Abstract
Purpose
Benign lesions of the proximal femur region, such as simple bone cysts, aneurysmal bone cysts, and fibrous dysplasia, are common in children. Benign lesions may cause pathologic fractures, limb length inequities, and growth disturbances. Differential diagnoses, e.g., malignant bone tumors and osteomyelitis, are sometimes difficult to rule out.
Objective
We aimed to evaluate outcomes in children with benign lesions of the proximal femur treated with curettage, bone grafting, and plate fixation.
Methods
In this retrospective study, we included 30 children (median age 10.5 years; range 1.1–17.8 years) suffering from bone cysts and tumor-like lesions of the proximal femur region treated between 2002 and 2018. We analyzed plain X-ray images and CT scans in all children and obtained MRI scans in a selected group of children (63.3%). We examined histopathologic biopsy results for all bone lesions before initiating treatment. Surgical management comprised tumor curettage with adjuvant high-speed drilling and allogenic bone grafting supplemented by bone graft substitutes before plate fixation. Median follow-up interval was 87 months (range 24–156 months). We evaluated the healing of lesions according to Capanna’s classification and rated functional outcomes according to Merle d’Aubigné and Postel score.
Results
Overall, 25 of 30 (83.3%) patients were admitted to hospital because of a pathologic fracture. We diagnosed simple bone cysts in 15 (50.0%) patients, aneurysmal bone cysts in 7 (23.5%) patients, and fibrous dysplasia in 8 (26.5%) patients. Bone consolidation was achieved in 22 of 30 (73.3%) patients after a mean of 5 months (range 3–7 months). The main complication was recurrence of the lesion in 4 of 30 (13.3%) patients. With respect to the Merle d’Aubigné and Postel scores, 17 of 30 (56.7%) patients obtained an excellent result (18 points), while 12 (40.0%) patients had a good result (15–17 points) and only 1 (3.3%) patient had a fair result (14 points).
Conclusion
Surgical treatment of bone cysts and tumor-like lesions of the proximal femur by local resection or destruction of the lesion, followed by filling the defect with bone graft material and internal stabilization represents a safe and effective treatment option in children.
Level of evidence
Therapeutic, retrospective comparative study—Level III
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bone tumors in children occur with a reported incidence of 79.3 per 1 million children [1]. The proximal femur is among the most frequently affected locations for benign bone tumors and tumor-like lesions in children. Simple bone cyst (SBC), aneurysmal bone cyst (ABC), non-ossifying fibroma, and fibrous dysplasia (FD) represent the most common benign bone tumors and tumor-like lesions in children [2]. Pathologic fractures in children are caused by SBCs in approximately 75% of cases [3, 4].
The pertrochanteric and subtrochanteric regions are exposed to high tension and bending forces during weight-bearing. Pathologic fractures often constitute the first symptom of such lesions. Pathologic fractures are often diagnosed on the basis of plain X-ray images obtained after the injury. Varus deformity, limb length discrepancy, or femoral head avascular necrosis can complicate pathologic fractures of the proximal femur.
While plain radiographs are the basis for diagnosis, accurate assessment of the size, type, and location of the lesion may require the use of computer tomography (CT) or magnetic resonance imaging (MRI) [5, 6]. This article describes our management of SBCs, ABCs, and FD of the proximal femur in children.
Simple bone cyst
SBCs account for about 3% of all benign primary bone tumors [7]. As pointed out by Lokiec and Wientroub, SBCs represent an enigma for radiologists, pathologists, and orthopedic surgeons because the growth and clinical course of these cysts are difficult to predict [8]. Most pathologic fractures caused by SBCs heal spontaneously, but the SBC persists in 20–50% of patients [7]. In the majority of pathologic fractures caused by bone cysts or tumor-like lesions, the priority is to treat the fracture first, followed by treatment of the lesion. However, this rule does not apply to pathologic fractures in the proximal femur region because the lesion impairs the stability of the femur, and the child will not be able to walk safely due to the high risk of refracture.
