Abstract
There is substantial evidence implicating the urokinase system in tissue remodeling during neo-vascularization, inflammation, tumor invasion, and metastasis. Regulated degradation of the extracellular matrix at the leading edge of migrating cells, mediated by uPA and uPAR, is required for tissue remodeling, invasiveness, and angiogenesis. Psoriasis and basal cell carcinoma (BCC) are the most common skin diseases. Pathogenesis of both of them is associated with keratinocyte hyperproliferation, inflammatory cell migration, and angiogenesis—processes in which the plasminogen system (uPA, uPAR, tPA, and PAI-1) plays a crucial role. In the present study, the comparative analysis of uPA, uPAR, tPA, and PAI-1 expression in the normal skin, in the biopsies of patients with psoriasis vulgaris, and BCC was carried out. uPA, uPAR, and PAI-1 expression was up-regulated in the epidermis of psoriatic skin and in tumor cells in BCC. Increased uPAR expression was detected in the derma of psoriatic lesions and in the stroma surrounding tumor cells in BCC. Increased expression of uPA in epidermal cells in psoriasis and in tumor cells in BCC suggests an important role of the uPA system for aggressively proliferating and invading cells of epidermal origin. A possible activation of the stroma, as a result of uPA–uPAR interaction between tumor cells and the surrounding stroma, is suggested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is generally recognized that the plasminogen/plasmin system, consisting of urokinase plasminogen activator (uPA), uPA receptor (uPAR), tissue-type PA (tPA), and plasminogen activator inhibitors (PAI-1 and PAI-2), plays a crucial role in tissue repair and remodeling by regulating cell migration, extracellular matrix degradation, and activation of growth factors and metalloproteinases [2, 53, 54, 67]. Inactive proenzyme plasminogen can be converted into the active enzyme, plasmin. Plasmin is a trypsin-like serine protease with broad specificity. It can directly degrade matrix components such as laminin and collagen type IV, and is capable of activating latent collagenases and matrix metalloproteinases (MMPs). Two physiological plasminogen activators (PAs) have been identified: tissue-type PA (tPA) and urokinase (uPA), but only urokinase has a specific high affinity receptor on the cell surface (uPAR). The tPA-mediated pathway is primarily involved in fibrin homeostasis [1]. Precise temporal and spatial regulation of extracellular proteolisis mediated by uPA and its receptor is required for tissue remodeling, cell migration, e.g., inflammatory reactions, invasive growth of cancer cells and angiogenesis. Inhibition of plasminogen/MMP system at the level of PAs is mediated by specific plasminogen activator inhibitors (PAI-1 and PAI-2) at the level of plasmin primarily by α2-antiplasmin, and at the level of MMPs by tissue inhibitors of MMPs (TIMPs) [5, 12, 65].
It has been found that the urokinase system (uPA and uPAR) plays an important role in tumor growth and metastasis. Elevated expression of uPA and its receptor correlates with poor prognosis, and is associated with advanced cancers, including the occurrence of metastasis [7, 11, 13, 18, 27, 39]. The activation of uPA and uPAR in brain, lung, colorectal, prostate, ovarian, and breast cancers [18, 30, 31, 63, 71] has been reported. uPAR is unique as it is rarely expressed in normal quiescent tissues, whereas its expression is highly increased in several tumor types, suggesting uPAR as a potential marker of malignancy [12]. Though tPA has been detected in normal and some malignant tissues, uPA is more commonly associated with malignancies [39].
Non-melanoma skin cancer, in general, is an increasing problem for health care services in the world and its incidence is growing with the highest rates for BCC [36, 38, 40]. BCC is the most common skin carcinoma with low-grade malignancy [21]. The pathogenesis of both psoriasis and BCC is associated with keratinocytes hyperproliferation, inflammatory cells migration, and angiogenesis—the processes in which plasminogen system plays an important role [10, 16, 33, 56, 69]. Nevertheless, very few investigations of the plasminogen system in psoriasis and BCC have been carried out, and data on plasminogen system activity have been contradictory. Furthermore, no studies on uPAR expression either in normal skin or in psoriasis have been performed so far. Thus, to gain insight into the role of the plasminogen system in the pathogenesis of psoriasis and BCCs, we compared the expression pattern of uPA, uPAR, tPA, and PAI-1 in normal skin, BCC, and psoriatic skin samples.
