Abstract
Background
The risk of transfusion following total hip arthroplasty (THA) continues to be problematic. The best choice of anesthesia (spinal vs general) and impact of tranexamic acid (TXA) use in reducing transfusions following surgery remain unclear. Therefore, the purpose of this study was to compare rates of blood transfusion following THA via the anterior approach using three different anesthesia protocols with and without TXA.
Materials and methods
This retrospective review included 1399 patients (1659 hips), receiving spinal anesthesia (SA) without (248 patients) and with TXA (77 patients), general anesthesia (GA) without (151 patients) and with TXA (171) and general anesthesia with paravertebral block (GA-PVB) and TXA (748 patients). All procedures were performed by a single surgeon. Chi-Squared tests and logistic regression were performed to evaluate the rate and risks of transfusion between groups.
Results
Without TXA, transfusion rate with GA (24.5%) was higher than SA (13.4%) (p = 0.004). With TXA, there was no difference in transfusion rates between GA (4.6%), SA (3.9%) or GA-PVB (4.0%). The multivariable regression revealed bilateral (Odds Ratio (OR): 6.473; p < 0.001), female (OR: 2.046; p = 0.004), age (OR: 1.028; p = 0.012) and pre-operative anemia (OR: 2.604; p < 0.001) as increasing the risk of transfusion while use of TXA (OR: 0.168; p < 0.001) significantly reduced transfusion risk.
Conclusion
The use of TXA during THA via the anterior approach removed the influence of anesthesia type regarding risk of transfusion. The use of TXA may reverse presumed disadvantages of GA alone, potentially facilitating rapid discharge following surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rate of transfusion following total hip arthroplasty (THA) varies widely across the United States, with the median incidence rate for individual hospitals approximated at nearly 16% [1]. Due to the high incidence and associated poor post-operative outcomes [2], perioperative blood management strategies, to include type of anesthesia administered, are often evaluated and modifications recommended. Spinal anesthesia (SA) is most commonly performed and has reported lower transfusion rates (1.6 to 16.2%) when compared to general anesthesia (GA) (0 to 19.8%) following THA [3,4,5,6]. The benefit of lower blood loss associated with intraoperative hypotension afforded by SA [5] may be diminished postoperatively, however, as prolonged hypotension and urinary retention following SA often delay discharge.
The clinical disadvantages of SA and the push toward outpatient surgery has led to the renewed enthusiasm for use of GA to perform THA and has been combined with additional blood management strategies to decrease the risk of post-operative transfusion. Tranexamic acid (TXA), an antifibrinolytic agent, is now widely used and has been shown to significantly decrease blood loss and the incidence of transfusion [2, 7, 8]. While less recognized as an important factor in blood management protocols, peripheral nerve blocks, such at the paravertebral block (PVB), used in multimodal analgesic regimens [9] may also have an important role in mitigating blood loss [10]. Previous research evaluating a lumbar plexus block in combination with GA [11, 12] has reported lower blood loss compared to GA alone. However, the true risk of transfusion may have been underrepresented due to the inclusion of autogenic blood transfusions [12].
Currently, several meta-analyses have evaluated anesthesia methods and the use of TXA but often include heterogeneous or outdated blood management protocols in the evaluation of multiple orthopedic procedures and surgical approaches [10, 13, 14]. While a single surgeon cohort may be considered less generalizable, the influence of isolated, specific procedural changes on the incidence of transfusion may be easier to clarify. At the current study site, three such isolated procedural changes occurred over a ten-year-period, including the additional use of TXA, phasing out SA in favor of GA and adoption of use of the PNB. All other surgical details, including the exclusive use of the anterior approach was maintained. While anecdotal evidence would suggest a decrease in overall transfusion rate, it remains unclear which blood management strategy was most impactful in the effort to reduce the incidence of transfusion. Therefore, the purpose of this study was (1) to retrospectively report on the incidence of post-operative blood transfusion following anterior approach THA using three different anesthesia protocols and (2) to evaluate the individual influence of patient demographics on transfusion incidence.