Aneurysmal bone cyst
The incidence of ABCs is approx. 2% among all benign bone tumors [9]. The typical feature of an ABC is osteolytic lesion with multiple cavities and expansive growth [10]. MRI represents the most valuable diagnostic tool in the differential diagnosis of ABCs. Typical MRI findings are fluid-to-fluid levels and septations [10, 11]. However, it must be kept in mind that teleangiectatic osteosarcoma may also show fluid-to-fluid levels, but its growth is usually more aggressive [12].
Fibrous dysplasia
FD is a benign lesion with a prevalence between 5 and 7% among benign bone tumors [13, 14]. FD occurs in a monostotic or (less commonly) polyostotic type (McCune–Albright syndrome, Mazabraud syndrome) [13]. Monostotic FD leads to a pathologic fracture in approx. 50% of affected children. Pathologic fractures of FD frequently occur in lesions located in the proximal femur [15,16,17,18].
Almost all tumor-like lesions of bones require histopathologic evaluation before treatment. The timing of taking biopsies of the lesion is of primary importance. In case of lesions with radiologic signs of possible malignancy, the lesion should be biopsied at a specialized orthopedic oncology center.
The most common treatment option of benign bone lesions of the proximal femur is curettage and bone grafting, usually supported with internal stabilization. However, there are few reports describing treatment and outcome of such lesions in children [19]. Thus, we aimed to describe the indications, types of treatment, and outcomes of benign tumors and tumor-like lesions of the proximal femur in children and adolescents.
Patients and methods
Study method, data acquisition, and patient selection
This study was approved by the Ethics Committee of Silesian Medical University, Katowice, Poland (PCN/0022/KB/99/20 dated June 5th, 2020). We enrolled 30 patients (median age 10.5 years; range 1.1–17.8 years) with benign tumors and tumor-like lesions of the proximal femur treated in our department with open curettage, allogenic bone grafting, and plate fixation during a 17-year period (January 1, 2002, to December 3, 2018). Patients who had been operated at another hospital and required re-operation were excluded from the study.
Functional outcomes were classified according to Merle d’Aubigné and Postel score [20] (Table 1). We obtained plain a.p. X-ray images of the pelvis in all patients and additional a.p. and axial X-ray images of the affected femur. Pre-operative CT scans of the affected proximal femur were also obtained in all patients. In 19 of 30 (63.3%) patients, MRI was performed. All children were examined by a pediatric surgeon, and diagnostic results were discussed by an interdisciplinary group of consultants consisting of a pediatric radiologist, pediatric oncologist, pediatric surgeon, and orthopedic surgeon during a tumor board meeting. Based on the decision of this interdisciplinary expert group and radiological findings including conventional radiography, CT, or MRI scans, we performed preoperative needle biopsies in 16/30 (53.3%) children. After histopathologic confirmation of the benign nature of the lesion, surgery was performed. In all children, we performed tumor curettage with adjuvant high-speed drilling and allogenic bone grafting, supplemented with artificial bone graft substitutes (Calcibon®, Biomet, Warsaw, Poland). Overall, 22 of 30 (73.3%) patients underwent internal fixation of the proximal femur by implantation of an LCP Pediatric Hip Plate® (DePuySynthes, Johnson & Johnson, New Brunswick, NJ, USA) [21]. In 4 (13.3%) patients each, we inserted either a locking plate or an angular plate.
In all children suffering from acute pathologic fractures, surgery was performed using a traction table. Children with tumor-like lesions of the proximal femur who did not suffer from a pathologic fracture were placed on a radiolucent table for surgery in the supine position. We applied a lateral approach to the proximal femur in all children. For tumor curettage, we used an approach via the fracture gap or via a bone window cut into the lateral femoral cortex. After surgery, the patients were allowed to walk using two crutches without weight-bearing for an average of 31 days (range 20–63 days). All patients underwent radiologic examination on the first day after surgery and at 6, 12, and 20 weeks after surgery. After this period, the frequency of radiologic checkups was determined individually. At each assessment, we determined bone healing progress on plain radiographs based on Capanna’s classification [22], i.e., grade 1 = complete bone healing; grade 2 = incomplete healing with marginal cortex thickening and small residual areas of osteolysis; grade 3 = recurrence with large areas of osteolysis and cortex thinning; grade 4 = lack of response to treatment with persistence of cyst growth.
