Abstract
Background
Closed reduction and internal fixation (CRIF) is the preferred treatment to retain the native joint and maintain optimal functionality in femoral neck fractures. Sliding hip screw (SHS) and cannulated hip screws (CHS) are established CRIF options. SHS offer high biomechanical stability, whereas CHS are minimally invasive. These established systems have a 17–21% failure rate. The Femoral neck system (FNS) was recently developed to combine the advantages of both predecessors. The aim of this study was to describe the first clinical experience with this novel implant with special emphasis on the safety and efficacy.
Methods
During a 1-year period all patients in our level-2 trauma centre with a FNF indicated for CRIF were treated using the FNS and evaluated at 2, 6, 12 weeks, 6 months and 1 year postoperatively using patient and fracture characteristics, surgical notes and radiographic imaging.
Results
Thirty-four patients were included, mean age was 63 years (SD 8), 58.2% was female. Fractures were classified as Pauwels I (n = 10), Pauwels II (n = 15), Pauwels III (n = 9), Garden I (n = 1), Garden II (n = 17), Garden III (n = 12) and Garden IV (n = 4). Eight reoperations were reported after 1-year follow-up; osteosyntheses failed in 6 patients due to avascular necrosis (n = 4) and cut-out (n = 2). In two patients the implant was removed due to inexplicable pain. Age (< 65 years) was related to lower risk for failure. There was a trend for females having more failures.
Conclusion
This study indicates that the FNS is a potential safe and effective CRIF modality. Age (< 65 years) is an important factor to keep in mind when selecting patients for CRIF as it is related to lower risk for failure. Future long-term follow-up studies with larger populations should indicate if functional results and risk factors for failure are comparable to SHS or CHS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Femoral neck fractures (FNF) are the most common type of proximal femur fractures [1]. With increasing life expectancy it is estimated that worldwide incidences will increase to 6.26 million by 2050 [2]. The number living with hip fracture related disability is expected to rise to 21 million in the next 40 years [3]. Standard treatment for FNF is surgical, as dictated by international as well as national Dutch guidelines, offering early patient mobilization and decreased risk for complications [4]. Surgical treatment is a multifactorial decision based on fracture type, patient characteristics, local preferences and routines. Arthroplasty is the treatment of choice in biological elderly sustaining hip fractures and patients with symptomatic osteoarthritis in their medical history [5]. For younger, healthy and active patients who are likely to outlive their hip arthroplasty, joint preserving surgery by closed reduction and internal fixation (CRIF) is often preferred [6]. CRIF is increasingly applied to achieve better functional outcomes, less complications and prevent early hip arthroplasty revision surgery [7]. In current practice, CRIF typically involves fixation with 3 cancellous hip screws (CHS) or a sliding hip screw (SHS) principle [3]. CHS offers the advantage over SHS of a relatively minimal invasive technique and shorter operation time. On the other hand, SHS provides more biomechanical stability [3]. Both SHS and CHS are appropriate methods for Pauwels I–II type fractures, whereas SHS is also indicated for FNFs that are more vertically oriented or displaced [8, 9]. The recently introduced Femoral Neck System (FNS, DePuy Synthes, MA, USA) theoretically combines the stability of SHS with the minimal invasiveness of CHS [10, 11]. FNS is a CRIF modality using a lateral support plate and two screws entering the lateral cortex through the same entry point, subsequently deviating into the femoral head to provide rotatory stability. Intended use of FNS are all AO-31B fractures according to the manufacturer, thus also including more challenging Pauwels II–III and Garden III–IV.
Unfortunately, all types of joint preservation fixations for FNF carry risks of secondary failure due to avascular necrosis or non-union [3, 12, 13]. Violation of femoral head vasculature due to the fracture or secondary displacement is often held accountable [3, 12, 13] Moreover, several factors are known to increase the risk of failure after SHS and CHS, such as female sex, body mass index (BMI), fracture displacement, quality of reduction, high age, kidney failure, chronic pulmonary issues, smoking and alcohol abuse [3, 12,13,14,15]. Until now, publications on FNS are limited to in vitro studies testing its stability [10, 11]. Clinical experience and failure rates of FNS are consequently lacking.