Materials and methods
Informed consent was obtained from each patient before inclusion in the study. Skin biopsies were obtained from Caucasian patients (ages 40–65). Tissue samples from 8 patients with psoriasis vulgaris, 6 patients with basal cell carcinomas (BCCs), and 11 healthy adult volunteers were involved. Patients were required to have psoriasis on the trunk of body and extremities (progressive stage in three cases, stationary stage in five cases) with a clinical diagnosis of at least 6 month duration. Psoriasis vulgaris was diagnosed based on clinical criteria and was confirmed histologically in each case. The samples of psoriatic skin were collected under local anesthesia. All patients received standard topical treatments with corticosteroid-containing creams. Patients were excluded if they had received systemic treatments with possible effects on psoriasis vulgaris (e.g., corticosteroids, retinoids, and immunosuppressants) within the last 4 weeks. Patients were excluded in case of a history of cardiovascular disease, diabetes, renal impairment, liver failure, or inflammatory disease. BCCs and samples of healthy volunteers’ skin were collected at surgeries. All BCCs were confirmed histologically. The study included biopsy specimens from the skin involved with psoriasis and BCC as well as from normal skin adjacent the lesion. All tissue samples were embedded in OCT Tissue Tek, immediately frozen in liquid nitrogen, and stored at −70 °C. Cryostat sections (6 μm thick) were mounted on silane-coated glasses, air-dried, and stained.
The following murine monoclonal antibodies were used: rabbit polyclonal to human uPA (Santa Cruz sc-14019), mouse monoclonal to uPA receptor (Abcam ab82220), mouse monoclonal to tPA (Abcam ab82249), and rabbit polyclonal to PAI-1 (Novus Biologicals NBP1-19773). Secondary affinity-purified, biotinylated horse anti-mouse IgG were purchased from Vector Laboratories, Inc., Burlingame, CA. Avidin–biotin blocking kit, normal horse serum, and biotin avidin-peroxidase staining kit (Vectastain® ABC-Kit) were obtained from Vector Laboratories, Inc., Burlingame, CA. Mouse and rabbit purified IgG (Sigma, Inc.) were used for control staining.
Immunohistochemical staining
Sections were first fixed in 4% paraformaldehyde for 2 min at room temperature, then in 96% ethanol for 30 min at −20 °C, and rinsed in phosphate-buffered saline (PBS) 3 times. To quench endogenous peroxidase activity, the slides with sections were incubated in 3% H2O2 in PBS for 30 min at room temperature. The slides were sequentially incubated in 10% normal horse serum and in 1% bovine serum albumin (BSA) for 30 min, then in avidin–biotin blocking kit for 15 min, and finally with primary antibodies or control IgG (mouse or rabbit) for 1 h. The slides were washed with PBS, incubated with secondary biotinylated anti-mouse antibody for 15 min, washed in PBS, and incubated with Vectastain® ABC-Kit and after—shortly with 0.1% H2O2 in a standard solution of DAB. The sections were counterstained with Mayer’s hematoxylin. Quantification of immune-positive cells was performed on cryosections using 20× or 40× objectives. At least five fields of view were used per each section. The images were obtained using a Leica DMI 6000B microscope equipped with a DFC 7000T digital camera and the image analysis program LAS X (Leica Microsystems).
Statistical analysis
The sections were analyzed using MetaMorph 5.0 (Universal Imaging, USA). The percentage of immune-positive cells in the epidermis and in the derma on sections was calculated for all skin samples (normal skin, psoriasis, and BCC). For statistics, five fields of view on five random sections for each sample were analyzed. All values are expressed as mean + SEM. Statistical analysis was performed by one-way ANOVA followed by Bonferroni/Dunn post hoc testing. The p value of <0.05 was considered significant.
Results
In this study, skin biopsies of 8 patients with psoriasis vulgaris, 11 healthy adults, and samples of BCCs from 6 patients were used for comparative analysis of uPA, uPAR, tPA, and PAI-1 expression. It is well known that the staining pattern of samples from different patients can vary significantly; therefore, we calculated the percentage of immune-positive cells in the epidermis, tumor masses, in the derma of normal skin, and in the skin samples from patients with psoriasis and BCC.
The key histopathological changes in lesional psoriatic skin were epidermal hyperplasia and expansion of the superficial dermal microvasculature. In the normal skin, the microvessels in the superficial, papillary dermis were short. Dilated and elongated superficial capillaries, passing into dermal papillae with multiple vascular segments in the papillary tips, were typical for psoriatic skin.
uPA and uPAR in normal skin, psoriatic skin, and BCCs
In the normal skin, uPA was detected in the derma of all samples (Fig. 1a). Staining from faint to moderate was found in the dermal vessels, and weak staining was detected in sweat and sebaceous glands. uPAR staining in normal skin samples was weak (Fig. 2a) and was observed only in some dermal vessels; in one skin, sample moderate staining was noted in the basal cell layer and in some dermal fibroblasts (not shown).