Twelve months after surgery, each patient also underwent assessment of clinical outcome according to Merle d’Aubigné and Postel scores [20]. In patients requiring re-operation, we evaluated the outcome after the second surgery.
Statistical analysis
Using package R version 3.6.1 on x86_64-conda6-linux-gnu platform, we investigated if there was any statistical difference regarding the different lesion types with respect to radiologically confirmed healing signs categorized according to Capanna’s classification (grade 1–4) and Merle d’Aubigné and Postel score obtained at follow-up examination [20, 22]. Since our data did not meet the requirements for the 2-way ANOVA test, we adopted the Kruskal–Wallis test. We applied Tukey’s post hoc test to calculate confidence intervals (CIs) for the comparison of Merle d’Aubigné and Postel scores between the three groups of patients as defined by different Capanna’s grades. Interaction plots were obtained using “interaction.plot” by R-function [23]. To examine nontrivial interaction between type of lesion (ABC, SBC, and FD), Capanna’s grade, and Merle d’Aubigné and Postel score, we applied a nonparametric Adonis test [24] for the following model: score ~ grade + type + grade:type.
Data are presented as median (range). Due to the small number of patients within the three groups, we did not analyze the data statistically but presented them in a table (Table 2).
Results
Patient demographics and type of lesion
We included 23 (76.7%) boys and 7 (23.3%) girls in this study. Median age at presentation was 10.5 years (range 1.1–17.8 years). Median follow-up lasted 87 months (24–156 months). Overall, 25 of 30 (83.3%) patients were admitted to hospital because of a pathologic fracture after minor trauma. The remaining 5 (17.7%) patients underwent X-ray examination because of sports injuries (3 children), persistent knee pain (1 child), or persistent hip pain (1 child). We obtained preoperative CT scans for all children (Figs. 1, 2).
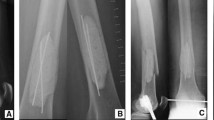
a Boy aged 5.6 years diagnosed with ABC (patient no. 27). He sustained a torsional trauma when walking downstairs at school and suffered a proximal femur fracture (O). Computed tomography revealed an ABC extending from the intertrochanteric region to the femoral neck. b 3-D reconstruction of CT scans showing mild varus angulation of the pathologic fracture. c Plain a.p. X-ray image obtained 5 months after curettage and high-speed drilling of the cyst wall, allogenic bone grafting supplemented by bone graft substitutes, correction of varus angulation, and stabilization by pediatric hip plate. d Axial X-ray image of the right hip showing no residual cyst and consolidation of fracture 5 months after surgical treatment of the pathologic fracture of ABC
a Boy aged 8.7 years diagnosed with SBC of the intertrochanteric region and femoral neck (patient no. 11). He sustained an undisplaced fracture (O) of the cyst wall. He was hit at the region of the hip when playing football. b CT scan of the right hip obtained 2 weeks after fracture and hip spica cast immobilization. The impaction at the fracture site had increased and callus formation became visible. Mild varus formation occurred. c Moderate antecurvation occurred at the site of the pathologic fracture during spica cast immobilization necessitating reduction and internal stabilization. d Plain X-ray a.p. image obtained 4 months after surgical treatment by curettage and high-speed drilling of the cyst wall, allogenic bone grafting supplemented by bone graft substitutes, correction of varus angulation, and stabilization by Pediatric Hip Plate [21]. Residual parts of the cyst were visible at the femoral neck and intertrochanteric region (indicated by arrows). e Residual SBC was confirmed 5 months after surgical treatment of bone cyst. Size and position of the residual cyst were visualized by CT scan. f Plain X-ray image of right proximal femur obtained 16 months after surgical treatment of bone cyst, showing partially consolidated lesion. Shortly thereafter we removed the plate. g CT scan obtained 2 years after the operation showing residual bone cyst at the region of the intertrochanteric region and distal femoral neck. h Plain a.p. and oblique X-ray images of right hip obtained after 2nd operation of right proximal femur performed 2 years after the first surgery. i X-ray image of right proximal femur obtained 5 months after surgical treatment of recurrent SBC. Plain X-ray image showing stable consolidation of cystic lesion with minimal shortening of femoral neck
Histopathologic findings
In response to the consultants’ suggestions at the tumor board meeting, we obtained pre-operative biopsies in 16 of 30 (53.3%) children. In the remaining 14 (46.7%) children, histopathologic evaluation of the lesion took place during surgery.
