Abstract
Background
Microbiological profile of pathogens causing periprosthetic joint infection (PJI) after primary total hip (THA) and knee (TKA) arthroplasty varies in different regions, clinics and even departments. The objective of this study was to analyze the pathogen structure in patients with PJI after primary THA and TKA and its influence on the effectiveness of the infection eradication after two-stage reimplantation.
Materials and methods
We collected the retrospective data of 364 patients—161 with PJI after primary TKA (113 treated in two stages 48 with failure after spacer implantation) and 203 patients with infected THA (127 after successful two-stage reimplantation and 76 with PJI recurrence after the first stage) within the time period from January 2012 to December 2017, treated with two-stage protocol in the single center. A comparative analysis of pathogen structure was performed between cohorts of patients with hip and knee PJI. A subanalysis was made between the subsets comprised from patients with successful two-stage treatment and the subsets with failure to treat the infection.
Results
Staphylococcus epidermidis was the most commonly identified pathogen in the full hip and knee cohorts: 30.1% and 32.5%, respectively. However, the percentage of methicillin-resistant Staphylococcus epidermidis (MRSE) among all S. epidermidis isolates was higher in the hip cohort—50% compared with 35% in the knee cohort (p = 0.073). Other coagulase-negative Staphylococci were more common to patients with PJI after primary TKA—10.3% compared with 5% (p < 0.02). Streptococcus sp. caused hip PJI in a larger percentage of cases than in knee PJI (p < 0.01)—7% and 2%, respectively (p < 0.01).
Polymicrobial associations were significantly more common in hip PJI compared to knee PJI: 45.3% and 14% of cases, respectively (p < 0.001). The presence of polymicrobial infection significantly raised the risk of PJI recurrence [OR 2 (95% CI from 1.24 to 3.24)] in knee PJI patients and reduced the effectiveness of infection eradication from 73.9% to 47.8%.
Conclusion
Comparative analysis showed significant differences in the structure of PJI pathogens in the hip and knee. These findings are useful when choosing treatment strategies and empirical antibiotics regimens, in the management of patients with PJIs after primary hip and knee arthroplasty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prosthetic joint infection (PJI) is currently the leading cause of early re-operations after primary and revision total hip (THA) and knee (TKA) arthroplasty. Up to 36.1% of all revision TKAs performed within 1 year after primary implantation were due to PJI. In revised joints this complication accounted for 39.6% of all surgical procedures [7, 10, 23]. Patients with PJIs have 1.87 higher relative risk for mortality than patients re-operated for an aseptic complication. Moreover, the presence of Enterococci increases this risk to 3.18 when compared to PJI patients infected by another pathogen [14].
Among the existing surgical treatment strategies of debridement, antibiotics and implant retention (DAIR) procedure, one stage reimplantation, in patients with PJI two-stage reimplantation demonstrates comparable effectiveness and remains the «gold standard» for the majority of chronic PJIs [2, 3, 11].
The role of pathogen type, together with other known risk factors, is critical in the development and recurrence for the PJIs. Chen et al. found that early mortality in patients with hospital strain of methicillin-resistant S. aureus (MRSA) bacteremia was significantly higher compared to patients with bacteremia caused by methicillin sensitive S. aureus (MSSA) [12]. Some authors claim that up to 54% of all identified Staphylococci were MR in patients with PJI [5, 16]. Along with known risk factors such as liver disease, prior debridement with prothesis retention, pathogen type (especially Gram-negative bacilli) the presence of the sinus tract is associated with treatment failure [18]. Similar data were obtained by Jhan et al. who analyzed the results of two-stage treatment in 62 patients and concluded that obesity, liver cirrhosis, Gram-negative infection, and the presence of a sinus tract were significantly related to failure [17].
