasis, among others. It has been proposed that the keratolytic effect of salicylic acid is based on the reduction of the intracellular cohesion of the corneocytes [27, 28]. Furthermore, salicylic acid can decrease the pH of the stratum corneum, promoting the increase of the hydration and softness of the skin. Bashir et al. [27] evaluated the skin desquamation produced by salicylic acid; these authors changed the pH of the salicylic acid formulations and found that efficacy was not modified by this parameter. However, local dermal toxicity was increased in the acidic pH. Nevertheless, the use of salicylic acid is recommended neither as treatment for neonates and infants, nor as a whole-body treatment due to the metabolic acidosis that it could produce.
Dexpanthenol
This drug is an alcoholic analog of vitamin B5 (pantothenic acid), which is converted into a component of coenzyme A (CoA) when it is absorbed by the skin. Formulations with dexpanthenol have been applied to improve the hydration of the skin in skin disorders, particularly in the stratum corneum, and also to reduce the TEWL [29]. In 2002, Proksch et al. [30] tested skin-barrier repair after a dexpanthenol cream treatment (containing 5% dexpanthenol) and a dexpanthenol-free cream (placebo) in 20 subjects with skin injury induced by sodium lauryl sulphate. The authors found that dexpanthenol cream treatment significantly increased hydration in the stratum corneum compared to placebo. Moreover, the dexpanthenol 5% cream enhanced skin-barrier repair and reduced skin redness. Similarly, Biro et al. [31] compared the effects of Bepanthol® (5% dexpanthenol) and its placebo. These authors reported that the hydration of the stratum corneum remained stable in the sites treated with dexpanthenol 5%, whereas hydration decreased at the sites with placebo treatment. It is noteworthy that the efficiency of dexpanthenol as skin repairer could be related with the gene modulation by this component. In this regard, it has been shown that dexpanthenol promotes the expression of some interleukins (IL-6 and IL-8) and chemokines (CCL2 and CXCL1), which may contribute to the skin-repair properties of this compound [32].
Retinoids
The retinoid family comprises vitamin A (retinol) and its natural and synthetic derivatives. The effects of the retinoids on the skin have been extensively studied for many years and it has been shown that these stimulate the synthesis of procollagen, increase the fibroblast population, inhibit enzymes such as matrix-degrading metalloproteinases, facilitate desquamation, thin the stratum corneum, and possess anti-inflammatory properties [33]. These characteristics render the retinoids an excellent option for the management of ichthyosis. In this respect, tretinoin is one of the most employed retinoids; however, it presents some adverse events, such as burning, stinging, peeling, redness, and dryness of the skin. In addition, it may produce birth defects in newborns, such as cranial abnormalities if used during pregnancy. For these reasons, in recent years, researches have focused on looking for alternative compounds such as adapalene and tazarotene. Adapalene is a third-generation retinoid derived from naphthoic acid [34, 35]. This synthetic retinoid analogue modulates cellular keratinization and the anti-inflammatory process [35, 36], and it has been reported that its application is effective in the management of acne, photoaging, and psoriasis. With respect to side effects, Verschoore et al. [37] compared skin irritation after treatments with tretinoin (0.025%) and adapalene (0.1%) gels. The authors found that adapalene gels were only slightly irritating, whereas the tretinoin gel produced strong irritation reactions in the majority of subjects. The possible therapeutic usefulness of adapalene was explored by Ogawa et al. [36], who analyzed the progression of a topical adapalene based-treatment in a 14-year-old male patient with epidermolytic ichthyosis. These authors reported that, after 6 months of treatment, the patient’s facial skin became smother. These results suggested that adapalene could be applied as an effective keratolytic treatment, with fewer harmful effects than classic retinoids. On the other hand, tazarotene is a member of a novel class of retinoids, the acetylenic retinoids, which has been used as treatment for different skin disorders [38, 39]. These retinoids have been applied in patients who present ectropion, which is a complication associated with certain subtypes of ichthyosis and that trigger medical and cosmetic consequences such as keratitis, conjunctivitis, and ephiphoria. Craiglow et al. [40] reported a case of a 77-year-old woman presenting disorders of keratinization and bilateral lower-eyelid ectropion. The treatment was based on the daily application of tazarotene 0.1% cream. After 2 weeks of treatment, the patient showed improvement in bilateral eyelid ectropion. Similarly, Behera et al. [41] evaluated the effect of application of tazarotene 0.1% gel twice daily for a case of lamellar ichthyosis in a 6-month-old male. Prior to that, the child was under oral acitretin treatment during 4 months, but he developed pseudoainhum during this time. After the usage of tazarotene, the authors observed a complete resolution of the pseudoainhum condition. However, as an adverse effect, tazarotene could induce local irritation, burning, erythema, and contact dermatitis, depending on the concentration and frequency of administration.