Histopathologic evaluation confirmed the presence of SBCs in 15 of 30 (50.0%) patients, ABCs in 7 (23.5%) patients, and FD in 8 (26.5%) patients.
Follow-up results
In 22 of 30 (73.3%) patients, postoperative bone consolidation was achieved after an average of 5 months (range: 3 to 7 months), as determined by plain X-ray images and clinical evaluation. At the follow-up examination 20 weeks after surgery, we recorded grade 1 bone healing in 17 (56.6%) patients, grade 2 in 5 (16.6%) patients, and grade 3 in 4 (13.3%) patients, according to Capanna’s classification [22]. In 4 patients, we obtained CT scans of the proximal femur 6 months after surgery to confirm consolidation of the pathologic fracture. We removed implants after a median interval of 211 days (range 92–541 days).
At the follow-up examination 12 months after surgery, we evaluated the clinical outcome according to Merle d’Aubigné and Postel score [20] (Table 1). Overall, 17 of 30 (56.7%) patients obtained an excellent result (18 points), while 12 (40.0%) patients had a good result (15–17 points) and only 1 patient had a fair result (14 points; Table 2). All patients had regained good hip range of motion with no pain at the final follow-up examination.
Using Kruskal–Wallis test, we inferred that there was no statistically significant difference in the Merle d’Aubigné score between the different patient groups suffering from SBC, ABC, or FD (p = 0.30). However, there was a statistically significant difference in the Merle d’Aubigné and Postel scores for groups of patients having different Capanna’s grades (p = 0.0025; Figs. 3, 4). Table 3 shows the CIs for the comparison of Merle d’Aubigné and Postel scores for the three groups of patients characterized by different Capanna’s grades.
The interaction plots indicated nontrivial interaction between Capanna’s grade and type of lesion (SBC, ABC, and FD) influencing the score. In particular, inspection of Figs. 3, 4 allowed to associate the most significant effect of interaction with the ABC type of lesion. This notion was supported by results of a non-parametric Adonis test [24] for the following model: score ~ grade + type + grade:type.
For additive effects, Adonis test confirmed the results of the Kruskal–Wallis test (additive effect of grade but no additive effect of type) and indicated a statistically significant and nontrivial interaction between grade and type (Figs. 5, 6).
Complications
One patient suffering from ABC achieved bone healing but developed deep wound infection 11 months after surgery. Due to completed bone consolidation, we removed the PHP plate and performed surgical wound debridement combined with deep tissue sampling for microbiologic analysis [25]. Subsequently, the patient received antibiotics and achieved stable bone healing and cure of infection.
In 4 of 30 (13.3%) children (2 with SBCs, 1 with ABC, and 1 with FD), we observed local recurrences of the lesions. All recurrences were diagnosed between 4 and 6 months after surgery. The patient with recurrent FD 4 months after surgery developed a fatigue femoral neck fracture due to bone graft resorption. After re-operation, complete bone consolidation was achieved in all patients after an average interval of 5.5 months (4.0–7.0 months; Table 2).
In 4 of 30 (13.3%) patients, we observed limb length discrepancies (median 1.25 cm; range 1.0–2.0 cm). The patient with the largest discrepancy required drill epiphysiodesis of the other limb at the level of the distal femur 20 months after primary surgery. We did not observe any implant failures among the study participants.