The objective of this study is to provide our first year of clinical experience using FNS at a large level II trauma teaching hospital, with a special focus on implant failure in perspective to commonly known risk factors.
Methods
Patients
FNS was introduced in November 2018 at Zuyderland Medical Center (Heerlen, the Netherlands), a level 2 trauma teaching hospital. Since its introduction in November 2018, all FNF indicated for CRIF were fixed using FNS. Inclusion of patients for this study started when the first implant was used. All included patients were mono-trauma patients. Procedures were performed by trauma or orthopaedic surgeons or a directly supervised resident-in-training. During the period of November 2018–November 2019, all FNS-treated patients were included in this retrospective analysis of prospectively collected data. Clinically obtained data of patients included: age, gender, BMI, ASA-classification, pretraumatic housing and mobility, medical history, fracture type, Almelo hip fracture score (early mortality prediction score based on patient specific variables), surgery time, length of incision as described in the operative report, tip–apex index, position of the implant on the anteroposterior (cranial, central or caudal) and lateral radiographs (ventral, central or dorsal), length of hospital stay and complications (both intra-operative and postoperative)[16]. Fracture type was distinguished using the AO-foundation classification and by the straightforward Pauwels or Garden classification [17,18,19]. Criteria for CRIF were according to the intended use criteria of the FNS, as described by its producer, i.e., FNF AO type 31-B and national guidelines [20]. Patients with Garden III/IV, Pauwels III or other typical SHS/CHS failure risk factors were extensively counselled according to shared decision principles concerning their potentially increased likelihood of implant failure [14, 15] and were only considered for FNS when young of age (≤ 65 years), no extensive medical history was present and were in good physical condition. In patients between 65 and 85 years of age a decision would be made depending on a combination of biological age, amount of fracture dislocation and comorbidities. Criteria for hip arthroplasty included existence of Kellgren–Lawrence grade 3–4 osteoarthritis on plain radiograph, a medical history of symptomatic osteoarthritis, reduced mobility, increased biological age or patients explicitly indicating the desire for a total hip arthroplasty (THA) [16]. Patients were transferred to the orthopaedic department if hip arthroplasty was indicated. In total, 35 patients met CRIF criteria and were treated using FNS. This study was approved by the local medical ethical committee (METCZ; ID: METC Z2020051, date of approval: April 6, 2020) and the need for informed consent was waived. This report was written in compliance with the STROBE-guidelines (Strengthening the Reporting of Observational Studies in Epidemiology) [21].
Surgery in brief
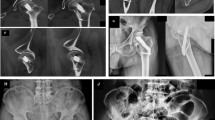
The patient was placed supine on a conventional traction table with the affected leg in a traction shoe and the contralateral leg was put in an elevated leg rest. Closed fracture reduction was monitored using fluoroscopy in two views. A standard sterile surgical field was set up. An antirotation Kirschner wire was inserted in the superior–anterior part of the head through a small stab incision. A lateral—approximately 4 cm—incision was made starting 20–30 mm distal to the centre of the femoral neck axis. Subcutaneous layers, fascia and vastus lateralis muscle were split to reach the lateral cortex of the proximal femur. A guidewire was inserted using the 130 degree angled guide, aiming for biplanar central position through the femoral neck and head (Fig. 1). Guidewire depth was measured to determine the required construct size, after which the bone was reamed and the implant—comprised of the bolt engaged in the lateral support plate (Fig. 1A)—was placed using the insertion handle. A locking antirotation screw was subsequently inserted through the insertion handle using the same entry as the bolt. Finally, the distal locking screw through the plate was inserted using an additional protection sleeve through the insertion handle. All surgeons used the same technique during the study period. Senior surgeons involved had surgical experience ranging between 4 and 20 years with different forms of CRIF. None of these surgeons had prior experience with the FNS. Typical intra-operative steps are presented in Fig. 2A, B.