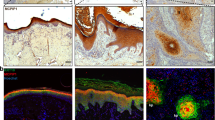
uPA expression in normal skin (a), psoriatic skin (b), and in BCC (c). Immunohistochemical staining was performed using avidin–biotin–peroxidase method (black or dark brown color). Nuclei were counterstained with hematoxylin. In normal skin, uPA staining was detected in the dermal vessels (arrow), in sweat and sebaceous glands (arrowhead). uPA expression in psoriatic samples was prominent in epidermal suprabasal cell layers, in cells of inflammatory infiltrate, and in the dermal blood vessels (arrow). Elevated uPA expression was noted in tumor cells in BCC samples. Scale bar 20 µm. d Percentage of immune-positive cells in the epidermis and in the derma of normal skin, psoriatic skin, and BCC. All values are expressed as means + SEM. Asterisk significant difference (p < 0.001) in the amount of positive cells in normal skin compared to psoriatic skin or BCC samples
uPAR expression in normal skin (a), psoriatic skin (b), and in BCC (c). Immunohistochemical staining was performed using avidin–biotin–peroxidase method (black or dark brown color). Nuclei were counterstained with hematoxylin. Weak uPAR staining was detected in normal skin. In psoriatic skin, uPAR expression was significantly increased in the epidermis and especially in the derma: in the vessels (arrow) and in the cells of inflammatory infiltrate (arrowhead). uPAR positive staining of different intensity was evident in tumor cells, surrounding extracellular matrix, in the dermal blood vessels (arrow), and in the derma bordering the tumor area in BCC samples. Scale bar 20 µm. d Percentage of immune-positive cells in the epidermis and in the derma of normal skin, psoriatic skin, and BCC. All values are expressed as means + SEM. Asterisk significant difference (p < 0.001) in the amount of positive cells in normal skin compared to psoriatic skin or BCC
In lesional psoriatic skin, uPA expression was increased and was detected in all biopsies. uPA positive immunostaining was observed in the epidermis of psoriatic skin in suprabasal cell layers (Fig. 1c, d). In the derma, uPA was found in blood vessels. uPA staining of non-lesional skin from patients with psoriasis was less pronounced than in psoriatic skin lesions; the staining was detected in the dermal vessels and occasionally in the epidermal suprabasal cell layers (not shown). uPAR was expressed in all samples from patients with psoriasis (Fig. 2c). uPAR expression was significantly increased in the epidermis and particularly in the derma of psoriatic skin (Fig. 2d). Intensive immunostaining was noted in the dermal vessels.
In BCCs, uPA elevated expression was detected in five of the six examined tumors (Fig. 1c, d). Positive staining of different intensity was found in the tumor cells and in the surrounding extracellular matrix (Fig. 2b). Faint staining was detected in the dermal blood vessels adjacent to the tumor. uPAR was detected in five of the six examined BCCs. In some samples, no uPAR expression was found in the tumor cells; however, intensive uPAR staining was observed in the surrounding stromal cells and in the dermal vessels (Fig. 2b, d).
As shown in Fig. 1d, uPA expression was increased in the epidermis of psoriatic lesions and in the tumor cells in BCC, while in the derma of BCC and psoriatic samples, uPA expression was decreased. In contrast, uPAR expression in the derma of psoriatic samples and BCC was up-regulated (Fig. 2d).
tPA in normal skin, psoriatic skin, and BCCs
In the normal skin, tPA staining was observed only in the endothelium of the dermal vessels (Fig. 3a), and prominent staining was observed in large vessels. tPA expression was also detected in the cells of sweat glands and in hair bulbs. No staining was found in the epidermis.
tPA expression in the normal skin (a), psoriatic skin (b), and in BCC (c). Immunohistochemical staining was performed using avidin–biotin–peroxidase method (black or dark brown color). Nuclei were counterstained with hematoxylin. In the normal skin, tPA staining was detected in endothelial cells of the dermal vessels (arrow). In biopsies of psoriatic skin, tPA expression was evident in the basal and suprabasal epidermal layers (arrowhead). In BCCs, tPA expression was revealed in the endothelium of the dermal vessels (arrow). In tumor cells, it was not detected. Scale bar 20 µm. d Percentage of immune-positive cells in the epidermis and in the derma of normal skin, psoriatic skin, and BCC. All values are expressed as means + SEM. Asterisk significant difference (p < 0.001) in the amount of positive cells in normal skin compared to psoriatic skin or BCC
Staining for tPA was observed in all biopsies of lesional (Fig. 3c) and non-lesional (not shown) skin of patients with psoriasis. In biopsies of lesional skin, the most prominent staining was detected in the endothelium of large dermal vessels. In smaller vessels, the staining was faint or even non-detectable. Faint tPA expression in the basal and suprabasal epidermal layers as well as in the sweat glands was detected in half of the psoriatic samples. In non-lesional skin, tPA was found in endothelial cells of dermal vessels and occasionally in the epidermal basal layer.
In BCCs, tPA immunostaining was found in the endothelium of dermal vessels and in the sweat glands (Fig. 3b). In the tumor cells, no tPA expression could be detected.
As shown in Fig. 3d, tPA expression was increased in the epidermis of psoriatic skin and in the derma around the tumor cells in BCC.
PAI-1 in normal skin, psoriatic skin, and BCCs
In normal skin, weak PAI-1 staining could be detected in the epidermis and derma (Fig. 4a).