Propylene glycol
It has been demonstrated that propylene glycol is a potent skin-penetration enhancer and that, at certain concentrations, it can induce keratolysis. Keratolytic effects have been related to conformational changes of α-keratin and other keratinized proteins of the stratum corneum. The authors mentioned that these keratinized proteins changed from α-helix into β-sheet [42]. Therefore, treatments based on propylene glycol may be effective in cases of hyperkeratosis [43]. It should be noted that formulations with propylene glycol are often in combination with another excipient. For example, when propylene glycol is combined with salicylic acid, it is an effective treatment for hyperkeratotic disorders [44]. However, its use (on topical, oral, and intravenous administration) has been related to toxic effects, including central nervous system toxicity, hemolysis, hyperosmolarity [45, 46], arrhythmia, lactic acidosis, and eczematous skin reactions [47].
Glycerol
Glycerol is a hygroscopic compound capable of absorbing water from the environment, as well as from deeper parts of the stratum corneum. This compound presents important pharmacological properties, such as hydration of the stratum corneum, a keratolytic effect, and a protective function against irritations. In addition, it presents a desmolytic effect, causing a decrease in intraepidermal pressure on the intracellular lipids and triggering an increase in the liquid crystalline state of lipids [4, 48]. Additionally, it stimulates the gene expression of aquaporines AQP3 and AQP10, which play a significant role in water homeostasis [20]. Glycerol has been used for several years in topical dermatological formulations. Blanchet-Bardon et al. [19] evaluated the efficacy of Dexeryl® (an emollient cream containing glycerol 15% and paraffin 10%) as treatment for non-bullous forms of ichthyosis in subjects under the age of 18 years. The test consisted of the application of Dexeryl® and a placebo (Dexeryl® excipients only). The study demonstrated that the excipients alone exhibited lower therapeutic action compared with the results of the glycerol and paraffin mixture. Likewise, Lodén et al. [49] studied the effect of a cream containing 20% glycerol on skin hydration and the skin-barrier function, compared with its placebo cream. After 10 days of treatment, the patients’ results suggested that glycerin significantly increased skin hydration and restored the epidermal barrier.
Systemic treatments
As mentioned previously, ichthyosis therapy could involve the combination of hydration, lubrication, and keratolytic agents. Thus, in some cases, systemic treatments combined with topical therapy are needed. Systemic therapy is usually based on oral retinoids such as etretinate and acitretin. Etretinate is an aromatic retinoid that is slowly eliminated from the body (approximately after 120 days), whereas acitretin is a metabolite of etretinate and has a half-life of 2 days in humans [50]. It has been observed that patients with ichthyosis show phenotypic improvement with systemic retinoids. Usually, at the beginning of the treatment, a low dosage of the retinoid is prescribed, which can be modified according to the satisfactory benefit observed. Virtanen et al. [51] evaluated retinoid therapy (topical and oral) in patients with epidermolytic ichthyosis. These authors applied, as topical retinoid therapy, 0.05% tretinoin (Aberela®, Janssen-Cilag) and 0.05% tazarotene (Zorac®, Allergan) and, as oral retinoid therapy, 5–25 mg/day of acitretin (Neotigason®, HoOEman-LaRoche). The authors found greatest improvement with treatments based on both topical and oral retinoid therapy. However, the oral use of these retinoids could trigger severe dryness of the skin and mucous membranes, joint pain, and epidermal fragility, among others. In particular, the major side effect of the acitretin use is teratogenicity.
In another study conducted by Vahlquist et al. [52], the efficacy of oral liarozole in the treatment of patients with lamellar ichthyosis was determined by a phase II/III trial. The study included 64 patients, who were administered once-daily oral liarozole (75 or 150 mg) or placebo. The results of these authors demonstrated a decrease in the overall severity of ichthyosis and scaling, but a therapeutic effect on erythema or pruritus was not found. Interestingly, the efficacy of oral liarozole 150 mg a day corresponds to that reported in another clinical trial by Verfaille et al. [53]. Other studies that have been classified as well-executed have involved a very small number of patients (< 20); thus, their results lack representativeness [5]. Figure 3 presents different topical and systemic approaches based on the patients’ needs.
Toxicity hazards
Since regardless of the etiology of the ichthyosis the barrier function of the skin is compromised, pharmaceutical treatments involve the administration of dermal systems on damaged skin. In this regard, approaches such as baths with salts or mild cleansers, petrolatum-based emollients, and topical formulations, followed by adherent wraps or gauzes, are frequently applied. These latter are mainly directed toward maintaining the normal humidified, healthy, and temperature-controlled state of the skin [54], in order to reduce TEWL and heat loss, to remove cellular turnover, or to avoid infections.
Although topical treatments are designed to improve skin-barrier properties, different reports highlight the high risk of the systemic absorption of the components of these formulations, due to the large areas of altered skin that are exposed and susceptible to injury from even minor trauma. However, to our knowledge, few findings have been described or no toxicological studies have been performed to date in patients with ichthyosis [55]. Therefore, only reports of adverse events after the administration of commercial or test formulas have been reported.
