Abstract
Accumulating studies have indicated that vitiligo, especially non-segmental vitiligo (NSV), is one kind of autoimmune diseases and CD4+ T cells play important roles in the pathogenesis. However, there have been very limited data on the detailed changes of each of the CD4+ T cell subsets in periphery in active NSV. To clarify this issue, we collected the peripheral blood mononuclear cells (PBMCs) from 30 patients with active NSV and 30 age- and sex-matched healthy controls. The percentages of circulating Th1, Th2, Th17 and Tregs were evaluated using flow cytometry and the expressions of their specific transcription factors T-bet, GATA3, RORγt and FOXP3 at mRNA level and protein levels were qualified by qPCR and flow cytometry, respectively. Meanwhile, the expression levels of IFN-γ, IL-4, TGF-β, and IL-17A in serum were measured. We found that in patients with NSV, the percentages and absolute numbers of circulating Th1 and Th17 were both significantly higher than those of healthy controls, while the percentages of Th2 and Tregs and absolute numbers showed no significant difference compared to healthy controls. Moreover, the ratios of Th1/Tregs and Th17/Tregs in circulation were both statistically elevated in active NSV. Similar results were got in qualification of their corresponding transcription factors at mRNA level and protein levels. Compared with healthy controls, the expression level of IL-17A was significantly increased in serum of patients with NSV, while the productions of IFN-γ, IL-4, TGF-β had no significant change. These data suggested that in circulating CD4+ T cell subsets, Th1 and Th17 played the major role in cellular immunity in the progression of vitiligo. The immune lever in circulation was inclined to effector CD4+ T cells not suppressor CD4+ T cells that may result in the loss of self-tolerance to melanocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the pathogenesis of vitiligo is incompletely deciphered, accumulating clinical and laboratory data have indicated that autoimmune responses against melanocytes, especially T cell-based cellular immunity, play important roles in the loss of functional melanocytes in the skin. Different from consensus on the role of cytotoxic CD8+ T cells in vitiligo [23, 42], there are more complicated and even controversial reports on the status of CD4+ T cell subsets, which consist of effector CD4+ T cells (i.e., Th1, Th2 and Th17) and suppressor CD4+ T cells (i.e., regulatory T cells, Tregs).
The earlier theory of the immune response in vitiligo was inclined to type 1-like T cell profile [39]. Morphological studies have confirmed the presence of IFN-γ secreting CD4+ T cells (i.e., Th1) and CD8+ T cells in perilesional and lesional skin in vitiligo [36, 41]. Increased expression of IFN-γ mRNA was found both in vitiligo skin and PBMCs [11, 29]. However, up to now, no direct evidence supports the participation of circulating Th1 in the pathogenesis of vitiligo. After the discovery of Th17, a distinct lineage of effector CD4+ T helper cells, it was found that Th17 also took part in the pathogenesis of vitiligo with increased IL-17 expression both in lesional skin and serum [20, 37]. Moreover, Th17-associated cytokines, such as IL-22 and IL-23 greatly increased in serum in vitiligo [8, 35]. However, the data of proportion of peripheral Th17 in patients with vitiligo were still controversial [15, 44]. Although it was reported that IL-4 could directly inhibit melanogenesis of normal melanocytes in vitro [6], there were limited studies about Th2 in vitiligo. Some studies showed significantly increased IL-4 mRNA and serum IL-4 in vitiligo patients [13, 17], while some reported low GATA3 expression and IL-4 production in serum [29]. As the most important inhibitory T cells, Tregs have been paid more attention in vitiligo. Some groups found decreased Tregs in skin tissue [1, 19] and one of them pointed out that decrease of chemokine CCL22 in perilesional skin reduced Tregs infiltration at skin lesions [19], while another study found that significant increase of Foxp3 expression in the skin of vitiligo patients with defect function [5]. Others reported that either there were significantly reduced Tregs in CD4+ T cells in periphery with low expression of FOXP3 mRNA [5, 23], or low production of TGF-β in serum [3, 24]. The frequency of Tregs in peripheral blood was also in dispute [23, 27, 43].