PAI-1 expression in the normal skin (a), psoriatic skin (b), and in BCC (c). Immunohistochemical staining was performed using avidin–biotin–peroxidase method (black or dark brown color). Nuclei were counterstained with hematoxylin. In the normal skin, weak PAI-1 staining could be detected in small dermal vessels (arrow) and in the basal cell layer (arrowhead). In psoriatic skin, PAI-1 staining was observed in single cells (arrowhead) of the basal and suprabasal epidermal cell layers (arrow). In BCC samples, PAI-1 was expressed in tumor cells and in the surrounding extracellular matrix (arrowhead). Scale bar 20 µm. d Percentage of immune-positive cells in the epidermis and in the derma of normal skin, psoriatic skin, and BCC. All values are expressed as means + SEM. Asterisk significant difference (p < 0.001) in the amount of positive cells in normal skin compared to psoriatic skin or BCC
In psoriatic skin samples, prominent PAI-1 staining was observed in single cells in the basal and suprabasal epidermal cell layers (Fig. 4c), as well as in dermal blood vessels. In biopsies of non-lesional skin of patients with psoriasis, PAI-1 was not found.
In most of the BCC samples, no PAI-1 expression could be detected in the tumor cells (Fig. 4b). PAI-1 was expressed in single cells around the tumor, in the derma, and in endothelial cells of the blood vessels.
Similarly to tPA, PAI-1 expression was up-regulated in the epidermis of psoriatic skin and in the derma surrounding the tumor masses in BCC (Fig. 4d).
Discussion
Pathogenesis of psoriasis and BCC is associated with keratinocytes hyperproliferation and their differentiation disorders, activation, adhesion, and migration of keratinocytes and endothelial cells, chronic inflammation, and angiogenesis [23, 26, 43, 55, 58]. The plasminogen system is known to be involved in all of these processes. uPAR is particularly significant due to its ability to localize the proteolytic effects of uPA to the leading edge, which activates latent growth factors and proteases and mediates angiogenesis, matrix degradation, activation of intracellular signaling, tumor cell invasion, and metastasis [2, 7, 53, 54, 67].
In the present study, an immunohistochemical assessment and a quantitative evaluation of uPA, uPAR, tPA, and PAI-1 expression in the normal skin, psoriasis, and BCC have been performed. Since the staining pattern varied according to the patient, the percentage of immune-positive cells for each type of staining in each sample was calculated.
The obtained results show that uPA, uPAR, tPA, and PAI-1 are present in the epidermis of psoriatic skin, whereas in the normal epidermis, they are undetectable in most of the cases or are expressed at a low level. These results correlate with data obtained by other groups [24, 64]. Using in situ hybridization and immunohistochemical staining, it was shown that uPA and tPA expression was elevated in psoriatic skin [9, 25, 28, 34, 35, 42, 57, 66]. mRNA for tPA expression was detected throughout the epidermis but concentrated in the superficial stratum spinosum. uPA mRNA was noted primarily in the basal layer. Immunohistochemical analysis showed that the uPA and tPA were expressed in the cytoplasm of keratinocytes in the psoriatic lesions before treatment and in the lesions not cleared after treatment, while it was undetectable in the normal epidermis, in unaffected psoriatic epidermis, and in the cleared psoriatic skin [24, 42, 64]. Another study showed that uPA staining was present in the basal layer of keratinocytes in psoriatic skin, in the extracellular matrix, and in the suprapapillary epidermal layers, while tPA was detected in the superficial keratinizing cells, including both the stratum spinosum and the parakeratotic layer [29]. However, some previously published results obtained by Northern blot analysis and immunohistochemistry revealed uPA expression in the normal human epidermis as a predominant plasminogen activator [24].
Our data on elevated PAI-1 expression in psoriatic skin support previously published ELISA and immunohistochemistry results which demonstrated that the plasma level of PAI-1 was significantly increased in patients with psoriasis compared to healthy donors; initially, elevated level of PAI-1 decreased after treatment and during recovery period [25, 52]. In the other study, PAI-2, but not PAI-1, was detected by mRNA analysis and biochemical assays in the normal and psoriatic epidermis [44]. Increase in PA activity especially due to the increase in uPA and tPA expression levels in psoriatic epidermis may cause non-localized degradation of extracellular matrix and facilitate the turnover of keratinocytes [25, 28, 29, 65].
The prominence of dermal microvascular expansion in psoriasis suggests that it is an angiogenesis-dependent disease. Our data indicate that tPA, uPAR, and PAI-1 are coordinately increased in the dermal microvessels of psoriatic skin, suggesting that PA system is involved in psoriatic angiogenesis. Recent studies have shown the existence of an additional non-proteolytic function of the uPA system [2, 53]. uPA is known to stimulate vascular cell proliferation through direct activation of latent growth factors (HGF, VEGF189) or by releasing growth factors bound to matrix (VEGF isoforms, TGF-β, bFGF) [4, 15, 17, 19, 20, 37, 50]. Endothelial cells of blood vessels in psoriatic skin demonstrate upregulation of both VEGF receptors [VEGFR-1 (flt-1) and VEGFR-2 (KDR/flk-1)], angiopoietin receptor Tie2, angiopoietin 2, and altered pattern of integrin expression (upregulation of αvβ3 and β1 integrins and downregulation of β4 integrin) [14, 51]. Many growth factors (such as TGF-α and VEGF) can feedback to plasminogen system increasing the expression of both, uPA and uPAR. Thus, the uPA system could be implicated in the activation of endothelial cells, in their increased proliferation, in new vessel formation, and in tissue remodeling in psoriatic skin.