To minimize toxicity risks, the employment of bland emollients appears to be a good choice, because they create an occlusive lipid layer that minimizes the signs of the disease, whereas it is recommended to reduce or avoid the use of silver sulfadiazine, lactic acid, and salicylic acid preparations, and antibacterial soaps [56]. The use of silver topical cream with sulfadiazine as an antibacterial agent was evaluated in children with burns and scalds, with a mean area of injury of 13%. The study found neutropenia and erythema multiforme rash due to drug sensitivity, while absorption studies revealed a mean urinary concentration of sulfadiazine of 31.8 mg/l, indicating high systemic absorption of the drug [4]. Likewise, lactic acid is a great moisturizer and amphiphilic cream bases with concentrations of 5% have shown good efficacy in reducing hyperkeratosis. However, topical preparations have been associated with the development of lactic acidosis in patients with severe skin impairment [57] and their use should be also avoided in newborns [4]. On the other hand, intoxication with topical salicylic acid has been more frequently reported in the literature [55, 58]. It has been used topically as a keratolytic, bacteriostatic, fungicidal, and photoprotector. Nevertheless, high blood concentrations of this drug are dangerous, and it has been reported that concentrations greater than 35 mg/dl may produce the salicylic acid toxicity (salicylism) syndrome, which gives rise to symptoms such as nausea, vomiting, dizziness, psychosis, coma, and even death. Moreover, metabolic damage includes acidosis, hypoglycemia in children, and hyperglycemia in adults [55]. Therefore, extreme care should be taken in prescribing topical salicylic acid for conditions involving large body-surface areas (such as ichthyosis) and particularly for infants [59]. Intoxication could be explained by the half-life of salicylic acid, which can range from 2–12 h depending on the dose. Hence, if a quantity of lotion is applied twice a day and corresponds to the minimal toxic concentration in blood, salicylic acid could accumulate in the body and levels could even become sufficiently high to cause death [55].
On the other hand, topical or systemic retinoids also comprise a therapy considered for infants with harlequin ichthyosis or, less often, collodion babies (e.g., tazarotene or acitretin, respectively), and other ichthyoses. The major benefit of retinoid administration is that they can aid in accelerating the shedding of the thick scale, but the main drawback in the administration of retinoids lies the risk for systemic adverse effects [56]. Vitamin A has demonstrated efficacy in the treatment of some ichthyoses, but high doses are required and it is stored in the liver, causing toxicity. Therefore, the design of synthetic retinoids was the option for improving efficacy and limiting toxicity. Examples of synthetic retinoids include acitretin, isotretinoin and, in the past, etretinate. These have been employed for decades for the therapy of some ichthyoses in patients of all ages, including newborns. Notwithstanding this, the use of systemic retinoids may cause acute and chronic toxicities, producing a variety of symptoms. For example, treatment with acitretin may cause severe hair loss, although this is reversible after the discontinuation of therapy. In addition, abnormalities in laboratory blood tests, such as blood cell counts, blood chemistry, and lipids, are frequently encountered. In cases of chronic toxicity, the skeletal system is mainly affected, with an enthesopathy similar to diffuse idiopathic skeletal hyperostosis [50]. Interestingly, these drawbacks are less common with the administration of isotretinoin.
As previously mentioned, AHA are keratolytics that reduce ichthyotic scaling by drawing water into the stratum corneum and increasing exfoliation by reducing corneocyte cohesion. Rare cases of metabolic acidosis are reported with the cutaneous administration of AHA; thus, caution should be taken with their use in young children and patients with large body surfaces affected [16].
As a general issue in ichthyoses therapy, toxicity is developed with many of the drugs, mainly due to the severity of barrier impairment (as with newborns) or the large surface of the skin to be treated with a high frequency of administration. A novel approach to overcome this issue is designing drug-delivery systems, as colloidal carriers, that can control the rate and site of drug delivery. Polymeric or lipid nanoparticles, liposomes, and niosomes, etc. can be introduced into conventional pharmaceutical dosage forms such as creams, ointments, hydrogels, or sprayable solutions to achieve effective therapeutic concentrations without reaching undesirable toxic effects.
Novel formulations
As mentioned in “Topical treatments” and “Systemic treatments”, there are various pharmaceutical dosage forms to administer drugs for the treatment of ichthyoses and novel formulations should consider some important features in this condition, mainly the large surface of the skin to be treated and severe skin damage. Likewise, advances in the knowledge of molecular and genetic events involved in skin functions may provide the tools for selecting effective drugs and designing suitable pharmaceutical-dosage forms. In this regard, the development of new gene and pharmacological therapeutic strategies could focus on the modifier effects of gene mutations, protein replacement, molecular chaperones, and protein catabolism systems (e.g., ubiquitin–proteasome system) [60].