Postoperative follow-up
All patients were followed and evaluated at 2-, 6- and 12-week postoperative at the outpatient department (OPD). Only if pain persisted after 12 weeks, follow-up was prolonged to 6 months. 1 year after surgery all patient files were once again checked for complications. If none were reported, patients were contacted to evaluate complaints. Standard plain anteroposterior and lateral radiographs were obtained directly after surgery and after 6 weeks. If pain persisted after 6 weeks, radiographs were obtained after 12 weeks. When fracture healing was deemed uncertain or delayed after 4 months, additional imaging was obtained. Clinical signs of delayed fracture were defined as persisting pain at the fracture site at rest or on palpitation with limitations in weight bearing. Radiological signs of delayed union were defined as absence of callus, bone or trabeculae on both anteroposterior and lateral plain radiographs. When conventional radiographs failed to detect abnormalities, an additional computed tomography was obtained, on indication complemented using single photon emission computed tomography. Failure was defined as avascular necrosis or non-union. In case of failure, patients were referred to an orthopaedic surgeon for further treatment. Trombo-embolic prophylaxes (low molecular weight heparins) were administered for 6 weeks according to local protocols. Physiotherapy was issued for all patients and permissive weightbearing was allowed directly after surgery. Hospital discharge criteria were normal vital parameters, dry wound, safe ambulant mobilization or further rehabilitation in a dedicated clinic.
Statistical methods
All statistical analysis were performed using SPSS (IBM Corp. IBM SPSS Statistics for MacOS, Version 25.0, Armonk, NY, USA). Normality was tested using the Kolmogorov–Smirnov test. Continuous variables were denoted as mean and standard deviation (SD) if normally distributed. Non-normally distributed variables were reported as median and interquartile range (IQR). Categorical variables were reported as frequencies and percentages of the total. Differences between groups were analysed using Pearson χ2 test for dichotomous variables. A p value < 0.05 was considered statistically significant. Missing data were reported as such.
Results
Patient and fracture characteristics
Between November 2018 and November 2019, 35 patients were treated using FNS. One patient was excluded from evaluation for being a multi-trauma case with prolonged admission to the intensive care unit due to additional injuries. Thirty-four patients completed 6-month follow-up, none were lost to follow-up. The majority was female (58.8%) with a mean age of 63 years. Nineteen patients had a BMI > 25 (kg/m2). Six patients (17.6%) classified as ASA III. Thirty-one patients (91.2%) lived at home at time of trauma.
Twelve fractures were classified as Garden III (35.3%) and 4 as Garden IV (11.8%). All patients scored below 9 on the AHFS implicating low risk for early mortality. Patient demographics and fracture specifications are listed in Table 1.
Surgery
Mean surgical time was 34 min (SD 9.4 min). Blood loss was negligible. Incision length ranged between 30 and 80 mm (mean 45.3 mm, SD 8.8 mm). The majority of procedures (n = 20) was performed by senior surgeons. Altogether, 5 senior surgeon were involved. No intraoperative complications were observed.
Postoperative care
Thirty-two out of 34 patients (94.1%) were allowed permissive weightbearing after surgery. Two patients were restricted to reduced mobilization (< 50% weightbearing) due to the surgeons preference. Both procedures were performed by one orthopaedic surgeon. The restriction of reduced mobilization was solely based on the surgeons preference. Sixteen patients out of 34 were referred to a rehabilitation clinic specialized in rehabilitation after hip fracture surgery. Other patients received standardised care at home including physiotherapy.