The differences in microbiological profiles between hip and knee were mentioned by Tsai et al. In their study on 294 hip and knee PJI patients, they found that the number of infections caused by anaerobes was higher in hip PJI—11 vs 0 in knee PJI. Polymicrobial cases were also higher in hip PJI—22 vs 6 [26]. Despite the large body of data on causative pathogens in patients with PJI after primary total knee and hip arthroplasty, there is a lack of studies, comparing the effect of microorganism profile and types of pathogens on failure after spacer implantation in these groups of PJI patients. This study is designed to assess the hypothesis that localization of periprosthetic infection influences on pathogen structure. The objective of this study was to analyze the pathogens causing PJIs after primary total hip and knee arthroplasty and their influence on the effectiveness of two-stage reimplantation for eradication of the infection.
Materials and methods
From the database of our institutional archives, we collected the patients with primary TKA and THAs who were diagnosed with PJIs within the time period from January 2012 to December 2017 treated with two-stage protocol in the single center. PJI was diagnosed according to criteria of the Philadelphia International Consensus Meeting—2013: two positive periprosthetic cultures with phenotypically identical microorganisms or a sinus tract communicated with the joint; or at least three minor criteria; elevated serum C-Reactive Protein (CRP) and Erythrocyte Sedimentation Rate (ESR), elevated synovial fluid White Blood Cell count or positive mark of Leucocyte Esterase test strip, elevated synovial fluid polymorphonuclear neutrophil percentage [21].
PJI was classified according to the time of manifestation since index surgery: early (< 3 months after surgery), delayed (3–12 months after surgery) and late (> 12 months after surgery) [29].
A comparative analysis of the pathogens was performed between the cohorts of patients with hip and knee PJIs. An analysis was also made between the subsets comprised from patients with successful two-stage treatment and the subsets with failure to treat the infection. Patients treated with DAIR procedures were excluded (Fig. 1).
All patients with confirmation of the PJI diagnosis passed through the first of the two-stage surgical treatment, which included skin incision with the resection of the old scar, arthrotomy (extended approaches were used in case of severe contracture), implant components removal with its further microbiological analysis along with five infected tissue samples taken from different aspects of the joint. After extensive surgical debridement of all affected tissues and further jet lavage with 5–7 L of normal saline, an antibiotic loaded cement spacer was implanted (Refobacin bone cement/DePuy CMW 3 bone cement with gentamycin and additional 4 g of vancomycin in each batch of 40 g). In cases of high hip dislocation risk, joint instability, or microsurgical coverage with soft tissue flaps immobilization was employed until the second stage of treatment.
Microorganisms were isolated from homogenized intraoperative tissue biopsies and from the surface of the removed implants after sonication. Microbial species were identified by the staff microbiologist from cultures with the use of selective media and biochemical test panels.
All patients received intravenous antibiotic therapy from the operation day up to 2 weeks, followed by 4–6 weeks of oral therapy. Initial antibiotic regimen included combinations of vancomycin with beta-lactam antibiotics or quinolones; alternatively, beta-lactam antibiotics with quinolones. Antibacterial therapy was corrected after receiving the results of bacteriological examination of intraoperative samples if necessary.
The first stage of treatment was considered to be successful if there were no clinical and laboratory signs of reinfection, including negative culture growth after routine joint aspiration, at the moment when the patient was invited for the second stage of treatment. The outcome was interpreted as unsuccessful when inflammatory signs remained or reappeared during the period between the first step and reimplantation. These signs included the presence of acute inflammation with high levels of serum CRP, development of a sinus tract and relapse or reinfection, depending on the isolated microorganisms. In this case, patient underwent spacer exchange instead. The time period without antibiotics was not less than 2 weeks.
The second stage of surgical treatment included spacer removal, debridement, jet lavage, and implantation of the revision system with required level of constraint and compensation of bone defects with augments if needed. Antibiotic prophylaxis was provided, taking into consideration the results of bacteriological examination on the first stage of treatment. The mean follow up period was 5.6 (2.4–7.2) and 4 (2.1–6.9) years for knee and hip patients, respectively.
All characteristics of investigated cohorts are illustrated in Table 1.