Bassotti et al. [61] studied the efficacy of N-acetylcysteine in urea in a sample of five children with congenital lamellar ichthyosis. The cream containing the drug was topically administered on predetermined body-surface areas twice daily for 6 weeks, followed by a daily maintenance application. After 4 months, notorious improvement in all the treated areas was observed, whereas slight adverse effects, such as pruritus, slight burning, and irritations, were observed in two patients. Therefore, the authors concluded that their cream was beneficial and safe as a therapeutic option for lamellar ichthyosis (on skin lesions) and for ectropion; notwithstanding this, data should be confirmed in a larger group of patients. It is noteworthy that N-acetylcysteine possesses poor bioavailability (< 3%) when applied topically with hepatic metabolism and renal excretion [61]. Due to that this molecule has an extremely unpleasant odor that may cause low adherence to the treatment by patients. Thus, Batalla et al. [62] undertook a study using carbocysteine instead of N-acetylcysteine in a cream to improve this feature. Carbocysteine is an analogous amino acid derivative; it possesses anti-inflammatory, antioxidant, and mucolytic properties. In addition, this molecule does not produce the disgusting odor that is characteristic of N-acetylcysteine. The formulation was tested on four patients with several types of ichthyosis (self-improving congenital ichthyosis = 1, lamellar ichthyosis = 2, and IV = 1), resulting in positive recovery, good tolerability, and no local adverse effects in the majority of individuals (three of four patients).
On the other hand, a high frequency of vitamin D deficiency in children with congenital ichthyosis has been shown [63]. In this regard, Sethuraman et al. [64] reported an excellent response with regard to skin scaling and stiffness in seven children with congenital ichthyosis and severe vitamin D deficiency, when the children were administered 60,000 IU of oral colecalciferol daily for 10 days. At day 5, improvement in scaling was noted and, after 1 month of treatment, the skin was nearly normal in all the cases and a reduction of stiffness was also observed in all the children. Therefore, the authors stated that congenital ichthyosis with a vitamin D deficiency can be managed effectively with supplementation with high-dose vitamin D (followed by the recommended daily allowance).
On the other hand, the admixture of proteases into skin care formulations could represent another interesting novel approach for improving the efficacy of ichthyosis treatments. Namely, the presence of desquamated corneocytes in ichthyoses leads to the high presence of proteins (particularly keratin), which would be degraded by the proteases added to topical formulations. A study demonstrated that proteases reduced visible skin scaling and dryness, among other important effects. Proteases have been included in skin cleansers or leave-on formulations, but more clinical tests are needed to provide conclusions on their clinical usefulness [65].
With respect to leave-on products, a no-rinse chemical foam has been recently patented for the administration of the novel retinoid trifarotene to treat ichthyosis. The invention highlights the advantages of using a foam formulation to obtain the patient’s compliance, since the dose can be controlled better, usage is rapid and more practical, and the formulation is well tolerated and also less irritating. Moreover, according to the authors, the spreading of the foam onto the skin is more comfortable compared to other pharmaceutical treatments [66]. However, although this approach represents an interesting therapeutic alternative, its efficacy has not yet been demonstrated. More recently, Ramot et al. [67] made the assumption that arachidonyl-2′-chloroethylamide, a cannabinoid receptor 1 agonist, could decrease the expression of mutated keratin 1 and upregulate that of functional keratin 10 in cases of epidermolytic ichthyosis; however, further and extensive work needs to be done to emit an appropriate conclusion about usefulness of modulation of cannabinoid signaling in ichthyosis [67].
To prepare more effective carriers to overcome issues that conventional dosage forms cannot solve, many researches have been conducted in the field of nanomedicine. The systems developed have shown promising results, mainly due to their small size and their topical approach. The cutaneous use of nanoparticles as drug-delivery systems (polymeric and lipid, solid lipid nanoparticles, and nanostructured lipid carriers) has been extensively reviewed [60, 68-70]. These systems present low viscosity and comprise aqueous dispersions that are usually employed by means of semi-solid formulations with adequate consistency for application on the skin. Whether polymeric or lipid, nanoparticles represent an excellent alternative for topical drug delivery, due to that their surface is modifiable with chemical or physical features that can protect the drug from degradation. Moreover, the adverse effects of toxic drugs can decrease because of the possibility of controlled release, and cutaneous penetration of the drugs across the skin is enhanced due to increasing the concentration gradient. Concerning this, some drugs for the treatment of diseased skin have successfully been incorporated into natural polymeric nanoparticles of chitosan. Likewise, retinol has been encapsulated for anti-acne and anti-wrinkle treatment. More recently, TyroSpheres™, nanocarriers made of tyrosine-derived polymers from naturally occurring metabolites, have proved to reduce the proliferation of keratinocytes by the sustained release of paclitaxel [68].
In a similar way, Wyatt et al. [71] prepared silicon particles loaded with retinol, so as to protect it from degradation and to reduce its toxicity at high concentrations. The authors demonstrated that retinol was slowly released for several hours from the particles and that these reduced irritation in a double-blind skin study performed in 20 healthy subjects [71]. Likewise, a nanoemulsion containing the anti-inflammatory and keratolytic agent salicylic acid was successfully prepared to improve its stability and water solubility as well as to produce a topical localized anti-inflammatory effect in a mouse model [72]. On the other hand, as previously mentioned, mutation in the TGM1 gene produces a defective TGM1 enzyme that leads to ARCI. In this regard, replacement therapy of enzyme TGM1 encapsulated within thermo-responsive nanogels of poly(N-isopropylacrylamide)-polyglycerol has shown promising results for the treatment of ARCI in 3-D full-thickness skin models. The authors reported that the enzyme-loaded nanogel delivered TGM1 to the intercellular spaces between keratinocytes and improved the barrier function in the skin model [73, 74].