In summary, to date, detailed profile of CD4+ T cell subsets in peripheral blood in active vitiligo is still obscure. In this study, we investigated the frequencies of Th1, Th2, Th17 and Tregs and the expressions of their corresponding specific transcription factors in periphery, and their relative specific cytokine production in serum from patients with progressive NSV and healthy controls.
Materials and methods
Subjects
Thirty patients with active NSV (15 males and 15 females, aged 18–45 years) and 30 age- and sex-matched healthy controls were enrolled in our clinical control study after written informed consent using protocols approved by the Hospital’s Protection of Human Subjects Committee (The First Hospital of Jilin University, Clinical Ethics Committee). For all included patients, new lesions and/or enlargement of pre-existing lesions of vitiligo emerged within last 6 months and no immunosuppressive treatment was administered in last 2 months. Patients with chronic inflammatory and autoimmune diseases except vitiligo were excluded in this study. None of the healthy individuals had any evidence of vitiligo or any other autoimmune disease.
PBMCs stimulation
PBMCs were isolated from peripheral blood by Ficoll density gradient centrifugation (Ficoll, Sigma-Aldrich Company, US) and were resuspended with RPMI 1640 medium containing 10 % FBS. After adjusting the cell concentration, cells were cultured in 96-well plates as 4 × 105 per well in the medium supplemented with PMA and ionomycin in the presence of GolgiPlug (Leukocyte activation cocktail with BD GolgiPlug, BD Pharmingen, US) for 4 h before FACS analysis.
FACS analysis for cytokine staining and transcript factor staining
For surface staining, 4 × 105 freshly isolated PBMCs were directly incubated with FITC, PE, PE-Cy5.5 or APC-conjugated mAbs to human CD4, CD25, and CD127 (eBioscience, US) for 30 min at 4 °C. After surface staining of CD4, activated PBMCs were fixed and permeabilized with BD Cytofix/Cytoperm Plus (BD Bioscience, US) according to the manufacturer’s instructions for cytokine staining. Cells were incubated with PE-Cy5.5-conjugated anti-human IFN-γ mAb, PE-conjugated anti-human IL-4 mAb, and APC-conjugated anti-human IL-17A mAb, or control isotypes (all mAbs were purchased from eBioscience, US). For transcript factor staining, after surface staining of CD4, 4 × 105 PBMCs were fixed and permeabilized with Foxp3 staining buffer set (eBioscience Bioscience, US) according to the manufacturer’s instructions. Cells were incubated with PE-Cy5.5-conjugated anti-human T-bet mAb, PE-conjugated anti-human RORγt mAb, and APC-conjugated anti-human GATA3 mAb, or control isotypes (all mAbs were purchased from eBiboscience, US). FACS analysis was performed with a FACSCalibur flow cytometer (BD Biosciences, US) using FlowJo software.
Real-time PCR
Total RNA was extracted from freshly isolated PBMCs (from 15 active NSV patients and 15 healthy controls) using the TRIzol regent (Invitrogen, US). First-strand cDNA was synthesized from 1 µg DNAse-treated total RNA using an oligo-dT18 primer and RevertAidTM M-MLV reverse transcriptase. Transcripts were quantified by real-time quantitative PCR on an ABI PRISM 7700 Sequence Detector (Perkin-Elmer Applied Biosystems) with Applied Biosystems predesigned TaqMan Gene Expression Assays and reagents according to the manufacturer’s instructions. The real-time PCR included an initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 30 s, 55 °C for 1 min, and 72 °C for 1 min, and one cycle of 95 °C for 1 min, 55 °C for 30 s, and 95 °C for 30 s. The relative abundance of transcript was normalized and calculated against that of the β-actin gene as an endogenous reference using the 2 −ΔΔCT method. T-bet, GATA3, RORγt, FOXP3 and β-actin primers were purchased (Sangon Biotech, Shanghai, China).
Enzyme-linked immunosorbent assay (ELISA)
Cytokines in serum were measured by human IFN-γ, IL-4, IL-17A, TGF-β1 ELISA Ready-SET-Go! Kits (eBioscience, US) according to the manufacturer’s instructions. All samples were in triplicates. Data were analyzed with ELISA CALC software.