The role of the uPA system in cancer has been investigated in different in vitro assays and in tumors. These studies indicated that uPA binding to uPAR was necessary for the primary tumor growth, cell invasion, and neo-vascularization [53]. Elevated uPA expression is associated with poor prognosis and metastasis in many tumors [30, 39, 59, 63, 71]. It was demonstrated that uPA overexpression in ovarian cancer SKOV3 cells enhanced greatly their invasion potential, migration, and adhesion in vitro. These results correlated with high expression level of uPA and PAI-1 in the malignant tumor samples and their serum concentration in patients compared to the benign tumors and controls [71].
Several retrospective and prospective studies have demonstrated that the elevated levels of uPA and PAI-1 in breast tumors are the most reliable prognostic biomarkers and correlate with poor patient outcome in lymph node-negative breast cancer [18, 32, 39]. At the concentrations detected in tumor samples, PAI-1 and uPA promoted cancer progression and metastasis [18]. The testing of uPA and PAI-1 levels by ELISA has been recommended by the American Society of Clinical Oncology (ASCO) for evaluation of the reoccurrence risk in breast cancer patients [62].
Quantitative immunohistochemistry analysis of the expression of PA system proteins was also evaluated in resected colorectal cancer and correlated with clinicopathological parameters and patient outcome. It was shown that the overexpression of uPA, uPAR, and PAI-1 was significantly associated with cancer progression and liver metastasis [63].
In the two-stage model of skin carcinogenesis in mice, it was found that uPAR deficiency dramatically reduced susceptibility to tumor formation and increased keratinocyte differentiation. These data were confirmed clinically: it was found that human differentiated keratoacanthomas expressed low levels of uPAR [47].
The main feature of BCC is the low frequency of metastasis; however, it may be locally aggressive and, if untreated, may penetrate into the bones [45]. Like other carcinomas, BCC is characterized by malignant cell proliferation and enhanced angiogenesis. The data on PA expression in BCC have been contradictory. In early studies in fibrin lysis assay, no statistical difference in the activity of PA from BCC and normal skin samples could be detected [49]. Moreover, in later studies, no significant difference in the biochemical activity or expression level of PA from skin samples of seborrheic keratosis, BCC, and squamous cell carcinoma was observed [48, 68]. Comparative analysis of uPA mRNA content from different types of BCC revealed its presence only in the nodular subtype of BCC (in tumor islands, fibroblast-like stromal cells, and in the basal epidermal cell layer) [64]. It is notable that tPA mRNA was detected in tumor cells [65]. However, zymography and in situ hybridization demonstrated expression and activity of uPA and PAI-1 in malignant cells from squamous cell carcinomas, but not in BCCs. tPA expression was detected only in the derma of non-malignant tissue samples [60].
In tumors, the urokinase system can be assembled by cooperation between different cell types, each producing a different component of the uPA system. This study demonstrates that the level of uPA expression is increased in tumor cells and in the surrounding extracellular matrix in BCCs samples, thus suggesting the role of uPA in facilitating tumor cell migration and invasion into the underlying derma. The intense uPAR staining in the derma bordering the tumor can be attributed to uPA–uPAR interaction that mediated tumor growth and invasion, uPAR-mediated desmoplasia, and stromal remodeling. The presence of uPAR on the endothelial cells in BCC samples suggests that it also plays a role in tumor angiogenesis. It was hypothesized that PAI-1 may contribute to the defense mechanisms of normal tissue against invading tumor cells [2]. Therefore, it is tempting to speculate that the up-regulated PAI-1 expression in some samples correlates with the slow growth pattern and low frequency of metastasis in BCC.
High levels of uPA and uPAR expression in tumors are commonly associated with cancer progression [8, 61]. The most interesting fact of the present study was that uPA, especially uPAR staining in tumor cells in BCCs, was visually lower than in psoriasis, a benign hyperproliferative skin disease. This finding suggests that uPAR may represent a new therapeutic target for the development of anti-psoriatic therapeutic agents.
In conclusion, psoriasis and BCC are complicated biological processes that are regulated by many different factors. The PA system can be one of these important factors that are involved in regulation of cell growth and invasion. In the present study, we addressed the PA system function using immunohistochemical staining and calculated the percentage of positive cells in the derma and epidermis in human skin samples. uPA, uPAR, and PAI-1 were up-regulated in the epidermis of psoriatic skin and in the tumor cells in BCC; high levels of uPAR expression were detected in the derma of psoriatic lesions and in the stroma surrounding tumor cells in BCC samples. These data suggest a possible activation of the stroma, uPA–uPAR interaction between tumor cells, and the surrounding stroma and its involvement in tumor growth. Increased expression of uPA in the epidermal cells in psoriasis and that in the tumor cells in BCC suggest the important role of uPA system for aggressively proliferating and invading cells of epidermal origin. These assumptions need further investigation. Gene expression and proteome analysis as well as in vitro cell culture experiments are necessary to confirm the involvement of uPA system in pathogenesis of psoriasis and BCC.