In addition, various approaches have been developed to treat other skin disorders; however, the principles of nanomedicine employed could be applied to create specific formulations to treat ichthyosis. In this regard, curcumin has demonstrated anti-psoriatic activity in molecular and cellular pharmacological studies due to the inhibition of pro-inflammatory TNF-α; however, some limitations for topical curcumin administration include its low water solubility and poor skin-penetration capability. Thus, to improve drug retention, two research groups proposed the encapsulation of curcumin in nanoparticles blended into hydrogels [75, 76]. In both researches, favorable results for blended systems of nanoparticles-hydrogel were found, as they performed better in achieving high skin permeation, high therapeutic effect, and a slow release profile when compared to their counterpart non-blended nanoparticles [75, 76]. Likewise, dendritic core-multishell nanocarriers (CMN) have been proposed by Hönzke et al. [77] as an alternative to the effective delivery of drugs to treat damaged skin. The authors prepared biodegradable CMN containing dexamethasone as model drug and found a more efficient delivery profile for the nanocarriers compared to dexamethasone cream. They also achieved a significant reduction of IL-8 with dexamethasone-loaded CMN in comparison with a commercial dexamethasone cream in the inflammatory skin model [77]. Therefore, these systems could be employed for administration of specific therapeutic compounds against ichthyosis.
In the case of approaches based on gene therapy, many gene-correction agents penetrate cellular membranes deficiently, have low bioavailability, or are formulated in undesirable solvents. Thus, nanocarriers may be an option to formulate effective therapies against keratin genodermatoses. For example, antisense oligonucleotides and plasmid DNA have been formulated into chitosan nanoparticles for topical gene therapy; however, many more studies are needed to determine their therapeutic efficacy and the potential negative adverse effects [60], especially in an organ such as the skin. Finally, although many studies have revealed the improvement of topical delivery via nanoparticles, the safety issues deriving from this approach need to be addressed.
Conclusions
Inherited ichthyoses cause a destabilization of the lipid composition that impacts the barrier function of the skin, its hydration, and desquamation. Their pathogenesis involves a chronic condition where prolonged pharmacological treatments are needed. Currently, there are various drugs available with varied mechanisms of action for the treatment of ichthyoses; however, to our knowledge, there are no specific treatments for a determined type of ichthyosis and there are few clinical trials showing a convincing efficacy of each of the substances applied, highlighting the possible need for a multi-therapy. In addition, due to the destabilization of the protective barrier, the skin of patients with ichthyosis is highly sensitive; therefore, the prolonged application of exogenous substances may easily cause irritation, a common adverse effect observed in clinical trials. Promising advances in the development of nanocarriers for topical use could aid in solving this question; notwithstanding this, further investigation is required. This context reveals a panorama with the need for more clinical investigation in the search for effective drugs and innocuous vehicles for the medical treatment of ichthyosis.
References
Akiyama M (2011) Updated molecular genetics and pathogenesis of ichthiyoses. Nagoya J Med Sci 73(3–4):79–90
Yoneda K (2016) Inherited ichthyosis: syndromic forms. J Dermatol 43(3):252–263
Oji V, Tadini G, Akiyama M, Blanchet Bardon C, Bodemer C, Bourrat E et al (2010) Revised nomenclature and classification of inherited ichthyoses: results of the first ichthyosis consensus conference in Soreze 2009. J Am Acad Dermatol 63(4):607–641
Oji V, Preil M, Kleinow B, Wehr G, Fischer J, Hennies HC et al (2017) S1 guidelines for the diagnosis and treatment of ichthyoses—update. J Ger Soc Dermatol 15(10):1053–1065
Hernández-Martin A, Aranegui B, Martin-Santiago A, Garcia-Doval I (2013) A systematic review of clinical trials of treatments for the congenital ichthyoses, excluding ichthyosis vulgaris. J Am Acad Dermatol 69(4):544–549
Kolarsick PAJ, Kolarsickk MA, Goodwin C (2011) Anatomy and physiology of the skin. J Dermatol Nurses Assoc 3(4):203–213