Discussion
Imaging methods and histopathologic evaluation
We conducted plain X-ray images and CT scans in all 30 patients but MRI scans in only 19 (63.3%) patients. In contrast, Erol et al. obtained plain X-ray images and MRI scans in all patients and additionally conducted CT scans in a few patients [19]. In our study, the bone lesions in all children were evaluated and discussed in a tumor board meeting by a consultant group consisting of a pediatric radiologist, pediatric oncologist, and pediatric orthopedic surgeon. Overall, 16 (53.3%) patients underwent biopsy before the scheduled surgery. Location, size, and cortical destruction (≥ 50%) of the lesion are known risk factors for pathologic fractures [26]. Snyder et al. analyzed the fracture risk based on assessment of the lesions’ cross-sectional geometry on CT scans in relation to the unaffected contralateral bone [5]. Pireau et al. described the bone cyst index (BCI; measured by dividing the cyst area by the diameter of the diaphysis squared on T1 MRI scans) as a predictor of pathologic fracture risk. In their study, BCI > 3.5 was associated with a greater risk of pathologic femur fracture [6]. However, Vasconcellos et al. did not confirm BCI to be a valuable predictor of fracture risk [27]. We were not able to assess the fracture risk in our study group because 25 of 30 (83.3%) patients were admitted to hospital for treatment of a pathologic fracture of the femur. The fracture rate of 83.3% observed in our investigation was similar to the pathologic fracture rate of 77.4% reported by Erol et al. for benign bone lesions of the proximal femur in children [19].
Simple bone cysts
Current treatment options for SBCs include mechanical disruption of the cyst wall, injections (steroids, bone marrow aspirate, bone substitutes), percutaneous intramedullary decompression, and curettage with allogenic bone grafting [22, 28,29,30]. Canavese et al. compared outcomes of patients treated with autologous bone marrow injection, methylprednisolone injection, and percutaneous curettage and obtained best results with percutaneous curettage (70% of healing) [31]. The recurrence rate of lesions after percutaneous techniques ranges from 8 to 30% [32, 33]. In a series of 12 SBCs located in the trochanteric and femoral neck regions treated with intramedullary nails, Roposch et al. observed complete healing or healing with residuals in all children [34]. Time to healing was 38.8 months on average [34].
We observed a recurrence rate of 13.3% (2 of 15 patients) for SBCs. Dormans and Pill presented a system of guidance for management of proximal femur lesions in children with open and closed physes [26]. Based on our study results, we hypothesize that the key steps are to remove the lesion completely, fill the cavity with osteoconductive material, and stabilize the proximal femur region by plate fixation.
Aneurysmal bone cysts
Rapid growth and progressive destruction of bone resemble the features of a malignant bone tumor. If an ABC is suspected, MRI scans should be obtained to demonstrate the typical feature of ABCs, such as multiple cavities filled with fluid showing fluid-to-fluid levels [31]. To exclude malignant bone tumors, e.g., giant cell tumor, a biopsy is strongly recommended [35].
In our investigation, all patients with suspected ABC were biopsied pre-operatively to exclude malignancy. For most cases of ABCs, surgical treatment is recommended; thus, spontaneous healing cannot be assessed [36, 37]. ABCs lead to a pathologic fracture in 36–72% of affected patients [12, 38]. Most pathologic fractures occur in the active phase of ABC [38]. In contrast to SBCs, ABCs will hardly heal during fracture consolidation. Currently, curettage with bone grafting is the most popular treatment strategy. In excessively large ABCs, pre-operative arterial embolization should be performed one day before surgery to limit blood loss during the operative intervention [10, 12]. Recurrence rate after curettage with bone grafting ranges from 18 to 20% [10, 38]. We observed ABC recurrence in 1 of 7 patients. With large ABCs located close to the growth plate, complete resection may be impossible, resulting in recurrence or persistence of the ABC, which might entail further investigation or other forms of treatment [39].
We performed internal fixation of all ABCs located in the proximal femoral region because varus formation has been described after ABC treatment without stabilizing the proximal femur [12]. We did not apply cryosurgery to treat proximal femoral ABCs, but we are aware that Marcove et al. reported promising results for local intralesional excision followed by application of liquid nitrogen [40]. Recently published reports describe MRI-guided percutaneous cryoablation of ABCs in children. Interventional MRI facilitates exact visualization and cryoablation of ABCs, thus allowing accurate monitoring of the size and location of the growing ice ball within the lesion [41].
SBCs and ABCs can be stabilized with intramedullary nails or plates [19, 34, 38]. We admit that stabilization with plates as applied in our patients requires larger incisions than that for insertion of intramedullary nails and thus results in poorer esthetic outcomes.