Postoperative outcome and complications
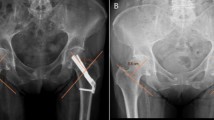
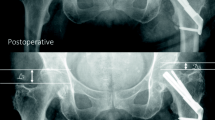
Patients were hospitalized for a mean of 4 days (SD 2.8 days). Five patients were hospitalized over 7 days due to unavailable rehabilitation places, but were clinically ready for hospital discharge after 4 days. One patient developed pneumonia and an urinary tract infection postoperatively which were both successfully treated using antibiotics. No intra-operative complications were observed. Eight patients (23.5%) presented themselves within 12 months due to persisting pain in the affected hip. In six of these patients, additional radiological imaging showed both biological failure, namely, avascular necrosis (n = 4), and implant failure, defined as cut out (collaps of the neck–shaft angel into varus) (n = 2). Two out of these eight patients showed no radiological abnormalities in fracture healing after 8 months but complained of the sensation of irritating osteosynthesis material. In these two patients, implants were removed 10 months after primary surgery and both patients rehabilitated uneventful after removal without further pain during follow-up. These patients were not included in the failure group as no clinical or radiological cause for their complaints could be found. With regard to the failure of osteosyntheses, four out of six cases had suboptimal implant positioning (increased tip–apex distance or the implant not centrally positioned, see (Fig. 3)). In those cases, 3 patients showed avascular necrosis, one cut-out. All six patients with CRIF failure underwent conversion to total hip arthroplasty. No additional interventions were required. No factors were found to significantly increase the risk for complications (see Table 2).
Discussion
The present 1-year follow-up study showed that FNS is a minimally invasive and successful treatment option for Garden I–IV and Pauwels I–III type FNF in a variety of patients, with failure rates and patterns comparable to those observed in established CRIF systems. The overall failure rate for FNS was 17.6% after 1 year. As these rates are comparable to the established CRIF systems, it is safe to say this technique is at least comparable in use as other systems. To the best of our knowledge, this was the first study describing the clinical experience using this novel implant.
Failure of CRIF in FNF is commonly caused by biological failure due to avascular necrosis of the femoral head or non-union of the fracture. It is a severe, and the most common complication of CRIF. Failure rates vary in literature but are estimated to be 17–21% after 6–24 months [3, 5]. Our failure rate falls at the lower limit of this range. However, future follow-up should indicate whether failure rates remain similar at 24 months. The largest study investigating the risk factors for revision was derived from the FAITH trial study group and found generic risk factors for failure, including female sex, higher BMI, increased age, more displaced fractures and suboptimal fracture reduction and implant placement [3, 12]. Present study also included kidney function, COPD and time to surgery as risk factor based on the study by Duckworth et al. [14]. Although not significant, females in present study also showed a trend towards having a higher risk for failure. Of the results of other known factors, only age was related to an increased risk for failure in present study. Being the first clinical experience study, the number of patients was probably too low to reach significance for female sex and perhaps other risk factors as well, i.e., our study could have been prone to level II error being underpowered for these sub-analyses. Nevertheless, medical professionals should thoroughly discuss the typical risk factors for failure during hospital admittance and facilitate a shared decision making for the eventual treatment of choice. As Dutch guidelines suggest CRIF in patients < 65 years of age and in certain cases older patients depending on a combination of biological age, amount of fracture dislocation and comorbidities, we have observed in this study that there is a significant difference in age in both groups. Especially younger patients (< 65 years of age), tend to have a lower risk for failure compared to the older patients (> 65 years of age). Avascular necrosis of the femoral head has in some studies been observed to be higher in SHS than in CHS [3]. This observation has been rationalized to originate from more violation of vasculature due to thicker diameter in SHS column screw compared to screws in CHS [3]. Typical SHS systems employ 13 mm diameter column screws (cross-sectional surface area 133 mm2), the three screws in CHS systems are commonly 6.5 mm in diameter (sum of cross-sectional surface areas 100 mm2) and the FNS is comprised of a central bolt measuring 10 mm in diameter with a 6.4 mm antirotation screw (sum of cross-sectional surface areas 111 mm2) [22, 23]. Future bone scintigraphy studies or clinical comparative studies with sufficient power should clarify how FNS acts on the vascularization in comparison to existing CRIF systems.