Materials and methods
This retrospective, institutional review board approved study evaluated a consecutive cohort of patients having undergone primary unilateral or bilateral THA from 2011 to 2020. All patients had indications to undergo THA for hip arthritis, including avascular necrosis diagnoses. Patients requiring THA for femoral neck fractures were excluded. No patients were excluded due to demographics or body habitus. All procedures were performed at a tertiary community hospital by a single, high volume arthroplasty surgeon exclusively using anterior approach for all primary THA since 2005. Patients underwent a standard pre-operative medical clearance for surgery performed by the patient’s primary care physician and any required medical specialist. All patients were screened and classified by a core group of experienced anesthesiologists as part of the Perioperative Surgical Home initiative put forth by the American Society of Anesthesiologists (ASA) [15].
Prior to surgery, all patients received pre-operative antibiotics and 1000 mg of acetaminophen, unless contraindicated. Patients did not undergo autologous donation prior to surgery nor did they receive medications to increase red blood cell concentration. Intraoperative cell salvage technology such as cell saver devices were not used in any unilateral or bilateral procedures. All THAs were performed using the anterior approach on a specialized fracture table (Hana®, Mizuho OSI, Union City, CA, USA), as described by Matta et al. [16]. Intraoperative fluoroscopy was used in all cases to assess cup and femoral stem positioning, leg length and hip offset.
Surgery was performed under GA or SA without TXA (2011–2013), under GA or SA with TXA (2014–2015) and GA-PVB (2015–present). The type of anesthesia was considered the standard of care during each date range; therefore, all patients received the same treatment unless specifically contraindicated. Beginning in 2014, use of TXA was adopted and all patients received one gram of intravenous tranexamic acid prior to incision and before closure of the surgical wound. Patients with a history of recurrent deep vein thromboses, pulmonary embolism or less than a three month history of thrombotic cerebrovascular stroke were excluded from receiving TXA and excluded from data analysis for this time period. Use of the PVB was implemented in 2015, containing bupivacaine 0.5% (20 cc), epinephrine (100 mcg) and Clonidine (1 mcg/kg), the maximum dose divided in half for bilateral procedures. Throughout the entire study period, all patients received a pericapsular injection with a max dose of bupivacaine 0.15% (1 cc/kg) and toradol (30 mg), again, divided in half for bilateral procedures. Additionally, a bipolar sealant device (Aquamantys™ Bipolar Sealer, Medtronic, Minneapolis, MN) was used to control intraoperative bleeding.
All patients received press fit acetabular and femoral implants utilizing neutral faced acetabular inserts. The hip capsule was preserved in all cases and closed using braided suture. The tensor fascia was closed with a running barbed suture or running braided suture. The subcutaneous layer was closed with either interrupted braided suture or a running monocryl. Final wound closure was completed using either staples or a zipper method (ZipLine® Medical, Silicon Valley, CA). Upon wound closure, each patient was given dexamethasone (4 mg) intravenously to control post-operative nausea. Patients receiving SA had routine placement of Foley catheters prior to surgery. Catheters were discontinued approximately 24 h following surgery with confirmation of normal urination ability prior to discharge. No patient undergoing GA or GA-PVB received a Foley catheter. Wound drains were not used in any patient.
Post-operative pain management included primarily acetaminophen with oral narcotics provided only upon patient request for break through pain. Post-operative nausea was symptomatically treated with anti-emetics. Immediate, unrestricted weight bearing as tolerated was allowed following adequate recovery from anesthesia. Intermittent mechanical foot compression was used for all patients until discharge. Deep vein thrombosis chemoprophylaxis was initiated on post-operative day one or two based on patient risk stratification. Patients were prescribed Aspirin 325 mg for 30 days after surgery, unless other risk factors were present. Aspirin 81 mg was utilized in selected cases of intolerance, bleeding, or bruising issues with higher doses of aspirin. Patients with additional venous thromboembolic risk factors such as cancer, prior history of venous thromboembolic, hormone replacement therapy or other high risk medications were prescribed a direct oral anticoagulant for 21 days. Low molecular weight heparins for 14 days was utilized if there were concerns of post-operative bleeding, primarily for those going to an inpatient acute rehabilitation facility. Patients requiring chronic anticoagulation resumed post-operative anticoagulants on post-operative day one or two.