Fibrous dysplasia
Surgical management of proximal femur FD is discussed controversially. The most frequently recommended method of treatment, especially in cases of fractures, is curettage and bone grafting, but this treatment is associated with a high risk of recurrence of the lesion. Guille et al. reported that 66.6% of patients with FD and Shepherd’s crook deformity treated by different methods required re-operation [16]. In our study, FD recurred in 1 of 8 patients.
Type of treatment and outcomes
Due to high forces acting at the proximal femur during walking and sports, most lesions located in this region require surgical stabilization. In contrast to Erol et al. who inserted titanium nails, sliding screws, and screws for stabilization of lesions [19], we used different types of plates for internal stabilization. The lesions healed in 26 of 30 (86.7%) patients after the first treatment, which agrees well with the healing rate (90.3%) reported by Erol et al. [19].
We classified outcomes according to the original Merle d’Aubigné and Postel score [20] and did not apply the modified Merle d’Aubigné score proposed by Matta et al. [42] because Øvre et al. demonstrated a close correlation between the original and modified scores [43]. However, we employed a longer follow-up interval (87 months; range 24–156 months) than that reported by Erol et al. (45 months; range 25–89 months) [19].
Study limitations
Retrospective assessment with inadvertent biases was the main limitation of our study. The heterogeneous study group with a limited number of patients in each of the three groups represented another limitation. Therefore, it is difficult to compare the results between groups and to choose appropriate clinical classification. Thus, our findings need to be confirmed by adequately powered, prospective multicenter studies.
Conclusion
Surgical treatment of tumor-like lesions of the proximal femur is challenging but provides good mid-term results. The main stem of management of these benign lesions is to exclude malignant tumors with adequate imaging and biopsy, as well as discussion of diagnostic results and treatment options in a specialized tumor board. We showed that treatment of benign bone lesions of the proximal femur by local resection or destruction of the lesion, followed by filling the defect with bone graft material and internal stabilization is a safe and effective treatment option in children.
Abbreviations
- ABC:
-
Aneurysmal bone cyst
- BCI:
-
Bone cyst index
- CT:
-
Computer tomography
- FD:
-
Fibrous dysplasia
- MRI:
-
Magnetic resonance imaging
- SBC:
-
Simple bone cyst
References
van den Berg H, Kroon HM, Slaar A, Hogendoorn P (2008) Incidence of biopsy-proven bone tumors in children: a report based on the Dutch pathology registration “PALGA.” J Pediatr Orthop 28(1):29–35. https://doi.org/10.1097/BPO.0b013e3181558cb5
De Mattos CBR, Binitie O, Dormans JP (2012) Pathological fractures in children. Bone Joint Res 10:272–280
Jackson WF, Theologis TN, Gibbons CL, Mathews S, Kambouroglou G (2007) Early management of pathological fractures in children. Injury 38:194–200
Ortiz EJ, Isler MH, Navia JE, Canosa R (2005) Pathologic fractures in children. Clin Orthop Relat Res 432:116–126
Snyder BD, Hauser-Kara DA, Hipp JA et al (2006) Predicting fracture through benign skeletal lesions with quantitative computed tomography. J Bone Joint Surg [Am] 88:55–70
Pireau N, De Gheldere A, Mainard-Simard L, Lascombes P, Docquier PL (2011) Fracture risk in unicameral bone cyst: is magnetic resonance imaging a better predictor than plain radiography? Acta Orthop Belg 77:230–238
Wilkins RM (2000) Unicameral bone cysts. J Am Acad Orthop Surg 8:217–224
Lokiec F, Wientroub S (1998) Simple bone cyst: etiology, classification, pathology, and treatment modalities. J Pediatr Orthop B 7(4):262–273