References
Al-Horani RA (2014) Serpin regulation of fibrinolytic system; implications for applications in cardiovascular diseases. Cardiovasc Hematol Agents Med Chem 12(2):91–125
Andreasen PA, Kjoller L, Christensen L, Duffy MJ (1997) The urokinase-type plasminogen activation system in cancer metastasis; a review. Int J Cancer 72:1–22
Barker JNWN (1991) Pathophysiology of psoriasis. Lancet 338:227–230
Bhushan M, Mclaughlin B, Weiss JB (1999) Level of endothelial cell stimulating angiogenesis factor and vascular endothelial growth factor are elevated in psoriasis. Br J Dermatol 141:1054–1060
Blasi F, Vassalli J-D, Dano K (1987) Urokinase-type plasminogen activator; proenzyme, receptor, and inhibitors. J Cell Biol 104:801–804
Blasi F, Carmeliet P (2002) uPAR; a versatile signaling orchestrator. Nat Rev Mol Cell Biol 3:932–943
Blasi F, Sidenius N (2010) The urokinase receptor; focused cell surface proteolysis, cell adhesion and signaling. FEBS Lett 584(9):1923–1930
Buo L, Meling GI, Karlsrud TS (1995) Antigen level of urokinase plasminogen activator and its receptor at the tumor-host interface of colorectal adenocarcinomas are related to tumor aggressiveness. Hum Pathol 26:1133–1138
Chen CS, Jensen PJ (1996) Serum is a potent stimulator of keratinocyte tissue plasminogen activator expression. J Investig Dermatol 106(2):238–242
Choi J, Koo JY (2003) Quality of life issues in psoriasis. J Am Acad Dermatol 49:57–61
Collen D (1999) The plasminogen (fibrinolytic) system. Thromb Haemost 82(2):259–270
Collen D (2001) Role of the plasminogen system in fibrin-homeostasis and tissue remodelling. Hematology 1(1):1–9
Colman RW, Wu Y, Liu Y (2010) Mechanisms by which cleaved kininogen inhibits endothelial cell differentiation and signaling. Thromb Haemost 104:875–885
Creamer D, Allen M, Sousa A (1995) Altered vascular endothelium integrin expression in psoriasis. Am J Pathol 147:1661–1667
Creamer D, Jaggar M, Allen M (1997) Overexpression of angiogenic growth factor platelet-derived endothelial cell growth factor/thymidine phosphorylase in psoriatic epidermis. Br J Dermatol 137:8851–8855
Creamer D, Sullivan D, Bicknell R, Barker J (2002) Angiogenesis in psoriasis. Angiogenesis 5:231–236
Detmar M, Brown LF, Claffey KP (1994) Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptor in psoriasis. J Exp Med 180:1141–1146
Duffy MJ, McGowan PM, Harbeck N, Thomssen C, Schmitt M (2014) uPA and PAI-1 as biomarkers in breast cancer: validated for clinical use in level-of-evidence-1 studies. Breast Cancer Res 16(4):428
Elder JT, Fisher GJ, Lindquist PB (1989) Overexpression of transforming growth factor-a in psoriatic epidermis. Science 243:811–814
Ettehadi P, Greaves MW, Wallach D (1994) Elevated tumor necrosis factor-a (TNF-a) biological activity in psoriatic lesions. Clin Exp Immunol 96:146–151
Feller L, Khammissa RA, Kramer B, Altini M, Lemmer J (2016) Basal cell carcinoma, squamous cell carcinoma and melanoma of the head and face. Head Face Med 12:11
Fraki JE, Lazarus GS, Gilgor RS, Marchase P, Singer KH (1983) Correlation of epidermal plasminogen activator activity with disease activity in psoriasis. Br J Dermatol 108:39–44
Ganzetti G, Campanati A, Molinelli E, Offidani A (2016) Psoriasis, non-alcoholic fatty liver disease, and cardiovascular disease: three different diseases on a unique background. World J Cardiol 8(2):120–131
Gilhar A, David M, Kalish RS, Weisinger G (1996) In vivo effects of cytokines on psoriatic skin grafted on nude mice; involvement of the tumour necrosis factor (TNF) receptor. Clin Exp Immunol 106(1):134–142
Gissler HM, Frank R, Kramer MD (1993) Immunohistochemical characterization of the plasminogen activator system in psoriatic epidermis. Br J Dermatol 128(6):612–618
Godi A (2004) New approaches to psoriasis treatment. Acta Dermatovenerol 13(2):50–57
Gramling MW, Church FC (2010) Plasminogen activator inhibitor-1 is an aggregate response factor with pleiotropic effects on cell signaling in vascular disease and the tumor microenvironment. Thromb Res 125:377–381
Grondahl-Hansen J, Nielsen LS, Kristensen P, Grondahl-Hansen V, Andreasen PA, Dano K (1985) Plasminogen activator in psoriatic scales is of the tissue-type PA as identified by monoclonal antibodies. Br J Dermatol 113(3):257–263