Females were more prevalent in knee group (74.5%), while gender distribution in the hip group was equal. The mean age in full hip and knee cohort was 61 years. Osteoarthritis was the main reason for the index surgery in both groups. Late PJI with the onset of the symptoms more than 12 months after primary arthroplasty was most common in both groups: 55.6% of all hip patients and 41% of all knee patients.
Statistical methods
Statistical analysis of the results was performed with STATISTICA 9.0 software (StatSoft, USA). Categorical data are presented as proportions, which were analyzed with Fisher exact test. The association of clinical factors with successful outcomes of the surgery is shown as odds ratios (OR) given with 95% CI. Reported P values are two-tailed. The p value below 0.05 was considered significant.
Results
We have retrospectively collected the data of 364 patients. 161 with PJI after primary total knee arthroplasty (TKA) were enrolled: 113—treated in two stages and 48—with failure after spacer implantation. 203 patients with infected total hip arthroplasty (THA) were enrolled: 127 after successful two-stage reimplantation and 76 with PJI recurrence after the first stage.
Overall, 341 isolates were identified in the hip cohort patients: 202—in successfully treated patients (n = 127) and 139 in patients with PJI recurrence after spacer implantation (n = 76). In the knee cohort patients, 193 pathogens were detected: 128 in the group with success (n = 113) and 65 in the PJI recurrence group (n = 65). Among 364 PJI patients included in our investigation 115 (31.6%) were polymicrobial, 92 of which belonged to hip and 23 to knee PJI groups (p < 0.001).
Staphylococcus epidermidis was the most frequently identified pathogen in the full cohort of hip and knees (Table 2). However, the percentage of methicillin-resistant Staphylococcus epidermidis (MRSE) among all S. epidermidis isolates was higher in the hip cohort—50% compared with 35% in the knee cohort (p = 0.073). Staphylococcus aureus more often caused knee than hip PJI (p = 0.007) with a comparable proportion of MRSA in both groups: 18% and 22%, respectively. Other coagulase-negative Staphylococci were also more common to patients with PJI after primary TKA. Streptococcus sp. caused hip PJI in a larger percentage of cases than in knee PJI (p < 0.01).
On the next stage of investigation, the comparative analysis between successful treatment and recurrence of infection in knees was carried out. S. epidermidis was the predominant pathogen for patients with eradicated infection while in the reinfected group S. aureus was the most common pathogen. 40% of S. aureus isolates in the recurrent group were methicillin-resistant compared to 17.7% MRSA cases in successful group (p = 0.086). The ratio of MRSE was comparable for successful treatment and for recurrent knee PJI. The proportion of Corynebacterium and fam. Enterobacteriaceae was higher in patients who received more than one spacer implantation (Table 3). Polymicrobial infection was twice as much in patients with PJI recurrence—22.6% compared to 10.6% of successfully treated patients (p = 0.05).
Staphylococci were most common in hip PJIs, both those with infection recurrence and effectively treated. The frequency of MRSA isolation was twice as much in case of PJI relapse—30.3% (p = 0.291). In contrast, the proportion of MRSE in the group with failed hip spacer implantation was lower than in infection eradication: 29.4% and 70.5%, respectively (p < 0.0001). Bacteria from the fam. Enterobacteriaceae were more likely to be isolated in hip patients with failed spacer implantation (p = 0.048), while Enterococcus sp. was more characteristic of patients without hip PJI recurrence (p = 0.051) (Table 4). Polymicrobial infection in failed spacer implantation cases was similar to the group of patients who reached reimplantation stage—46.0% and 44.8%, respectively.