Stoffel et al. [10] published a cadaveric biomechanical study comparing FNS with CHS and dynamic hip screw as SHS system with either antirotation screw or blade in simulated Pauwels III fractures. In terms of axial loaded cycles until 15 mm femoral neck or 15 mm leg shortening, the FNS was comparable to the SHS systems and significantly more resilient to loading compared to CHS. Schopper et al. (2020) compared the mechanical failure in the FNS to Hansson Pins in a simulated Pauwels II model with posterior fracture comminution. Again, the FNS was considered biomechanically superior in terms of cycles till 10° angular failure. If this is also applies in vivo should be subject of future investigations. Namely, an important finding of previous research was that smokers treated by CHS had a higher change of failure than when treated by SHS [3, 12]. In general, smokers have lower bone mineral densities (BMD) and impaired bone healing which jeopardizes postoperative mechanical stability, thus perhaps favouring implants with more primary mechanical stability [12]. Studies with subgroup analyses on BMD and smoking status can possibly clarify if FNS also has these biomechanical advantages over CHS.
The FNS shares several similarities to SHS and intramedullary nail surgeries for intertrochanteric fractures. For instance, patient and surgeon positioning and the pivotal closed reduction on the traction table are similar. Hence, 15 out of 35 surgeries were performed by residents in training who were all well familiar with intramedullary nailing procedures. The fact that there were no differences in failure between residents (under direct supervision) and senior surgeons, confirms the translation of previous techniques and the similar difficulty of the procedure. It should be noted that adequate positioning of the central bolt seems essential. Most of the failures in present study showed increased tip–apex distances or a bolt not in central position, hypothetically leading to decreased primary biomechanical stability as has been observed in SHS fixation of intertrochanteric fractures [24]. Similar to SHS, FNS allows for dynamic movement of facture ends in the femoral neck—i.e., relative stability—thereby stimulating endochondral ossification [25]. At the same time, FNS offers the potential benefit to dynamize only over 20 mm, therefore, reducing the risk of possible difference in leg length after fracture healing. Contrary to SHS, the combination of an integrated anti-rotation screw through the same entry as the column screw with a small lateral support plate allows a much smaller incision for the FNS. Minimally invasive incisions have been described for SHS but the conventional incision is typically around 57 mm and can go up to 150 mm [26, 27]. For FNS we found a mean incision length of 45 mm and we postulate that this can be decreased to lengths close to CHS values (27 mm according to Lee et al.) as experience with the surgery further increases [28]. Similar to many surgeries, larger incisions are required for obese patients, as was the case in one of our patients with a BMI of 33 kg/m2 with a 80 mm incision.
The major strength of present study is that it is the first report of a novel CRIF implant. We included all types of fractures according to the manufacturers intended use, thus also including severely displaced fractures. Every single patient treated with FNS in our centre was included in this study. Surgeries were performed by surgeons of different departments and with different levels of experience to expose the generalisability of the technique. Due to a certain degree of learning-curve, better outcomes are expected in future surgery. Most limitations were inherent to being the first report. Present study was potentially underpowered to reveal the typical CRIF risk factors. Smoking and alcohol status were not routinely obtained at the emergency department, while these are important risk factors. We did not include patient reported outcome measures (PROMs) and these should be included in future reports to objectify and distinguish more subtle subjective differences. Since all CRIF indications were treated using the FNS, there is a risk for selection bias at the OPD and an inherent lack of a dedicated control group. A prospective trial comparing the FNS with established CRIF systems should be part of future research such as the recently initiated multicentre trial for the Gannet, a dynamic locking blade plate [29].
Conclusion
This first study describing the use of FNS in patients with FNF with a 1-year follow-up. It showed that both failure rates and technical difficulty are at least comparable to established CRIF systems. Age (< 65 years) is an important factor to keep in mind when selecting patients for CRIF as it is related to lower risk for failure. Future clinical research with larger populations should clarify if FNS is superior to SHS and CHS, particularly bearing the mechanical stability in mind.