Grondahl-Hansen J, Ralfkiaer E, Nielsen LS, Kristensen P, Frentz G, Dano K (1987) Immunohistochemical localization of urokinase- and tissue-type plasminogen activators in psoriatic skin. J Investig Dermatol 88(1):28–32
Harbeck N, Kates RE, Schmitt M (2004) Urokinase-type plasminogen activator and its inhibitor type 1 predict disease outcome and therapy response in primary breast cancer. Clin Breast Cancer 5(5):348–352
Heissig B, Dhahri D, Eiamboonsert S, Salama Y, Shimazu H, Munakata S, Hattori K (2015) Role of mesenchymal stem cell-derived fibrinolytic factor in tissue regeneration and cancer progression. Cell Mol Life Sci 72(24):4759–4770
Hildenbrand R, Schaaf A (2009) The urokinase-system in tumor tissue stroma of the breast and breast cancer cell invasion. Int J Oncol 34(1):15–23
Hohler T, Marker-Hermann E (2001) Psoriatic arthritis; clinical aspects, genetics and the role of T-cells. Curr Opin Rheumatol 13:273–279
Jensen PJ, Baird J, Morioka S, Lessin S, Lazarus GS (1988) Epidermal plasminogen activator is abnormal in cutaneous lesions. J Investig Dermatol 90(6):777–782
Jensen PJ, Baird J, Belin D, Vassalli JD, Busso N, Gubler P, Lazarus GS (1990) Tissue plasminogen activator in psoriasis. J Investig Dermatol 95(5):13–14
Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R (2015) Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg 41(5):550–571
Kuroda K, Sapadin A, Shoji T (2001) Altered expression of angiopoietins and Tie2 endothelium receptor in psoriasis. J Investig Dermatol 116:713–720
Lanoue J, Goldenberg G (2016) Basal cell carcinoma: a comprehensive review of existing and emerging nonsurgical therapies. J Clin Aesthet Dermatol 9(5):26–36
Leurer C, Rabbani SA (2015) Plasminogen activator system—diagnostic, prognostic and therapeutic implications in breast cancer, a concise review of molecular pathology of breast cancer. In: Gunduz M (ed) A concise review of molecular pathology of breast cancer. ISBN 978-953-51-2030-8
Lomas A, Leonardi-Bee J, Bath-Hextall F (2012) A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 166(5):1069–1080
Lotti T, Bonan P, Panconesi E (1989) Plasminogen activation in psoriasis. Acta Derm Venereol Suppl (Stockh) 146:36–38
Lotti T, Bonan P, Cannarozzo G, Fedi AM, Panconesi E (1990) Antipsoriatic therapies inhibit epidermal plasminogen activator activity. Int J Dermatol 29(7):528–530
Lupu M, Caruntu C, Ghita MA, Voiculescu V, Voiculescu S, Rosca AE, Caruntu A, Moraru L, Popa IM, Calenic B, Greabu M, Costea DE (2016) Gene expression and proteome analysis as sources of biomarkers in basal cell carcinoma. Dis Mark. doi:10.1155/2016/9831237
Lyons-Giordano B, Loskutoff D, Chen CS, Lazarus G, Keeton M, Jensen PJ (1994) Expression of plasminogen activator inhibitor type 2 in normal and psoriatic epidermis. Histochemistry 101(2):105–112
Maguire T, Chin D, Soutar D, Duffy MJ (2000) Low levels of urokinase plasminogen activator components in basal cell carcinoma of the skin. Int J Cancer 85:457–459
Mazar AP, Henkin J, Goldfarb RH (1999) The urokinase plasminogen activator system in cancer; implication for tumor angiogenesis and metastasis. Angiogenesis 3:15–32
Mazzieri R, Pietrogrande G, Gerasi L, Gandelli A, Colombo P, Moi D, Brombin C, Ambrosi A, Danese S, Mignatti P, Blasi F, D’Alessio S (2015) Urokinase receptor promotes skin tumor formation by preventing epithelial cell activation of notch. Cancer Res 75(22):4895–4909
Miller SJ, Jensen PJ, Dzubow LM, Lazarus GS (1992) Urokinase plasminogen activator is immunocytochemically detectable in squamous cell but not basal cell carcinomas. J Investig Dermatol 98(3):351–358
Nagy B, Ban J, Brdar B (1977) Fibrinolysis associated with human neoplasia; production of plasminogen activators by human tumors. Int J Cancer 19:614–620
Nickoloff BJ, Mitra RS, Varani J (1994) Aberrant production of interleukin-8 and thrombospondin-1 by psoriatic keratinocytes mediates angiogenesis. Am J Pathol 144:820–828
Nickoloff BJ (2000) Characterization of lymphocyte-dependent angiogenesis using a SCID mouse; Human skin model of psoriasis. J Investig Dermatol 5:67–73