Discussion
Gram-positive microorganisms in our research were the main reason of PJI both: after primary THA and TKA, despite this fact comparative analysis showed that structure of causative pathogens during the last decade had changed. According to Rosteius T et al. in Germany coagulase-negative Staphylococci are the most frequently identified pathogens (up to 65% of all PJI). At the same time incidence of MRSE increased from 12.9% of all S. epidermidis isolates to 19.5% and MRSA percentage declined from 17.2% to 6.2% for the last eight years was marked [24]. Analysis of the leading pathogens causing orthopedic infection in Russia for the period 2012–2017 showed significant (p < 0.01) decrease in the frequency of S. aureus isolation from 34.5% in 2012–2013 up to 28.6% in 2016–2017. At the same time, the proportion of S. epidermidis increased significantly (p < 0.01) from 18.4% to 22.5%. During the period 2016–2017 methicillin-resistant strains accounted for 16.4 and 62.7% of S. aureus and S. epidermidis, respectively [8]. Zajonz et al. from Germany also confirmed this tendency noting an increase of S. epidermidis among PJI patients and the proportion of MRSE from 0% in 2001 to 74% in 2012 [28]. On the other hand, in Taiwan S. aureus plays the leading role with 29.9% [25].
We received comparable data from our research—63% of cases in the full cohort had Staphylococci as a causative pathogen, with 31% from S. epidermidis as the leading cause. The frequency of methicillin-resistant Staphylococci was comparable to the studies mentioned above, accounting for 29.5%. The proportion of MRSE and MRSA among all PJI cases included in the study was 44% and 20%, respectively. The percentage of methicillin-resistant forms of Staphylococci could be influenced by the localization of PJI. According to our research only 15.6% of all CoNS isolated in patients with knee PJI were MR compared with 34.5% in hip PJI group (p = 0.015). However, the presence of MR isolates in hip PJI patients didn’t influence the effectiveness of infection eradication—OR 1.5 (95% CI 0.9–2.5). In contrast, identification of MRSA in the knee PJI group significantly increased the risk of PJI recurrence—OR 2.2 (95% CI 1.3–3.7). Hischebeth GT et al. also illustrated the influence of MRSE isolates as a causative pathogen compared with MSSE on the effectiveness of spacer implantation—54.2% and 95.2%, respectively [15].
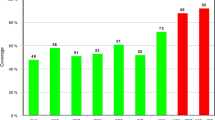
Treatment of MR staphylococcal prosthetic infection remains a great challenge, effectiveness of treatment according to Dubee et al. was 68% compared with 89% if susceptible microorganisms were identified [4, 13]. The difference in the identified pathogens among the patients of the compared groups resulted in the effectiveness of spacer implantation in our research. The higher two-stage success rate in the hip and knee PJIs from Enterococcus sp. (79.1%) cases could be explained by the high anti-enterococcal activity, of the combination of gentamycin and vancomycin added to bone cement spacer preparation. But this combination demonstrated low effectiveness towards fam. Enterobacteriaceae and non-fermenting bacteria, where the recurrence rate was 58.6% and 45.4%, respectively.
In knee PJI, the proportion of coagulase-negative Staphylococci (except S. epidermidis) was higher than in hip PJI, without a negative effect on the infection eradication—70%. The Streptococcus sp. isolates in knee PJIs were identified less frequently but their recurrence rate after two-stage treatment was extremely high—81.8%, comparable with report represented by Akgün et al. illustrating the difficulty to treat streptococcal PJIs [3].
In contrast to our data, Aggrawal et al. as well as Bjerke-Kroll et al. found no differences in the microbiological profile of hip and knee PJIs. [1, 6]. However, the pathogen spectrums included higher virulence and resistance organisms at a referral center in the United States compared with one in Europe. [1]
The polymicrobial infection in the study cohort was relatively high, the majority of which affected the THAs (p < 0.001) and was comparable to the study by Tsai et al. [25] Treatment of mono- and polymicrobial hip PJI showed equal effectiveness: 63% and 62%, respectively [OR 1.18 (95% CI from 0.88 to 1.6)]. Comparable data which confirmed predominance of polymicrobial cases and anaerobic microorganisms in hip PJI were published by Piper et al. and Lentino et al. as well [20, 22]. Multiple risk factors such as obesity and elevated CRP predispose patients to polymicrobial infections [9, 19, 27].