Statistical analysis
A standard two-tailed t test or a t test with Welch’s correction was used for statistical analysis with Graphpad Prism 5.0 software. Data were expressed as mean ± SD and differences at P < 0.05 or less were considered to be statistically significant.
Results
Altered distribution of peripheral CD4+ T cell subsets in active NSV
To fully evaluate the status of CD4+ T cell subsets in peripheral blood in progressive NSV, we first analyzed the frequencies of CD4+ CD25+ CD127− Tregs, CD4+ IFN-γ+ Th1, CD4+ IL-4+ Th2 and CD4+ IL-17A+ Th17 among total CD4+ T cells in 30 active NSV patients compared to 30 age- and sex-matched healthy controls in our study.
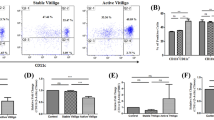
Part of freshly isolated PBMCs were directly used for detecting the frequency of CD4+ CD25+ CD127− Tregs and others were stimulated with PMA and ionomycin for 4 h and were analyzed for the frequency of CD4+ IFN-γ+ Th1, CD4+ IL-4+ Th2, CD4+ IL-17A+ Th17 by flow cytometry (Fig. 1). Significantly increased numbers of circulating Th1 [(8.68 ± 4.54) %, P < 0.01] and Th17 [(1.96 ± 1.07) %, P < 0.01] in CD4+ T cells were observed in progressive NSV patients compared with healthy controls [(5.64 ± 3.83) %; (1.17 ± 0.6) %]. However, the percentages of Th2 [(1.39 ± 0.49) %, P > 0.05] and Tregs [(4.46 ± 1.00) %, P > 0.05] had no statistical difference compared to the controls [(1.42 ± 0.68) %; (4.69 ± 0.96) %]. Moreover, we found that the ratio of Th1/Tregs and Th17/Tregs both significantly increased (2.38 ± 1.42 vs 1.32 ± 1.17, P < 0.01; 0.41 ± 0.22 vs 0.29 ± 0.12, P < 0.05) compared with healthy controls (Fig. 1c).
Peripheral CD4+ T cell subset distribution was altered in patients with progressive NSV. a PBMCs from active NSV patients (n = 30) and sex- and age-matched healthy controls (n = 30) were stained for CD4, CD25 and CD127 for the detection of Tregs. After 4-h stimulation by PMA and ionomycin in the presence of GolgiPlug in vitro, PBMCs were measured for expression of IFN-γ, IL-4 and IL-17A by intracellular staining. Representative dot plots obtained with samples from one healthy control and one active NSV patient were shown as an example. According to SSC and CD4 staining, cells were gated as CD4+ T lymphocytes. The number showed in the graph represented the mean value (%) of each CD4+ T cell subset; b diagrams showed the statistical results of various CD4+ T cell subsets; c diagrams showed the statistical results of the ratios of effector CD4+ T cells to suppressor CD4+ T cells. Data are expressed as mean ± SD. ns, P > 0.05; *P < 0.05; **P < 0.01
We also investigated the absolute numbers of Th1, Th2, Th17 and Tregs in circulation. Consistently, the absolute numbers of Th1 and Th17 were significantly elevated compared with their counterparts in healthy controls while no statistical difference was found in Th2 and Tregs between two groups (Table 1).
Specific transcription factors of different CD4+ T cell subsets in circulation
To solidify the observation by FACS analysis for the CD4+ T cell subsets, we further isolated total RNA from freshly isolated PBMCs and measured the expression levels of T-bet, GATA3, RORγt and FOXP3, which are the specific transcription factors for Th1, Th2, Th17 and Tregs, by qPCR, respectively (Fig. 2). We found that, normalized and calculated against that of housekeeping gene, mRNA expression levels of T-bet (1.542-fold, P < 0.05) and RORγt (1.2-fold, P < 0.05) were both significantly elevated compared with those of healthy controls, while GATA3 (1.093-fold, P > 0.05) and FOXP3 (1.1-fold, P > 0.05) had no statistical change compared with controls.