Nielsen HJ, Christensen IJ, Svendsen MN, Hansen U, Werther K, Brunner N, Petersen LJ, Kristensen JK (2002) Elevated plasma levels of vascular endothelial growth factor and plasminogen activator inhibitor-1 decrease during improvement of psoriasis. Inflamm Res 51(11):563–567
Parfyonova YV, Plekhanova OS, Tkachuk VA (2002) Plasminogen activators in vascular remodeling and angiogenesis. Biochemistry (Moscow) 67(1):139–156
Plekhanova OS, Parfenova EV, Tkachuk VA (2015) Mechanisms of vascular remodeling following arterial injury. Kardiologiia 55(7):63–77
Quinn AG, Perkins W (2010) Non-melanoma skin cancer and other epidermal skin tumors. In: Burns T, Breathnach S, Cox N, Griffiths C (eds) Rook’s textbook of dermatology. Blackwell Publishing Ltd, Chichester
Rogers HW, Weinstock MA, Feldman SR, Coldiron BM (2012) Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population. JAMA Dermatol 151(10):1081–1086
Rox JM, Reinartz J, Kramer MD (1996) Interleukin-1 beta upregulates tissue-type plasminogen activator in a keratinocyte cell line (HaCaT). Arch Dermatol Res 288(9):554–558
Ruano J, Suárez-Fariñas M, Shemer A, Oliva M, Guttman-Yassky E, Krueger JG (2016) Molecular and cellular profiling of scalp psoriasis reveals differences and similarities compared to skin psoriasis. PloS One 11(2):1–18
Santibanez JF (2013) Transforming growth factor-beta and urokinase-type plasminogen activator; dangerous partners in tumorigenesis. implications in skin cancer volume. Hindawi Publishing Corporation, UK, ISRN Dermatology. Article ID 597927
Sappino AP, Belin D, Huarte J, Hirschel-Scholz S, Saurat JH, Vassalli JD (1991) Differential protease expression by cutaneous squamous and basal cell carcinomas. J Clin Investig 88(4):1073–1079
Schmitt M, Harbeck N, Thompson C (1997) Clinical impact of the plasminogen activation system in tumor invasion and metastasis; prognostic relevance and target for therapy. Thromb Hemost 78(1):285–296
Schmitt M, Mengele K, Napieralski R, Magdolen V, Reuning U, Gkazepis A (2010) Clinical utility of level-of-evidence-1 disease forecast cancer biomarkers uPA and its inhibitor PAI-1. Expert Rev Mol Diagn 10(8):1051–1067
Seetoo DQ, Crowe PJ, Russell PJ, Yang JL (2003) Quantitative expression of protein markers of plasminogen activation system in prognosis of colorectal cancer. J Surg Oncol 82(3):184–193
Spiers EM, Lazarus GS, Lyons-Giordano B (1994) Expression of plasminogen activator enzymes in psoriatic epidermis. J Investig Dermatol 102(3):333–338
Spiers EM, Lazarus GS, Lyons-Giordano B (1996) Expression of plasminogen activators in basal cell carcinoma. J Pathol 178:290–296
Teofoli P, Mancini A, Lotti T (1996) Cyclosporine A inhibits tPA mRNA transcription in A431 cell line. Skin Pharmacol 9(2):137–140
Tkachuk VA, Plekhanova OS, Beloglazova IB, Parfenova EV (2013) A role of multi-domain structure of urokinase in regulating vascular growth and remodeling. Ukr Biokhim Zh 85:18–45
Tsuboi R, Yamaguchi T, Kurita Y, Nakao H, Ishihara K (1988) Comparison of proteinase activities in squamous cell carcinoma, basal cell epithelioma, and seborrheic keratosis. J Investig Dermatol 90:869–872
Tutrone WD, Saini R, Weinberg JM (2004) Biological therapy for psoriasis; an overview of infliximab, etanercept, efalizumab and alefacept. I Drugs 7(1):45–49
Vassalli J-D (1994) The urokinase receptor. Fibrinolysis 8(1):172–181
Zhang Y, Kenny HA, Swindell EP, Mitra AK, Hankins PL, Ahn RW, Gwin K, Mazar AP, O’Halloran TV, Lengyel E (2013) Urokinase plasminogen activator system-targeted delivery of nanobins as a novel ovarian cancer therapy. Mol Cancer Ther 12(12):2628–2639
Acknowledgements
Source of funding: Grant (No. 14-24-00086) of the Russian Science Foundation. The authors acknowledge that the work was carried out using equipment purchased with the funding from the Program of Development of M. V. Lomonosov Moscow State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the Bioethical Committee of Russian Cardiology Research Center and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Rubina, K.A., Sysoeva, V.Y., Zagorujko, E.I. et al. Increased expression of uPA, uPAR, and PAI-1 in psoriatic skin and in basal cell carcinomas. Arch Dermatol Res 309, 433–442 (2017). https://doi.org/10.1007/s00403-017-1738-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-017-1738-z