Controversially in the group of knee PJI patients with failure of the two-stage treatment the polymicrobial infections were twice as common as in the successfully treated PJIs. The presence of polymicrobial infection raised the risk of PJI recurrence [OR 2 (95% CI from 1.24 to 3.24)] and reduced the effectiveness of infection eradication in knee PJI from 73.9% to 47.8%. Moreover, the presence of Gram-negative isolates was an aggravating factor in knee PJI polymicrobial cases elevating the risk for failure—OR 2.2 (95% CI 1.32–3.35).
Conclusion
Most of the causative pathogens in patients with hip and knee PJI belonged to coagulase-negative Staphylococci. Its methicillin-resistant strains were more frequently identified in patients with PJI recurrence. Comparative analysis showed significant differences in the spectrum of PJI pathogens in the hip and knee. When choosing treatment tactics and antibiotics for empirical therapy it is necessary to take into account the high frequency of microbial associations in hip PJI, as well as the increased risk of infection recurrence in patients with polymicrobial knee PJI. Isolation of bacteria from the fam. Enterobacteriaceae, regardless of the infection localization, is an adverse prognostic factor in the treatment of patients with PJI after primary total hip and knee replacement.
Limitations
The study had a retrospective design, cohorts were formed with successful and recurrent hip and knee PJI patients to analyze causative pathogens depending on the localization and the outcome. In this situation, the effectiveness of treatment could not be analyzed properly and further randomized controlled investigations are necessary. Patients with potential confounding factors were excluded and not analyzed as a result. However, this study utilized strict criteria (first time PJI after primary total joint arthroplasty, absence of DAIR procedures before spacer implantation) to form cohorts with comparable initial data, and patient-related risk factors.
Availability of data and material
The dataset supporting the conclusions of this article is available from the corresponding author on reasonable request.
References
Aggarwal VK, Bakhshi H, Ecker NU, Parvizi J, Gehrke T, Kendoff D (2014) Organism profile in periprosthetic joint infection: pathogens differ at two arthroplasty infection referral centers in Europe and in the United States. J Knee Surg 27(5):399–406. https://doi.org/10.1055/s-0033-1364102
Akgün D, Müller M, Perka C, Winkler T (2019) High cure rate of periprosthetic hip joint infection with multidisciplinary team approach using standardized two-stage exchange. J Orthop Surg Res 14:78. https://doi.org/10.1186/s13018-019-1122-0
Akgün D, Trampuz A, Perka C, Renz N (2017) High failure rates in treatment of streptococcal periprosthetic joint infection: results from a seven-year retrospective cohort study. Bone Joint J 99-B(5):653–659. https://doi.org/10.1302/0301-620X.99B5.BJJ-2016-0851.R1
Antony SJ, Westbrook RS, Jackson JS, Heydemann JS, Nelson JL (2015) Efficacy of single-stage revision with aggressive debridement using intra-articular antibiotics in the treatment of infected joint prosthesis. Infect Dis (Auckl) 8:17–23. https://doi.org/10.4137/IDRT.S26824
Ascione T, Pagliano P, Balato G, Mariconda M, Rotondo R, Esposito S (2017) Oral therapy, microbiological findings, and comorbidity influence the outcome of prosthetic joint infections undergoing 2-stage exchange. J Arthroplasty 32(7):2239–2243. https://doi.org/10.1016/j.arth.2017.02.057
Bjerke-Kroll BT, Christ AB, McLawhorn AS, Sculco PK, Jules-Elysée KM, Sculco TP (2014) Periprosthetic joint infections treated with two-stage revision over 14 years: an evolving microbiology profile. J Arthroplasty 29(5):877–882. https://doi.org/10.1016/j.arth.2013.09.053