Cottalorda J, Kohler R, Sales de Gauzy J et al (2004) Epidemiology of aneurysmal bone cyst in children: a multicenter study and literature review. J Pediatr Orthop B 13:389–394
Mankin HJ, Hornicek FJ, Ortiz-Cruz E, Villafuerte J, Gebhardt MC (2005) Aneurysmal bone cyst: a review of 150 patients. J Clin Oncol 23:6756–6762
Sullivan RJ, Meyer JS, Dormans JP, Davidson RS (1999) Diagnosing aneurysmal and unicameral bone cysts with magnetic resonance imaging. Clin Orthop Relat Res 366:186–190. https://doi.org/10.1097/00003086-199909000-00024
Erol B, Topkar MO, Caliskan E, Erbolukbas R (2015) Surgical treatment of active or aggressive aneurysmal bone cysts in children. J Pediatr Orthop B 24(5):461–468. https://doi.org/10.1097/BPB.0000000000000173
DiCaprio MR, Enneking WF (2005) Fibrous dysplasia: pathophysiology, evaluation, and treatment. J Bone Joint Surg [Am] 87:1848–1864
Harris WH, Dudley HR, Barry RJ (1962) The natural history of fibrous dysplasia: an orthopaedic, pathological, and roentgenographic study. J Bone Joint Surg [Am] 44:207–233
Ippolito E, Bray EW, Corsi A et al (2003) Natural history and treatment of fibrous dysplasia of bone: a multicenter clinicopathologic study promoted by the European Pediatric Orthopaedic Society. J Pediatr Orthop B 12:155–177
Guille JT, Kumar SJ, MacEwen GD (1998) Fibrous dysplasia of the proximal part of the femur: long-term results of curettage and bone-grafting and mechanical realignment. J Bone Joint Surg [Am] 80:648–658
Kushare IV, Colo D, Bakhshi H, Dormans JP (2014) Fibrous dysplasia of the proximal femur: surgical management options and outcomes. J Child Orthop 8:505–511
Tong Z, Zhang W, Jiao N, Wang K, Chen B, Yang T (2013) Surgical treatment of fibrous dysplasia in the proximal femur. Exp Ther Med 5:1355–1358
Erol B, Topkar MO, Aydemir AN, Okay E, Caliskan E, Sofulu O (2016) A treatment strategy for proximal femoral benign bone lesions in children and recommended surgical procedures: retrospective analysis of 62 patients. Arch Orthop Trauma Surg 136(8):1051–1061. https://doi.org/10.1007/s00402-016-2486-9
Merle d’Aubigné R, Postel M (1954) Functional results of hip arthroplasty with acrylic prosthesis. J Bone Joint Surg Am 36:451–475
Rutz E, Brunner R (2010) The pediatric LCP hip plate for fixation of proximal femoral osteotomy in cerebral palsy and severe osteoporosis. J Pediatr Orthop 30(7):726–731. https://doi.org/10.1097/BPO.0b013e3181efb86b
Capanna R, Dal Monte A, Gitelis S, Campanacci M (1982) The natural history of unicameral bone cyst after steroid injection. Clin Orthop Relat Res 166:204–211
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at https://www.R-project.org/.
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
WJ Metsemakers M Morgenstern E Senneville et al Fracture-Related Infection (FRI) group (2020) General treatment principles for fracture-related infection: recommendations from an international expert group. Arch Orthop Trauma Surg. 140(8):1013–1027. https://doi.org/10.1007/s00402-019-03287-4 ([Epub 2019 Oct 29])
Dormans JP, Pill SG (2002) Fractures through bone cysts: unicameral bone cysts, aneurysmal bone cysts, fibrous cortical defects, and nonossifying fibromas. Instr Course Lect 51:457–467
Vasconcellos DA, Yandow SM, Grace AM, Moritz BM, Marley LD, Fillman RR (2007) Cyst index: a nonpredictor of simple bone cyst fracture. J Pediatr Orthop 27:307–310
Donaldson S, Wright JG (2011) Recent developments in treatment for simple bone cysts. Curr Opin Pediatr 23:73–77
Dormans JP, Sankar WN, Moroz L, Erol B (2005) Percutaneous intramedullary decompression, curettage, and grafting with medical-grade calcium sulfate pellets for unicameral bone cysts in children: a new minimally invasive technique. J Pediatr Orthop 25:804–811