Specific transcription factor’s expression of peripheral CD4+ T cell subsets. a After isolation of total RNA from PBMCs, the expression of Th1-specific transcription factor T-bet, Th2-specific transcription factor GATA3, Th17-specific transcription factor RORγt and Treg-specific transcription factor FOXP3 at mRNA level was measured in active NSV patients (n = 15) and healthy controls (n = 15). β-actin was used as internal control; b freshly isolated PBMCs from active NSV patients (n = 25) and sex- and age-matched healthy controls (n = 11) were stained for T-bet, GATA3, RORγt and FOXP3. Data are expressed as mean ± SD. ns, P > 0.05; *P < 0.05; ***P < 0.001
Except for mRNA level, we further collected PBMCs from additional 25 active NSV patients and 11 healthy controls and evaluated the expression of T-bet, GATA3, ROR-γt and FOXP3 at protein level by intracellular staining (Fig. 2b). We found that compared with healthy controls [(4.55 ± 3.04) %; (1.03 ± 0.32) %], the proportions of T-bet+ CD4 T cells and RORγt+ CD4+ T cells were both greatly increased in active NSV patients [(10.79 ± 7.98) %, P < 0.001; (2.08 ± 1.21) %, P < 0.001], while no significant change was found in the percentage of Foxp3+ CD4+ T cells [(2.94 ± 1.29) % vs (2.91 ± 1.45) %, P > 0.05] and GATA3+ CD4+ T cells [(1.45 ± 0.68) % vs (1.39 ± 0.65) %, P > 0.05] between active NSV patients and healthy individuals.
Differential expression of CD4+ T subset-associated cytokine in serum
To compare the function of cytokine production of different CD4+ T cell subset in patients and controls, we collected sera from active NSV patients and healthy controls and assessed the levels of IFN-γ, IL-4, IL-17A and TGF-β1, which are hallmarks of Th1, Th2 and Th17 polarization, respectively (Fig. 3). We found that only the level of IL-17A [(23.08 ± 5.80) pg/ml, P < 0.001] was greatly elevated in sera of patients with active NSV compared with that of healthy controls [(8.66 ± 1.83) pg/ml]. Although the level of IFN-γ [(34.95 ± 14.94) pg/ml] was increased in patients, it had no statistic significance compared with controls [(29.56 ± 14.76) pg/ml]. Neither the levels of IL-4 [(0.2911 ± 0.11) pg/ml, P > 0.05] nor TGF-β1 [(1121 ± 181.7) pg/ml, P > 0.05] had significant difference compared to healthy controls [(0.2868 ± 0.07) pg/ml; (1321 ± 140.8) pg/ml].
Cytokine productions by peripheral CD4+ T cell subsets. ELISA analysis for the cytokine production (pg/ml) of various CD4+ T cell subsets in serum from progressive NSV patients (n = 30) and healthy controls (n = 30). All samples were performed in triplicate. ns, P > 0.05; ***P < 0.001. All samples were performed in triplicate. ns, P > 0.05; ***P < 0.001
Discussion
To our knowledge, our work is the first report that presents circulating Th1, Th2, Th17 and Tregs along with the status of their respective transcript factors, at both mRNA and protein levels, and cytokines in circulation in patients with active NSV in a single study. Although skin biopsy can provide direct evidence for local immune responses in skin, blood samples are more convenient for clinical researchers and more easily be accepted by patients especially for patients at progressive stage. Moreover, any change of the immune cells in circulation reflects the status of homeostasis of peripheral immune system. In our study, we found that with no significant change of frequency and absolute number of CD4+ T cells in active NSV (Online Resource 1), other than Th2 and Tregs, Th1 and Th17 showed significant increase in proportions, absolute numbers, specific transcript factors and cytokine production. Moreover, the significantly elevated ratios of Th1/Tregs and Th17/Tregs observed in circulation indicate a loss of balance between effector and suppressor CD4+ T cells.