Boelch SP, Jakuscheit A, Doerries S, Fraissler L, Hoberg M, Arnholdt J, Rudert M (2018) Periprosthetic infection is the major indication for TKA revision—experiences from a university referral arthroplasty center. BMC Musculoskelet Disord 19(1):395. https://doi.org/10.1186/s12891-018-2314-1
Bozhkova SA, Kasimova AR, Tikhilov RM, Polyakova EM, Rukina AN, Shabanova VV, Liventsov VN (2018) Adverse trends in the etiology of orthopedic infection: results of 6 year monitoring of the structure and resistance of leading pathogens. Traumatol Orthop of Russia 24(4):20–31. https://doi.org/10.21823/2311-2905-2018-24-4-20-31 (In Russ)
Bozhkova S, Tikhilov R, Labutin D, Denisov A, Shubnyakov I, Razorenov V, Artyukh V, Rukina A (2016) Failure of the first step of two-stage revision due to polymicrobial prosthetic joint infection of the hip. J Orthop Traumatol 17(4):369–376. https://doi.org/10.21823/2311-2905-2018-24-4-20-31
Bozic KJ, Kamath AF, Ong K, Lau E, Kurtz S, Chan V, Vail TP, Rubash H, Berry DJ (2015) Comparative epidemiology of revision arthroplasty: failed THA poses greater clinical and economic burdens than failed TKA. Clin Orthop Relat Res 473(6):2131–2138. https://doi.org/10.1007/s11999-014-4078-8
Castellani L, Daneman N, Mubareka S, Jenkinson R (2017) Factors associated with choice and success of one- versus two-stage revision arthroplasty for infected hip and knee prostheses. HSS J 13(3):224–231. https://doi.org/10.1007/s11420-017-9550-z
Chen SY, Wang JT, Chen TH, Lai MS, Chie WC, Chien KL, Hsueh PR, Wang JL, Changv SC (2010) Impact of traditional hospital strain of methicillin-resistant Staphylococcus aureus (MRSA) and community strain of MRSA on mortality in patients with community-onset S aureus bacteremia. Medicine (Baltimore) 89(5):285–294. https://doi.org/10.1097/MD.0b013e3181f1851e
Dubée V, Zeller V, Lhotellier L, Kitzis MD, Ziza JM, Mamoudy P, Desplaces N (2013) Continuous high-dose vancomycin combination therapy for methicillin-resistant staphylococcal prosthetic hip infection: a prospective cohort study. Clin Microbiol Infect 19(2):E98-105. https://doi.org/10.1111/1469-0691.12071
Gundtoft PH, Pedersen AB, Varnum C, Overgaard S (2017) Increased mortality after prosthetic joint infection in primary THA. Clin Orthop Relat Res 475(11):2623–2631. https://doi.org/10.1007/s11999-017-5289-6
Hischebeth GT, Randau TM, Ploeger MM, Friedrich MJ, Kaup E, Jacobs C, Molitor E, Hoerauf A, Gravius S, Wimmer MD (2019) Staphylococcus aureus versus Staphylococcus epidermidis in periprosthetic joint infection—outcome analysis of methicillin-resistant versus methicillin-susceptible strains. Diagn Microbiol Infect Dis 93(2):125–130. https://doi.org/10.1016/j.diagmicrobio.2018.08.012
Ip D, Yam SK, Chen CK (2005) Implications of the changing pattern of bacterial infections following total joint replacements. J Orthop Surg (Hong Kong) 13(2):125–130. https://doi.org/10.1177/230949900501300204
Jhan SW, Lu YD, Lee MS, Lee CH, Wang JW, Kuo FC (2017) The risk factors of failed reimplantation arthroplasty for periprosthetic hip infection. BMC Musculoskelet Disord 18(1):255. https://doi.org/10.1186/s12891-017-1622-1
Kandel CE, Jenkinson R, Daneman N, Backstein D, Hansen BE, Muller MP, Katz KC, Widdifield J, Bogoch E, Ward S, Sajja A, Jeldes FG, McGeer A (2019) Predictors of treatment failure for hip and knee prosthetic joint infections in the setting of 1- and 2-stage exchange arthroplasty: a multicenter retrospective cohort. Open Forum Infect Dis 6(11):ofz452. https://doi.org/10.1093/ofid/ofz452
Kavolus JJ, Cunningham DJ, Rao SR, Wellman SS, Seyler TM (2019) Polymicrobial infections in hip arthroplasty: lower treatment success rate, increased surgery, and longer hospitalization. J Arthroplasty 34(4):710-716.e3. https://doi.org/10.1016/j.arth.2018.09.090