Availability of data and material
Not applicable
Code availability
Not applicable
References
Karagas MR, Lu-Yao GL, Barrett JA, Beach ML, Baron JA (1996) Heterogeneity of hip fracture: age, race, sex, and geographic patterns of femoral neck and trochanteric fractures among the US elderly. Am J Epidemiol 143(7):677–682
Cooper C, Campion G, Melton LJ 3rd (1992) Hip fractures in the elderly: a world-wide projection. Osteoporos Int 2(6):285–289. https://doi.org/10.1007/bf01623184
Nauth A, Creek AT, Zellar A, Lawendy A-R, Dowrick A, Gupta A et al (2017) Fracture fixation in the operative management of hip fractures (FAITH): an international, multicentre, randomised controlled trial. Lancet 389(10078):1519–1527. https://doi.org/10.1016/S0140-6736(17)30066-1
Verheyen CC, Smulders TC, van Walsum AD (2005) High secondary displacement rate in the conservative treatment of impacted femoral neck fractures in 105 patients. Arch Orthop Trauma Surg 125(3):166–168
Rogmark C, Kristensen MT, Viberg B, Rönnquist SS, Overgaard S, Palm H (2018) Hip fractures in the non-elderly—who, why and whither? Injury 49(8):1445–1450
Ghayoumi P, Kandemir U, Morshed S (2015) Evidence based update: open versus closed reduction. Injury 46(3):467–473
Bhandari M, Devereaux P, Swiontkowski MF, Tornetta P III, Obremskey W, Koval KJ et al (2003) Internal fixation compared with arthroplasty for displaced fractures of the femoral neck: a meta-analysis. JBJS 85(9):1673–1681
Aminian A, Gao F, Fedoriw WW, Zhang L-Q, Kalainov DM, Merk BR (2007) Vertically oriented femoral neck fractures: mechanical analysis of four fixation techniques. J Orthop Trauma 21(8):544–548
Stiasny J, Dragan S, Kulej M, Martynkiewicz J, Płochowski J, Dragan S (2008) Comparison analysis of the operative treatment results of the femoral neck fractures using side-plate and compression screw and cannulated AO screws. Ortop Traumatol Rehabil 10(4):350–361
Stoffel K, Zderic I, Gras F, Sommer C, Eberli U, Mueller D et al (2017) Biomechanical evaluation of the femoral neck system in unstable Pauwels III femoral neck fractures: a comparison with the dynamic hip screw and cannulated screws. J Orthop Trauma 31(3):131–137
Schopper C, Zderic I, Menze J, Muller D, Rocci M, Knobe M et al (2020) Higher stability and more predictive fixation with the Femoral Neck System versus Hansson Pins in femoral neck fractures Pauwels II. J Orthop Translat 24:88–95. https://doi.org/10.1016/j.jot.2020.06.002
Sprague S, Schemitsch EH, Swiontkowski M, Della Rocca GJ, Jeray KJ, Liew S et al (2018) Factors associated with revision surgery after internal fixation of hip fractures. J Orthop Trauma 32(5):223–230. https://doi.org/10.1097/BOT.0000000000001162
Gregersen M, Krogshede A, Brink O, Damsgaard EM (2015) Prediction of reoperation of femoral neck fractures treated with cannulated screws in elderly patients. Geriatr Orthop Surg Rehabil 6(4):322–327. https://doi.org/10.1177/2151458515614369
Duckworth A, Bennet S, Aderinto J, Keating J (2011) Fixation of intracapsular fractures of the femoral neck in young patients: risk factors for failure. J Bone Jt Surg Br Vol 93(6):811–816
Parker MJ, Raghavan R, Gurusamy K (2007) Incidence of fracture-healing complications after femoral neck fractures. Clin Orthop Relat Res 458:175–179
Nijmeijer W, Folbert E, Vermeer M, Slaets J, Hegeman J (2016) Prediction of early mortality following hip fracture surgery in frail elderly: the Almelo Hip Fracture Score (AHFS). Injury 47(10):2138–2143