With the advances in CD4+ T cell biology research, the initial Th1/Th2 classification has already been amended by ongoing discoveries of Tregs, Th17 and follicular T helper cells (Tfh) [18]. Because Tfh mainly takes parts in B cell immunity, we did not herein take this kind of CD4+ T cell subset into account. Inconsistent with our result, Ben et al. reported that the proportion of Tregs significantly decreased in the peripheral blood of vitiligo [5]. The difference may due to a slightly different definition of Tregs between us. They defined Tregs as CD4+ CD25+ T cells, while we defined them as CD4+ CD25+ CD127− T cells. Because the expression of CD25 will be upregulated after CD4+ T cells activation, our definition was more stringent.
In addition to the frequencies of CD4+ T cell subsets, their specific transcript factors were observed in our study. At mRNA level, we found both Th1-specific transcript factor, T-bet, and Th17-specific transcript factor, RORγt, were statistically increased compared with healthy controls. Although it is reported that RORγt could be expressed by a new kind of NK cells in mouse [16], this kind of cells does not exist in blood. T-bet also can be transiently expressed by primary B cells only in gut not in circulation [38]. Because we isolated the total RNA from PBMCs, not isolated CD4+ T cells, to exclude the possibility of contamination of other cells that may express T-bet and RORγt; we further limited the investigation by FACS analysis for these transcription factors only in CD4+ T cells. Using their corresponding antibodies to these transcription factors, we got similar results at protein level and further confirmed our previous data.
Interplay between two different CD4+ T cell subsets is very complicated. Tregs are potent suppressor CD4+ T cells, which can strongly inhibit effector CD4+ T cell subsets proliferation and induction to maintain the homeostasis of immune system. However, it was reported that Tregs could convert into Th17 in vitro [31, 33]. On the other hand, there exists competition among effector T cells for differentiation. It was reported that IL-4 and GATA3 could inhibit Th1 [25, 30, 40], Th17 [14] and Tregs [26] polarizations in vitro; Th1 and Th17 could inhibit Th2 polarization vice versa [9, 10, 28]. However, Th1 and Th17 often coexisted in some autoimmune diseases such as type 1 diabetes [22] and multiple sclerosis [7]. It was reported that IFN-γ could enhance Th17 induction in vitro [21]. Meanwhile, Th17 could not only induce Th1 but also directly transition into Th1 [12]. In our current study, we found both Th1 and Th17 increased in peripheral blood in patients with active NSV, which was consistent with others’ report on vitiligo skin biopsies [37]. The interaction between these two kinds of CD4+ T cell subsets in vitiligo and whether the increased Th17 is converted from Tregs need further investigations. On the basis of Th1 and Th17 profile in peripheral blood, it was not a surprise for us not finding a significant change of Th2 in our study.
Each CD4+ T cell subset secrets its lineage indicating cytokines such as IFN-γ (Th1), IL-4 (Th2), IL-17A (Th17) and TGF-β1 (Tregs). In vitro studies revealed that these four cytokines could affect normal functions or even the survival of melanocytes. It was reported that both IFN-γ and IL-17A could stimulate antimelanogenic cytokine IL-6 production in melanocytes and IL-4 could directly inhibit melanogenesis in melanocytes [41]. Both IFN-γ [41] and TGF-β1 could induce apoptosis in melanocytes [2] in vitro and TGF-β1 could inhibit the growth of melanocytes [32]. Thus, it may be expected that these cytokines may all increase in the sera of vitiligo patients. However, in our study, only IL-17A, Th17 mainly produced cytokine, was found significantly elevated in vitiligo. The change of serum IL-17A was consistent with others’ reports [4, 8, 44]. Although the percentage of IFN-γ-producing Th1 was significantly increased in active NSV patients, the level of IFN-γ in serum was higher than healthy controls with no statistical significance. The total level of IFN-γ in serum may be discounted by another IFN-γ-producing cell, i.e., CD8+ T cells, as we found no significant change in the frequency and the number of circulating CD8+ T cells in our study (Online resource1). Meanwhile, we found no significant change neither in the level of IL-4 nor TGF-β1 in serum compared with controls, which corresponded to our previous data of circulating Th2 and Tregs. Similar to IL-4, studies concerned in serum TGF-β1 indicated its level was either increased [44] or decreased [34] in patients with vitiligo. We found that TGF-β1 decreased a little in vitiligo; however, it had no statistical significance. These results further supported Th1 and Th17 polarization rather than Th2 and Tregs in active NSV.