Chavanas S, Bodemer C, Rochat A, Hamel-Teillac D, Ali M, Irvine AD et al (2000) Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet 25(2):141–142
De Laurenzi V, Rogers GR, Hamrock DJ, Marekov LN, Steinert PM, Comptom JG et al (1996) Sjögren–Larsson syndrome is caused by mutations in the fatty aldehyde dehydrogenase gene. Nat Genet 12:52–57
Hernández-Martín A, González-Sarmiento R (2015) Recent advances in congenital ichthyoses. Curr Opin Pediatr 27(4):473–479
Raghunath M, Hennies HC, Ahvazi B, Vogel M, Reis A, Steinert PM et al (2003) Self-healing collodion baby: a dynamic phenotype explained by a particular transglutaminase-1 mutation. J Investig Dermatol 120(2):224–228
Eckl KM, Tidhar R, Thiele H, Oji V, Hausser I, Brodesser S et al (2013) Impaired epidermal ceramide synthesis causes autosomal recessive congenital ichthyosis and reveals the importance of ceramide acyl chain length. J Investig Dermatol 133(9):2202–2211
Akiyama M (2014) The roles of ABCA12 in epidermal lipid barrier formation and keratinocyte differentiation. Biochim Biophys Acta Mol Cell Biol Lipids 1841(3):435–440
Deffenbacher B (2013) Successful experimental treatment of congenital ichthyosis in an infant. BMJ Case Rep. https://doi.org/10.1136/bcr-2013-008688
Fluhr JW, Gloor M, Lehmann L, Lazzerini S, Distante F, Berardesca E (1999) Glycerol accelerates recovery of barrier function in vivo. Acta Derm Venereol 79(6):418–421
Ganemo A, Virtanen M, Vahlquist A (1999) Improved topical treatment of lamellar ichthyosis: a double-blind study of four different cream formulations. Br J Dermatol 141:1027–1032
Fleckman P, Newell BD, Van Steensel MA, Yan AC (2013) Topical treatment of ichthyoses. Dermatol Ther 26(1):16–25
Vahlquist A, Gånemo A, Virtanen M (2008) Congenital ichthyosis: an overview of current and emerging therapies. Acta Derm Venereol 88(1):4–14
Björklund S, Engblom J, Thuresson K, Sparr E (2013) Glycerol and urea can be used to increase skin permeability in reduced hydration conditions q. Eur J Pharm Sci 50(5):638–645
Blanchet-Bardon C, Tadini G, MacHado Matos M, Delarue A (2012) Association of glycerol and paraffin in the treatment of ichthyosis in children: an international, multicentric, randomized, controlled, double-blind study. J Eur Acad Dermatol Venereol 26(8):1014–1019
Lorencini M, Brohem CA, Dieamant GC, Zanchin NIT, Maibach HI (2014) Active ingredients against human epidermal aging. Ageing Res Rev 15(1):100–115
Tadini G, Giustini S, Milani M (2011) Efficacy of topical 10% urea-based lotion in patients with ichthyosis vulgaris: a two-center, randomized, controlled, single-blind, right- vs. -left study in comparison with standard glycerol-based emollient cream. Curr Med Res Opin 27(12):2279–2284
Danby SG, Higgs-bayliss T, Chittock J, Albenali L, Cork J (2016) The effect of an emollient containing urea, ceramide NP, and lactate on skin barrier structure and function in older people with dry skin. Skin Pharmacol Physiol 29:135–147
Küster W, Bohnsack K, Rippke F, Upmeyer H-J, Groll S, Traupe H (1998) Efficacy of urea therapy in children with ichthyosis. Dermatology 196:217–222
Celleno L (2018) Topical urea in skincare: a review. Dermatol Ther 31(6):e12690
Data A, Bv I (2004) Emollients and keratolytics remain the mainstay of therapy for patients with ichthyosis. Drugs Ther Perspect 20(2):11–15
Khalil S, Bardawil T, Saade S, Chedraoui A, Ramadan N, Hasbani DJ et al (2018) Use of topical glycolic acid plus a lovastatin-cholesterol combination cream for the treatment of autosomal recessive congenital ichthyoses. JAMA Dermatol 154(11):1320–1323
Bashir SJ, Dreher F, Chew AL, Zhai H, Levin C, Stern R et al (2005) Cutaneous bioassay of salicylic acid as a keratolytic. Int J Pharm 292:187–194
Lebwohl M (1999) The role of salicylic acid in the treatment of psoriasis. Int J Dermatol 38(1):16–24
Proksch E, De Bony R, Trapp S, Boudon S (2017) Topical use of dexpanthenol: a 70th anniversary article. J Dermatol Treat 28(8):766–773
Proksch E, Nissen H (2002) Dexpanthenol enhances skin barrier repair and reduces inflammation after sodium lauryl sulphate-induced irritation. J Dermatolog Treat 13(4):173–178
Biro K, Thacy D, Ochsendorf FR, Kaufmann R, Boehncke W-H (2003) Efficacy of dexpanthenol in skin protection against irritation: a double-blind, placebo-controlled study. Contact Dermat 49:80–84
Heise R, Skazik C, Marquardt Y, Czaja K, Sebastian K, Kurschat P et al (2012) Dexpanthenol modulates gene expression in skin wound healing in vivo. Skin Pharmacol Physiol 25(5):241–248
Thomas JR, Dixon TK, Bhattacharyya TK (2013) Effects of topicals on the aging skin process. Facial Plast Surg Clin N Am 21(1):55–60