Lentino JR (2003) Prosthetic joint infections: bane of orthopedists, challenge for infectious disease specialists. Clin Infect Dis 36(9):1157–1161. https://doi.org/10.1086/374554
Parvizi J, Gehrke T (2014) International consensus group on periprosthetic joint infection. Definition of periprosthetic joint infection. J Arthroplasty 29(7):1331. https://doi.org/10.1016/j.arth.2014.03.009
Piper KE, Jacobson MJ, Cofield RH, Sperling JW, Sanchez-Sotelo J, Osmon DR, McDowell A, Patrick S, Steckelberg JM, Mandrekar JN, Fernandez Sampedro M, Patel R (2009) Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J Clin Microbiol 47(6):1878–1884. https://doi.org/10.1128/JCM.01686-08
Postler A, Lützner C, Beyer F, Tille E, Lützner J (2018) Analysis of total knee arthroplasty revision causes. BMC Musculoskelet Disord 19(1):55. https://doi.org/10.1186/s12891-018-1977-y
Rosteius T, Jansen O, Fehmer T, Baecker H, Citak M, Schildhauer TA, Geßmann J (2018) Evaluating the microbial pattern of periprosthetic joint infections of the hip and knee. J Med Microbiol 67(11):1608–1613. https://doi.org/10.1099/jmm.0.000835
Tsai JC, Sheng WH, Lo WY, Jiang CC, Chang SC (2015) Clinical characteristics, microbiology, and outcomes of prosthetic joint infection in Taiwan. J Microbiol Immunol Infect 48(2):198–204. https://doi.org/10.1016/j.jmii.2013.08.007
Tsai Y, Chang CH, Lin YC, Lee SH, Hsieh PH, Chang Y (2019) Different microbiological profiles between hip and knee prosthetic joint infections. J Orthop Surg (Hong Kong) 27(2):2309499019847768. https://doi.org/10.1177/2309499019847768
Wimmer MD, Friedrich MJ, Randau TM, Ploeger MM, Schmolders J, Strauss AA, Hischebeth GT, Pennekamp PH, Vavken P, Gravius S (2016) Polymicrobial infections reduce the cure rate in prosthetic joint infections: outcome analysis with two-stage exchange and follow-up ≥ two years. Int Orthop 40(7):1367–1373. https://doi.org/10.1007/s00264-015-2871-y
Zajonz D, Wuthe L, Rodloff AC, Prietzel T, von Salis-Soglio GF, Roth A, Heyde CE, Josten C, Ghanem M (2016) Infections of hip and knee endoprostheses. Spectrum of pathogens and the role of multiresistant bacteria. Chirurg 87(4):332–339. https://doi.org/10.1007/s00104-015-0126-5
Zimmerli W, Trampuz A, Ochsner PE (2004) Prosthetic-joint infections. N Engl J Med 351(16):1645–1654. https://doi.org/10.1056/NEJMra040181 (Review. PMID: 15483283)
Author information
Authors and Affiliations
Contributions
PP—development of the concept and study design of, collection, analysis and interpretation of the obtained data, statistical data processing, writing of the manuscript. SB—development of the concept and design of the study, writing and editing of the manuscript, interpretation the obtained data. AK—collection, analysis and interpretation of the obtained data, writing of the manuscript. RT—development of the concept and design of the study, editing of the manuscript. AK—development of the concept and design of the study, writing and editing of the manuscript. All authors read and approved the final version of the manuscript. All authors agree to be responsible for all aspects of the work to ensure proper consideration and resolution of all possible issues related to the correctness and reliability of any part of the work.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Preobrazhensky, P., Bozhkova, S., Kochish, A. et al. Comparative analysis of pathogen structure in patients with PJI after primary total hip and knee arthroplasty. Arch Orthop Trauma Surg 141, 1963–1969 (2021). https://doi.org/10.1007/s00402-021-04139-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-021-04139-w