In this study, we found enhanced Th1 and Th17 responses in circulation in active NSV and gave a detailed prospective in each of CD4+ T cell subsets. The correlation between the immunological changes in circulation and the skin tissue deserves further investigation. Additional studies for these CD4+ T cell subsets along with the status of their respective cytokines in circulation as well as in skin may provide better insight into the role of these cells in the pathogenesis of vitiligo.
References
Abdallah M, Lotfi R, Othman W, Galal R (2014) Assessment of tissue FoxP3+, CD4+ and CD8+ T-cells in active and stable nonsegmental vitiligo. Int J Dermatol 53:940–946
Alanko T, Saksela O (2000) Transforming growth factor beta1 induces apoptosis in normal melanocytes but not in nevus cells grown in type I collagen gel. J Invest Dermatol 115:286–291
Basak PY, Adiloglu AK, Ceyhan AM, Tas T, Akkaya VB (2009) The role of helper and regulatory T cells in the pathogenesis of vitiligo. J Am Acad Dermatol 60:256–260
Bassiouny DA, Shaker O (2011) Role of interleukin-17 in the pathogenesis of vitiligo. Clin Exp Dermatol 36:292–297
Ben Ahmed M, Zaraa I, Rekik R, Elbeldi-Ferchiou A, Kourda N, Belhadj Hmida N, Abdeladhim M, Karoui O, Ben Osman A, Mokni M, Louzir H (2012) Functional defects of peripheral regulatory T lymphocytes in patients with progressive vitiligo. Pigment Cell Melanoma Res 25:99–109
Choi H, Choi H, Han J, Jin SH, Park JY, Shin DW, Lee TR, Kim K, Lee AY, Noh M (2013) IL-4 inhibits the melanogenesis of normal human melanocytes through the JAK2-STAT6 signaling pathway. J Invest Dermatol 133:528–536
Edwards LJ, Robins RA, Constantinescu CS (2010) Th17/Th1 phenotype in demyelinating disease. Cytokine 50:19–23
Elela MA, Hegazy RA, Fawzy MM, Rashed LA, Rasheed H (2013) Interleukin 17, interleukin 22 and FoxP3 expression in tissue and serum of non-segmental vitiligo: a case–controlled study on eighty-four patients. Eur J Dermatol 23:350–355
Fitch F, Gajewski T, Nau G, Schell S, Otten G (1988) Nakahara memorial lecture. Regulation of T lymphocyte responses: interactions among receptors. Princess Takamatsu Symp 19:15–27
Geginat J, Paroni M, Maglie S, Alfen JS, Kastirr I, Gruarin P, De Simone M, Pagani M, Abrignani S (2014) Plasticity of human CD4 T cell subsets. Front Immunol 5:630
Grimes PE, Morris R, Avaniss-Aghajani E, Soriano T, Meraz M, Metzger A (2004) Topical tacrolimus therapy for vitiligo: therapeutic responses and skin messenger RNA expression of proinflammatory cytokines. J Am Acad Dermatol 51:52–61
Harbour SN, Maynard CL, Zindl CL, Schoeb TR, Weaver CT (2015) Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc Natl Acad Sci USA 112:7061–7066
Imran M, Laddha NC, Dwivedi M, Mansuri MS, Singh J, Rani R, Gokhale RS, Sharma VK, Marfatia YS, Begum R (2012) Interleukin-4 genetic variants correlate with its transcript and protein levels in patients with vitiligo. Br J Dermatol 167:314–323
Ivanov II, Zhou L, Littman DR (2007) Transcriptional regulation of Th17 cell differentiation. Semin Immunol 19:409–417
Jandus C, Bioley G, Rivals JP, Dudler J, Speiser D, Romero P (2008) Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum 58:2307–2317
Jetten AM (2009) Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal 7:e003