Orthostatic hypotension or other signs of symptomatic anemia were evaluated prior to each physical therapy session which occurred twice daily prior to discharge. The criteria for allogenic blood transfusion was individually assessed. Only patients with symptomatic anemia (persistent orthostatic hypotension, tachycardia, hypoxia unaffected by fluid resuscitation or medication management such as holding antihypertensive medications or narcotic medications) accompanied by hemoglobin levels less than 8.0 g/dL were transfused. Pre-operative hemoglobin and hematocrit levels were recorded as well as postoperative levels for each day following surgery until discharge. Pre-operative hemoglobin was used to classify patients as anemic according to the World Health Organization (WHO), with females and males having a hemoglobin ≤ 12 g/dL and ≤ 13 g/dL, respectively, considered anemic [17].
Data were compared between SA and GA groups, separated by the presence or absence of TXA (four groups total). Patients receiving SA or GA with TXA were also compared to the GA-PVB with TXA group. Patient demographics and perioperative variables were compared between groups, with continuous and categorical variables evaluated via independent t-tests and Chi-Squared tests, respectively. All data were then combined and several univariate logistic regressions were performed to determine individual contributors to the risk of required blood transfusion. Significant variables in the univariate analyses and those variables determined clinically relevant were included in the multivariable, stepwise logistic regression (Forward: Likelihood Ratio) to determine the variables’ influence for the risk of transfusion. Results were presented as odds ratios (OR) and 95% confidence intervals (CI). All statistical analyses were performed using SPSS version 25 with a significant level of p < 0.05.
Results
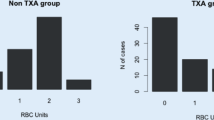
Overall, 1399 patients (1659 hips) were included in data analysis. Patient demographics and perioperative variables for each group are presented in Table 1. For patients not receiving TXA, the incidence of transfusion was significantly greater in the GA group (24.5%) compared to the SA group (13.4%) (p = 0.004). After the introduction of TXA, the incidence of transfusion significant decreased for both SA (13.4 to 3.9%, p = 0.012) and GA (24.5 to 4.6%, p < 0.001) patients, with the incidence of transfusion no longer different between SA and GA groups (p = 0.553). When compared to SA and GA with TXA, the incidence of transfusion was similar in the GA-PVB group (4.0%) (p = 0.941). Figure 1 shows the distribution between unilateral and bilateral transfusion incidence for each evaluated group.
When evaluating individual contributors to the risk of transfusion, female gender (p = 0.001), older age (p = 0.031), lower body mass index (p = 0.004) and undiagnosed pre-operative anemia (p < 0.001) were significant, patient specific variables (Table 2). Significant perioperative variables include an increased transfusion risk with bilateral procedures (p < 0.001) and lower risk with the use of TXA (p < 0.001). In reference to GA-PVB, patients receiving SA or GA regardless of TXA use were at a higher risk of transfusion (p < 0.001).
In the multivariable analysis, female gender (p = 0.004), increased age (p = 0.012) and pre-operative anemia (p < 0.001) remained significant patient specific contributors to the risk of transfusion. Additionally, bilateral procedures (p < 0.001) and the use of TXA (p < 0.001) remained significant perioperative contributors. However, anesthesia type was no longer significant, with no increased risk of transfusion with the use of SA (p = 0.757) or GA (p = 0.135) Table 3.
Discussion
Among all patients, including bilateral procedures, the total transfusion rate in the current study was 7.9%, falling within the range reported by previous studies of 1.0 to 9% in anterior approach THA [2, 5, 7, 8, 18, 19]. This overall rate, however, does not reflect the changes in blood management protocols at the study’s institution. Three main alterations were made to this protocol over the study period. First, the isolated transition from using SA to GA for THA resulted in an increase in post-operative transfusion rate from 13.4 to 24.5%, with the higher rate in GA supported by previous research [4,5,6, 13, 20]. However, the introduction of TXA decreased the overall transfusion rate and no statistical difference was noted between SA and GA (3.9% and 4.6%, respectively). These results are also consistent with previous research, citing a decrease in the rate of transfusion with the use of TXA during THA [2, 7, 8]. The lack of difference between SA and GA with the use of TXA increases the preference of GA, as previous research has reported lower occurrence of post-operative hypotension, no required use of urinary catheters leading to less urinary retention and fewer incidences of lower extremity weakness compared to SA, all of which are likely to delay discharge [3, 5].