Milanese A, Gorincioi E, Rajabi M, Vistoli G, Santaniello E (2011) New synthesis of 6[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid and evaluation of the influence of adamantyl group on the DNA binding of a naphthoic retinoid. Bioorg Chem 39(4):151–158
Piskin S, Uzunali E (2007) A review of the use of adapalene for the treatment of acne vulgaris. Ther Clin Risk Manag 3(4):621–624
Ogawa M, Akiyama M (2014) Successful topical adapalene treatment for the facial lesions of an adolescent case of epidermolytic ichthyosis. J Am Acad Dermatol 71(3):e103–e105
Verschoore M, Poncet M, Czernielewski J, Sorba V, Clucas A (1997) Adapalene 0.1% gel has low skin-irritation potential. J Am Acad Dermatol 36(6):S104–S109
Hofmann B, Stege H, Ruzicka T, Lehmann P (1999) Effect of topical tazarotene in the treatment of congenital ichthyoses. Br J Dermatol 141(4):642–646
Chandraratna RAS (1997) Tazarotene: the first receptor-selective topical retinoid for the treatment of psoriasis. J Am Acad Dermatol 37(2 III SUPPL.):12–17
Craiglow BG, Choate KA, Milstone LM (2013) Topical tazarotene for the treatment of ectropion in ichthyosis. JAMA Dermatol 149(5):598–600
Behera B, Chandrashekar L, Singh N, Thappa DM, Gochhait D (2017) Lamellar ichthyosis associated bilateral pseudoainhum of fingers and toes successfully treated with tazarotene. Dermatol Ther 30(5):3–5
Takeuchi Y, Yasukawa H, Yamaoka Y, Kato Y, Morimoto Y, Fukumori Y, Fukuda T (1992) Effects of fatty acids, fatty amines and propylene glycol on rat stratum corneum lipids and proteins in vitro measured by Fourier transform infrared/attenuated total reflection (FT-IR/ATR) spectroscopy. Chem Pharm Bull 40(7):1887–1892
Bowen JL, Heard CM (2006) Film drying and complexation effects in the simultaneous skin permeation of ketoprofen and propylene glycol from simple gel formulations. Int J Pharm 307(2):251–257
Catanzaro JM, Smith JG Jr, Augusta G (1991) Propylene glycol dermatitis. J Am Acad Dermatol 24(2):90–95
Lim TY, Poole RL, Pageler NM (2014) Propylene glycol toxicity in children. J Pediatr Pharmacol Ther 19:277–282
Fiume MM, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler D et al (2012) Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics. Int J Toxicol 31:245S–260S
Andersen KE, Storrs FJ (1982) Skin irritation caused by propylene glycols. Hautarzt 33(1):12–14
Roussel L, Atrux-Tallau N, Pirot F (2012) Glycerol as a skin barrier influencing humectant. In: Lodén M, Maibach HI (eds) Treatment of dry skin syndrome: the art and science of moisturizers. Springer, Berlin, pp 473–480
Lodén M, Wessman C (2001) The influence of a cream containing 20% glycerin and its vehicle on skin barrier properties. Int J Cosmet Sci 23(2):115–119
DiGiovana J, Mauro T, Milstone L, Schmuth M, Toro J (2013) Systemic retinoids in the management of ichthyoses and related skin types. Dermatol Ther 26(1):26–38
Virtanen M, Gedde-Dahl TJ, Mörk NJ, Leigh I, Bowden PE, Vahlquist A (2001) Phenotypic/genotypic correlations in patients with epidermolytic hyperkeratosis and the effects of retinoid therapy on keratin expression. Acta Derm Venereol 81(3):163–170
Vahlquist A, Blockhuys S, Steijlen P, Van Rossem K, Didona B, Blanco D et al (2014) Oral liarozole in the treatment of patients with moderate/severe lamellar ichthyosis: results of a randomized, double-blind, multinational, placebo-controlled phase II/III trial. Br J Dermatol 170(1):173–181
Verfaille CJ, Vanhoutte FP, Blanchet-Bardon C, Van Steensel MA, Steijlen PM (2007) Oral liarozole vs. acitretin in the treatment of ichthyosis: a phase II/III multicentre, double-blind, randomized, active-controlled study. Br J Dermatol 156(5):965–973
Bodemer C, Bourrat E, Mazereeuw-Hautier J, Boralevi F, Barbarot S, Bessis D et al (2011) Short- and medium-term efficacy of specific hydrotherapy in inherited ichthyosis. Br J Dermatol 165(5):1087–1094
Madan RK, Levitt J (2014) A review of toxicity from topical salicylic acid preparations. J Am Acad Dermatol 70(4):788–792
Craiglow BG (2013) Ichthyosis in the newborn. Semin Perinatol 37(1):26–31
Ramírez ME, Youseef WF, Romero RG, Martínez JMQ, González-Enseñat MA, Vilaplana XS et al (2006) Acute percutaneous lactic acid poisoning in a child. Pediatr Dermatol 23(3):282–285
Davies MG, Vella Briffa D, Greaves MW (1979) Systemic toxicity from topically applied salicylic acid. Br Med J 1:661
Fernandes NF, Janniger CK, Schwartz RA (2010) X-linked ichthyosis: an oculocutaneous genodermatosis. J Am Acad Dermatol 62(3):480–485