Dong C, Klimek P, Abächerli C, De Rosa V, Krieg AH (2020) Percutaneous cyst aspiration with injection of two different bioresorbable bone cements in treatment of simple bone cyst. J Child Orthop 14(1):76–84. https://doi.org/10.1302/1863-2548.14.190155
Canavese F, Wright JG, Cole WG, Hopyan S (2011) Unicameral bone cysts: comparison of percutaneous curettage, steroid, and autologous bone marrow injections. J Pediatr Orthop 31:50–55
Hou HY, Wu K, Wang CT et al (2011) Treatment of unicameral bone cyst: surgical technique. J Bone Joint Surg [Am] 93:92–99
Mik G, Arkader A, Manteghi A, Dormans JP (2009) Results of a minimally invasive technique for treatment of unicameral bone cysts. Clin Orthop Relat Res 467(11):2949–2954. https://doi.org/10.1007/s11999-009-1008-2
Roposch A, Saraph V, Linhart WE (2004) Treatment of femoral neck and trochanteric simple bone cysts. Arch Orthop Trauma Surg 124(7):437–442. https://doi.org/10.1007/s00402-004-0702-5
Luengo-Alonso G, Mellado-Romero M, Shemesh S, Ramos-Pascua L, Pretell-Mazzini J (2019) Denosumab treatment for giant-cell tumor of bone: a systematic review of the literature. Arch Orthop Trauma Surg 139(10):1339–1349. https://doi.org/10.1007/s00402-019-03167-x ([Epub 2019 Mar 15])
Biesecker JL, Marcove RC, Huvos AG, Miké V (1970) Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer 26(3):615–625. https://doi.org/10.1002/1097-0142(197009)26:3%3c615::aid-cncr2820260319%3e3.0.co;2-i
Gibbs CP Jr, Hefele MC, Peabody TD, Montag AG, Aithal V, Simon MA (1999) Aneurysmal bone cyst of the extremities factors related to local recurrence after curettage with a high-speed burr. J Bone Joint Surg Am. 81(12):1671–1678. https://doi.org/10.2106/00004623-199912000-00003
Dormans JP, Guirguis HB, Johnston DR, Khurana JS (2004) Surgical treatment and recurrence rate of aneurysmal bone cysts in children. Clin Orthop Relat Res 421:205–211
Ward AE; RATeS Study Group (2019) RATeS (Re-Admissions in Trauma and Orthopaedic Surgery): a prospective regional service evaluation of complications and readmissions. Arch Orthop Trauma Surg 139(10):1351–1360. https://doi.org/10.1007/s00402-019-03144-4 ([Epub 2019 Mar 20])
Marcove RC, Sheth DS, Takemoto S, Healey JH (1995) The treatment of aneurysmal bone cyst. Clin Orthop Relat Res 311:157–163
Fritz J, Sonnow L, Morris CD (2019) Adjuvant MRI-guided percutaneous cryoablation treatment for aneurysmal bone cyst. Skeletal Radiol 48(7):1149–1153. https://doi.org/10.1007/s00256-018-3115-1
Matta JM, Mehne DK, Roffi R (1986) Fractures of the acetabulum. Early results of a prospective study. Clin Orthop Relat Res 205:241–250
Øvre S, Sandvik L, Madsen JE, Røise O (2005) Comparison of distribution, agreement and correlation between the original and modified Merle d’Aubigné-Postel Score and the Harris Hip Score after acetabular fracture treatment: moderate agreement, high ceiling effect and excellent correlation in 450 patients. Acta Orthop 76(6):796–802. https://doi.org/10.1080/17453670510045390
Acknowledgement
All authors have read and agreed to the published version of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization WL, JM, RT, ER; formal analysis WL, RT, JD; investigation WL, RT; resources WL, JM, RT; statistical analysis, J.D.; writing—original draft preparation, WL, RT, ER; writing—review and editing JM; visualization WL, RT; supervision, JM, RT, ER.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This retrospective study was approved by the Ethics Committee of the Silesian Medical University, Katowice, Poland (PCN/0022/KB/99/20 dated June 5th, 2020).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tomaszewski, R., Rutz, E., Mayr, J. et al. Surgical treatment of benign lesions and pathologic fractures of the proximal femur in children. Arch Orthop Trauma Surg 142, 615–624 (2022). https://doi.org/10.1007/s00402-020-03687-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-020-03687-x