Peripheral nerve blocks, while not routinely performed during THA, are purported to decrease post-operative pain but may also have several physiological intraoperative influences [9]. Managing intraoperative pain can decrease heart and respiratory rates, as well as reduce peaks in blood pressure thereby limiting intraoperative blood loss [6, 14]. Limiting blood loss is especially important in the THA patient demographic of the current study. Approximately 12 to 18% of patients in each group underwent a simultaneous bilateral procedure. Identified as a significant risk factor for transfusion, a previous study identified bilateral procedures as having the greatest impact on transfusion risk, second only to TXA use [6, 20]. With over 50% of the patients undergoing THA being female and/or over the age of 65 coupled with approximately 20% having pre-operative anemia, the risk for transfusion is high, particularly for this group, therefore, the potential benefits of the PVB should not be ignored.
Under the current standard of care, the overall transfusion rate in the GA-PVB group was 4.0%, which is lower than most studies (0 to 19.8%) reviewing transfusion rates following THA performed under GA [2,3,4,5,6,7,8, 18, 19]. The reason for the discrepancy is likely multifactorial and not due to any one specific intervention. The interplay of specific variables such as (1) use of a specialized fracture table which may facilitate exposure, (2) use of a bipolar sealant device, TXA and/or PVB, (3) avoidance of wound drains, (4) individualized patient specific transfusion triggers and individualized anticoagulation strategies to avoid overtreatment of low risk patients, and (5) surgical experience with exclusive use of the anterior approach likely all contribute to decreased transfusion requirements [21]. Quantifying or adequately assessing how these variables individually impact the risk for transfusion following anterior approach THA is difficult and remains a focused area of research for the current study site.
The present study has several limitations. First, this was a retrospective chart review of a cohort consisting of consecutively performed anterior approach THA by a single surgeon and institution. While the surgical experience was high at the point data collection was started, incremental increases in surgical experience over time could have impacted the results to favor the latter cohorts. It is interesting to note that following the adoption of TXA, however, results appear identical among the SA, GA and GA-PVB groups indicating surgical consistency. Secondly, a full comorbidity review, aside from WHO pre-operative anemia, were not feasible. The percentage of patients with low hemoglobin prior to surgery did significantly decrease over the course of the study, therefore could have influenced the incidence of transfusions. Lastly, the significant interdisciplinary cooperation, including a core group of anesthesiologist with specific interests in use of PVB, may limit the generalizability of these results.
Conclusion
Intravenous TXA far exceeded all other evaluated methods for decreasing the incidence of post-operative transfusion, with its use eliminating the discrepancy in transfusion rate between SA and GA. When using TXA, supplementing GA with a PVB did not reduce the transfusion rate, yet the benefits in controlling post-operative pain and facilitating outpatient discharge encourage the continued use of a PVB. Methods to further decrease the risk of transfusion should focus on patient demographics, such as weight, age and gender, and the risk for underlying anemia that can predispose patients to required post-operative blood transfusions.