Khan R, Gupta S, Sharma A (2012) Circulatory levels of T-cell cytokines (interleukin [IL]-2, IL-4, IL-17, and transforming growth factor-beta) in patients with vitiligo. J Am Acad Dermatol 66:510–511
King C, Tangye SG, Mackay CR (2008) T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol 26:741–766
Klarquist J, Denman CJ, Hernandez C, Wainwright DA, Strickland FM, Overbeck A, Mehrotra S, Nishimura MI, Le Poole IC (2010) Reduced skin homing by functional Treg in vitiligo. Pigment Cell Melanoma Res 23:276–286
Kotobuki Y, Tanemura A, Yang L, Itoi S, Wataya-Kaneda M, Murota H, Fujimoto M, Serada S, Naka T, Katayama I (2012) Dysregulation of melanocyte function by Th17-related cytokines: significance of Th17 cell infiltration in autoimmune vitiligo vulgaris. Pigment Cell Melanoma Res 25:219–230
Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, Szeliga W, Wang Y, Liu Y, Welling TH, Elder JT, Zou W (2008) Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol 181:4733–4741
Labikova J, Vcelakova J, Ulmannova T, Petruzelkova L, Kolouskova S, Stechova K (2014) The cytokine production of peripheral blood mononuclear cells reflects the autoantibody profile of patients suffering from type 1 diabetes. Cytokine 69:189–195
Lili Y, Yi W, Ji Y, Yue S, Weimin S, Ming L (2012) Global activation of CD8+ cytotoxic T lymphocytes correlates with an impairment in regulatory T cells in patients with generalized vitiligo. PLoS One 7:e37513
Lin M, Zhang BX, Shen N, Dong XJ, Zhang C, Qi XY, Zhu J, Li YZ, Man MQ, Tu CX (2014) Regulatory T cells from active non-segmental vitiligo exhibit lower suppressive ability on CD8+CLA+T cells. Eur J Dermatol EJD 24:676–682
Lopez-Bravo M, Minguito de la Escalera M, Dominguez PM, Gonzalez-Cintado L, del Fresno C, Martin P, Martinez del Hoyo G, Ardavin C (2013) IL-4 blocks TH1-polarizing/inflammatory cytokine gene expression during monocyte-derived dendritic cell differentiation through histone hypoacetylation. J Allergy Clin Immunol 132:1409–1419
Mantel PY, Kuipers H, Boyman O, Rhyner C, Ouaked N, Ruckert B, Karagiannidis C, Lambrecht BN, Hendriks RW, Crameri R, Akdis CA, Blaser K, Schmidt-Weber CB (2007) GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol 5:e329
Moftah NH, El-Barbary RA, Ismail MA, Ali NA (2014) Effect of narrow band-ultraviolet B on CD4(+) CD25(high) FoxP3(+) T-lymphocytes in the peripheral blood of vitiligo patients. Photodermatol Photoimmunol Photomed 30:254–261
Noble A, Staynov DZ, Kemeny DM (1993) Generation of rat Th2-like cells in vitro is interleukin-4-dependent and inhibited by interferon-gamma. Immunology 79:562–567
Nouri-Koupaee A, Mansouri P, Jahanbini H, Sanati MH, Jadali Z (2015) Differential expression of mRNA for T-bet and GATA-3 transcription factors in peripheral blood mononuclear cells of patients with vitiligo. Clin Exp Dermatol 40:735–740
Paludan SR (1998) Interleukin-4 and interferon-gamma: the quintessence of a mutual antagonistic relationship. Scand J Immunol 48:459–468
Radhakrishnan S, Cabrera R, Schenk EL, Nava-Parada P, Bell MP, Van Keulen VP, Marler RJ, Felts SJ, Pease LR (2008) Reprogrammed FoxP3+ T regulatory cells become IL-17+ antigen-specific autoimmune effectors in vitro and in vivo. J Immunol 181:3137–3147
Rodeck U, Bossler A, Graeven U, Fox FE, Nowell PC, Knabbe C, Kari C (1994) Transforming growth factor beta production and responsiveness in normal human melanocytes and melanoma cells. Cancer Res 54:575–581