Chamcheu JC, Wood GS, Siddiqui IA, Syed DN, Adhami VM, Teng JM et al (2012) Progress towards genetic and pharmacological therapies for keratin genodermatoses: current perspective and future promise. Exp Dermatol 21(7):481–489
Bassotti A, Moreno S, Criado E (2011) Successful treatment with topical N-acetylcysteine in urea in five children with congenital lamellar ichthyosis. Pediatr Dermatol 28(4):451–455
Batalla A, Dávila-Pousa C, Feal C, Flórez Á (2018) Topical carbocysteine: a new option for the treatment of ichthyosis. Pediatr Dermatol 35(6):e357–e359
Chouhan K, Sethuraman G, Gupta N, Sharma VK, Kabra M, Khaitan BK et al (2012) Vitamin D deficiency and rickets in children and adolescents with ichthyosiform erythroderma in type IV and V skin. Br J Dermatol 166(3):608–615
Sethuraman G, Marwaha RK, Challa A, Yenamandra VK, Ramakrishnan L, Thulkar S et al (2016) Vitamin D: a new promising therapy for congenital ichthyosis. Pediatrics 137(1):e20151313
Del Rosso QJ (2013) Application of protease technology in dermatology: rationale for incorporation into skin care with initial observations on formulations designed for skin cleansing, maintenance of hydration, and restoration of the epidermal permeability barrier. J Clin Aesthet Dermatol 6(6):14–22
Buge JC et al (2018) No-rinse chemical foam containing trifarotene, and use thereof in the treatment of ichthyosis. Patent application publication. Pub. no. US2018/0333656 A1. US Pat. 1
Ramot Y, Oláh A, Paus R (2018) Cover image: neuroendocrine treatment of inherited keratin disorders by cannabinoids? Br J Dermatol 178(6):1469
Zhang Z, Tsai PC, Ramezanli T, Michniak-Kohn BB (2013) Polymeric nanoparticles-based topical delivery systems for the treatment of dermatological diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol 5(3):205–218
Garcês A, Amaral MH, Sousa Lobo JM, Silva AC (2018) Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: a review. Eur J Pharm Sci 112:159–167
Ganesan P, Narayanasamy D (2017) Lipid nanoparticles: different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain Chem Pharm 6:37–56
Shields CW, White JP, Osta EG, Patel J, Rajkumar S, Kirby N et al (2018) Encapsulation and controlled release of retinol from silicone particles for topical delivery. J Control Release 278:37–48
Sinha P, Srivastava N, Rai VK, Mishra R, Ajayakumar PV, Yadav NP (2019) A novel approach for dermal controlled release of salicylic acid for improved anti-inflammatory action: combination of hydrophilic-lipophilic balance and response surface methodology. J Drug Deliv Sci Technol 52:870–884
Plank R, Obst K, Yealland G, Calderón M, Hedtrich S, Martina Eckl K et al (2016) Nanogel-mediated protein replacement therapy for autosomal recessive congenital ichthyosis (ARCI). In: Proceedings of world congress on recent advances in nanotechnology 2016, pp 1–2, Paper No. NDDTE 105
Plank R, Yealland G, Miceli E, Lima Cunha D, Graff P, Thomforde S et al (2019) Transglutaminase 1 replacement therapy successfully mitigates the autosomal recessive congenital ichthyosis phenotype in full-thickness skin disease equivalents. J Investig Dermatol 139:1191–1195
Mao K, Fan Z, Yuan J, Chen P, Yang J, Xu J (2017) Skin-penetrating polymeric nanoparticles incorporated in silk fibroin hydrogel for topical delivery of curcumin to improve its therapeutic effect on psoriasis mouse model. Colloids Surf B Biointerfaces 160:704–714
Sun L, Liu Z, Wang L, Cun D, Tong HHY, Yan R et al (2017) Enhanced topical penetration, system exposure and anti-psoriasis activity of two particle-sized, curcumin-loaded PLGA nanoparticles in hydrogel. J Control Release 254:44–54
Hönzke S, Gerecke C, Elpelt A, Zhang N, Unbehauen M, Kral V et al (2016) Tailored dendritic core-multishell nanocarriers for efficient dermal drug delivery: a systematic top-down approach from synthesis to preclinical testing. J Control Release 242:50–63
Funding
This work was financially supported by a Grant from DGAPA-UNAM to Gerardo Leyva-Gómez (Grant number: PAPIIT TA 200318).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
This article does not contain any studies with human participants or animals performed by any of the authors; therefore, informed consent is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cortés, H., Del Prado-Audelo, M.L., Urbán-Morlán, Z. et al. Pharmacological treatments for cutaneous manifestations of inherited ichthyoses. Arch Dermatol Res 312, 237–248 (2020). https://doi.org/10.1007/s00403-019-01994-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-019-01994-x