Bartonícek J (2001) Pauwels’ classification of femoral neck fractures: correct interpretation of the original. J Orthop Trauma 15(5):358–360
Meinberg E, Agel J, Roberts C, Karam MD, Kellam J (2018) Fracture and dislocation classification compendium—2018. J Orthop Trauma 32:S1–S10
Garden RS (1961) Low-angle fixation in fractures of the femoral neck. J Bone Jt Surg Br Vol 43(4):647–663
Federatie Medisch Specialisten (2016) Proximale femurfracturen. In: Richtlijnendatabase. https://richtlijnendatabase.nl/richtlijn/proximale_femurfracturen/proximale_femurfracturen_-_startpagina.html. Accessed 01 Feb 2020
Vandenbroucke JP, Von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ et al (2007) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 4(10):e297
DePuySynthes (2020) DHS/DCS system surgical technique. Synthes
Acumed (2020) Cannulated Screw System Surgical Technique
Caruso G, Bonomo M, Valpiani G, Salvatori G, Gildone A, Lorusso V et al (2017) A six-year retrospective analysis of cut-out risk predictors in cephalomedullary nailing for pertrochanteric fractures: can the tip-apex distance (TAD) still be considered the best parameter? Bone Jt Res 6(8):481–488. https://doi.org/10.1302/2046-3758.68.BJR-2016-0299.R1
Foster AL, Moriarty TF, Zalavras C, Morgenstern M, Jaiprakash A, Crawford R et al (2020) The influence of biomechanical stability on bone healing and fracture-related infection: the legacy of Stephan Perren. Injury. https://doi.org/10.1016/j.injury.2020.06.044
Ho M, Garau G, Walley G, Oliva F, Panni AS, Longo UG et al (2009) Minimally invasive dynamic hip screw for fixation of hip fractures. Int Orthop 33(2):555–560. https://doi.org/10.1007/s00264-008-0565-4
Ma JX, Kuang MJ, Xing F, Zhao YL, Chen HT, Zhang LK et al (2018) Sliding hip screw versus cannulated cancellous screws for fixation of femoral neck fracture in adults: a systematic review. Int J Surg 52:89–97. https://doi.org/10.1016/j.ijsu.2018.01.050
Lee YS, Chen SH, Tsuang YH, Huang HL, Lo TY, Huang CR (2008) Internal fixation of undisplaced femoral neck fractures in the elderly: a retrospective comparison of fixation methods. J Trauma 64(1):155–162. https://doi.org/10.1097/TA.0b013e31802c821c
Kalsbeek JH, Roerdink WH, Krijnen P, van den Akker-van Marle ME, Schipper IB (2020) Study protocol for the DEFENDD trial: an RCT on the dynamic locking blade plate (DLBP) versus the dynamic hip screw (DHS) for displaced femoral neck fractures in patients 65 years and younger. BMC Musculoskelet Disord 21(1):139. https://doi.org/10.1186/s12891-020-3131-x
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, BM and RVV; data curation, RCS and RJ, RVV, BM; formal analysis, RCS, RJ, and RVV; methodology, RCS and RJ; supervision, RVV, EDL, BB, and BM; writing—original draft, RCS, RJ; writing——review and editing, RVV, BB, BM, and EDL. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest.
Ethics approval
pproval was given by a local ethical commission (METC Z2020051).
Consent to participate
Consent to participate was waived by the ethical commission.
Consent for publication
All authors have given their consent for publication
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stassen, R.C., Jeuken, R.M., Boonen, B. et al. First clinical results of 1-year follow-up of the femoral neck system for internal fixation of femoral neck fractures. Arch Orthop Trauma Surg 142, 3755–3763 (2022). https://doi.org/10.1007/s00402-021-04216-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-021-04216-0