Sharma MD, Hou DY, Liu Y, Koni PA, Metz R, Chandler P, Mellor AL, He Y, Munn DH (2009) Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood 113:6102–6111
Tu CX, Jin WW, Lin M, Wang ZH, Man MQ (2011) Levels of TGF-beta(1) in serum and culture supernatants of CD4(+)CD25 (+) T cells from patients with non-segmental vitiligo. Arch Dermatol Res 303:685–689
Vaccaro M, Cannavo SP, Imbesi S, Cristani M, Barbuzza O, Tigano V, Gangemi S (2015) Increased serum levels of interleukin-23 circulating in patients with non-segmental generalized vitiligo. Int J Dermatol 54:672–674
van Geel NA, Mollet IG, De Schepper S, Tjin EP, Vermaelen K, Clark RA, Kupper TS, Luiten RM, Lambert J (2010) First histopathological and immunophenotypic analysis of early dynamic events in a patient with segmental vitiligo associated with halo nevi. Pigment Cell Melanoma Res 23:375–384
Wang CQ, Cruz-Inigo AE, Fuentes-Duculan J, Moussai D, Gulati N, Sullivan-Whalen M, Gilleaudeau P, Cohen JA, Krueger JG (2011) Th17 cells and activated dendritic cells are increased in vitiligo lesions. PLoS One 6:e18907
Wang Z, Wang Z, Wang J, Diao Y, Qian X, Zhu N (2016) T-bet-expressing B cells are positively associated with Crohn’s disease activity and support Th1 inflammation. DNA Cell Biol. doi:10.1089/dna.2016.3304
Wankowicz-Kalinska A, van den Wijngaard RM, Tigges BJ, Westerhof W, Ogg GS, Cerundolo V, Storkus WJ, Das PK (2003) Immunopolarization of CD4+ and CD8+ T cells to Type-1-like is associated with melanocyte loss in human vitiligo. Lab Invest 83:683–695
Wurtz O, Bajenoff M, Guerder S (2004) IL-4-mediated inhibition of IFN-gamma production by CD4+ T cells proceeds by several developmentally regulated mechanisms. Int Immunol 16:501–508
Yang L, Wei Y, Sun Y, Shi W, Yang J, Zhu L, Li M (2015) Interferon-gamma inhibits melanogenesis and induces apoptosis in melanocytes: a pivotal role of CD8+ Cytotoxic T lymphocytes in vitiligo. Acta Dermato-Venereol 95:664–670
Zhang BX, Lin M, Qi XY, Zhang RX, Wei ZD, Zhu J, Man MQ, Tu CX (2013) Characterization of circulating CD8+ T cells expressing skin homing and cytotoxic molecules in active non-segmental vitiligo. Eur J Dermatol EJD 23:331–338
Zhou L, Li K, Shi YL, Hamzavi I, Gao TW, Henderson M, Huggins RH, Agbai O, Mahmoud B, Mi X, Lim HW, Mi QS (2012) Systemic analyses of immunophenotypes of peripheral T cells in non-segmental vitiligo: implication of defective natural killer T cells. Pigment Cell Melanoma Res 25:602–611
Zhou L, Shi YL, Li K, Hamzavi I, Gao TW, Huggins RH, Lim HW, Mi QS (2015) Increased circulating Th17 cells and elevated serum levels of TGF-beta and IL-21 are correlated with human non-segmental vitiligo development. Pigment Cell Melanoma Res 28:324–329
Acknowledgments
The authors thank all patients and healthy donors who participated in the current study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This work was supported by the grants from the National Natural Science Foundation of China (No. 81401351) and Health and Family Planning Commission of Jilin Province (2012Z079). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhen, Y., Yao, L., Zhong, S. et al. Enhanced Th1 and Th17 responses in peripheral blood in active non-segmental vitiligo. Arch Dermatol Res 308, 703–710 (2016). https://doi.org/10.1007/s00403-016-1690-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-016-1690-3