References
Menendez ME, Lu N, Huybrechts KF, Ring D, Barnes CL, Ladha K, Bateman BT (2016) Variation in use of blood transfusion in primary total hip and knee arthroplasties. J Arthroplasty 31(12):2757
Free MD, Owen DH, Pascoe E, Allen P, Yang L, Harvie P (2019) Transfusion rates with intravenous tranexamic acid in total hip arthroplasty performed using the direct anterior approach. Hip Int 29(5):511
Stambough JB, Bloom GB, Edwards PK, Mehaffey GR, Barnes CL, Mears SC (1889) Rapid recovery after total joint arthroplasty using general anesthesia. J Arthroplasty 34(9):2019
Basques BA, Toy JO, Bohl DD, Golinvaux NS, Grauer JN (2015) General compared with spinal anesthesia for total hip arthroplasty. J Bone Joint Surg Am 97(6):455
Turcotte JJ, Stone AH, Gilmor RJ, Formica JW, King PJ (2020) The Effect of neuraxial anesthesia on postoperative outcomes in total joint arthroplasty with rapid recovery protocols. J Arthroplasty 35(4):950
Haughom BD, Schairer WW, Nwachukwu BU, Hellman MD, Levine BR (2015) Does neuraxial anesthesia decrease transfusion rates following total hip arthroplasty? J Arthroplasty 30(9 Suppl):116
Dabash S, Barksdale LC, McNamara CA, Patel PD, Suarez JC (2018) Blood loss reduction with tranexamic acid and a bipolar sealer in direct anterior total hip arthroplasty. Am J Orthop (Belle Mead NJ). https://doi.org/10.12788/ajo.2018.0032
Zhao H, Xiang M, Xia Y, Shi X, Pei FX, Kang P (2018) Efficacy of oral tranexamic acid on blood loss in primary total hip arthroplasty using a direct anterior approach: a prospective randomized controlled trial. Int Orthop 42(11):2535
Hannon CP, Keating TC, Lange JK, Ricciardi BF, Waddell BS, Della Valle CJ (2019) Anesthesia and analgesia practices in total joint arthroplasty: a survey of the american association of hip and knee surgeons membership. J Arthroplasty 34(12):2872
Guay J, Johnson RL, Kopp S (2017) Nerve blocks or no nerve blocks for pain control after elective hip replacement (arthroplasty) surgery in adults. Cochrane Database Syst Rev 10:CD011608
Utebey G, Akkaya T, Alptekin A, Sayin M, Gumus H, Ates Y (2009) The effects of lumbar plexus block and epidural block on total blood loss and postoperative analgesia in total hip arthroplasty. Agri 21(2):62
Stevens RD, Van Gessel E, Flory N, Fournier R, Gamulin Z (2000) Lumbar plexus block reduces pain and blood loss associated with total hip arthroplasty. Anesthesiology 93(1):115
Hu S, Zhang ZY, Hua YQ, Li J, Cai ZD (2009) A comparison of regional and general anaesthesia for total replacement of the hip or knee: a meta-analysis. J Bone Joint Surg Br 91(7):935
Richman JM, Rowlingson AJ, Maine DN, Courpas GE, Weller JF, Wu CL (2006) Does neuraxial anesthesia reduce intraoperative blood loss? A meta-analysis J Clin Anesth 18(6):427
Kain ZN, Vakharia S, Garson L, Engwall S, Schwarzkopf R, Gupta R, Cannesson M (2014) The perioperative surgical home as a future perioperative practice model. Anesth Analg 118(5):1126
Matta JM, Shahrdar C, Ferguson T (2005) Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res 441:115
Blanc B, Finch C, Hallberg L, Herbert V, Lawkowicz W, Layrisse M (1968) Nutritional anaemias. Report of a WHO scientific group. WHO Tech Rep Serv. 405:1
Cheng TE, Wallis JA, Taylor NF, Holden CT, Marks P, Smith CL, Armstrong MS, Singh PJ (2017) A prospective randomized clinical trial in total hip arthroplasty-comparing early results between the direct anterior approach and the posterior approach. J Arthroplasty 32(3):883
Vles GF, Corten K, Driesen R, van Elst C, Ghijselings SG (2021) Hidden blood loss in direct anterior total hip arthroplasty: a prospective, double blind, randomized controlled trial on topical versus intravenous tranexamic acid. Musculoskelet Surg 105(3):267
Walsh M, Preston C, Bong M, Patel V, Di Cesare PE (2007) Relative risk factors for requirement of blood transfusion after total hip arthroplasty. J Arthroplasty 22(8):1162
Attenello J, Andrews S, Nishioka S, Mathews K, Nakasone C (2021) Perioperative strategies to reduce transfusion rates in one-stage bilateral total hip arthroplasty via direct anterior approach. J Orthop 23:118
Funding
This research received no specific grant from any funding agency in the public, commercial or non-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Each author certifies that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Ethical approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Hawai ‘i Pacific Health Research Institute (local Western Institutional Review Board) approved this study.
Informed consent
This was a retrospective chart review and data collected were deidentified and presented as large scale, aggregate data. Therefore, no informed consent was obtained or required by the IRB.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Combs, D.B., Hummel, A., Nishioka, S.T. et al. Reducing transfusion in hip arthroplasty: tranexemic acid diminishes influence of anesthesia administered. Arch Orthop Trauma Surg 143, 3535–3540 (2023). https://doi.org/10.1007/s00402-022-04591-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-022-04591-2