Abstract
Purpose
We aimed to develop a surgical treatment strategy for benign bone lesions of the proximal femur based upon retrospective review of our data in 62 children.
Methods
Sixty-two children [38 male, 24 female; median age 9 years (range 5–18 years)] with proximal femoral benign bone lesions were surgically treated between 2005 and 2013. Histopathological diagnoses were simple (31) or aneurysmal (27) bone cysts, and nonossifying fibromas (4). The pathological fracture rate was 77.4 %. Surgical treatment was determined due to four criteria, including patient’s skeletal maturity, localization and initial diagnosis of lesion, and amount of bone loss in the femoral neck and lateral proximal femur. Surgical procedure consisted of biopsy, curettage, bone grafting, and internal fixation when required. The median follow-up was 45 months (range 25–89 months).
Results
Complete clinical recovery was achieved in 56 (90.3 %) patients between 4 and 8 months postoperatively; full weight-bearing and mobilization, without pain and limping, was possible. The median preoperative and postoperative last follow-up Musculoskeletal Tumor Society (MSTS) scores were 13.3 % (range 10–23.3 %) and 96.6 % (range 90–100 %), respectively (p < 0.0001). The pathological fractures were healed in 10 weeks on average (range 8–12 weeks). Fifty-seven (92 %) patients demonstrated complete or significant partial radiographic healing between 5 and 7 months that maintained throughout follow-up. Local recurrence was not observed, and only 1 (1.6 %) patient required reoperation for partial cyst healing. There were 5 (8 %) complications, 1 (1.6 %) of which required reoperation.
Conclusions
This treatment strategy can provide good local control and excellent functional and radiological results in the management of benign bone lesions of the proximal femur in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proximal femur is one of the most common locations for benign bone lesions in children and adolescents. Bone lesions, such as simple bone cysts (SBCs), aneurysmal bone cysts (ABCs), fibrous dysplasias, and nonossifying fibromas (NOFs), often are seen in this region [1–3]. These tumors usually are small and asymptomatic, but moderate–large lesions with impending or actual fractures leading to pain, limp, and inability to walk are not uncommon.
When an enlarging benign lesion involves the proximal femur, major concerns are bone destruction and periarticular location. Surgical intervention frequently is required to manage these lesions in children. Surgical treatment should eradicate the lesion sufficiently to preclude local recurrence while restoring bone integrity to prevent a subsequent pathological fracture or deformity [2, 4–8]. It also should provide good stabilization when a pathological fracture occurs. Treatment challenges, including the risk of nonunion, varus malunion, and avascular necrosis of the femoral head, should also be considered [3, 9–13].
The literature on the treatment strategy of proximal femoral benign bone lesions in children is limited [2, 5, 9, 11, 13, 14]. Some problems remain unanswered. Is a separate biopsy procedure before definitive surgery required, how are these lesions best treated, and do the lesions with impending or actual pathological fractures routinely require internal fixation?
We retrospectively analyzed the long-term clinical, functional, and radiological results of 62 children treated surgically for bone cysts and NOFs of the proximal femur. Our choice of surgical procedures was based upon four criteria, including skeletal maturity of patient, localization and initial diagnosis of lesion, and amount of bone loss in the femoral neck and lateral proximal femur. Based on these retrospective data, we aimed to develop a treatment strategy with recommended surgical procedures.
Patients and methods
Sixty-two nonconsecutive children with SBCs, ABCs, and NOFs of the proximal femur were treated surgically at the authors’ institution from 2005 to 2013. Our institution is a reference center for bone tumors to which usually malignant and active or aggressive benign bone lesions are referred. The study population constituted 81.6 % of pediatric patients admitted to our orthopedic oncology unit for proximal femoral benign bone lesions. There were no fibrous dysplasias, chondroblastomas, giant cell tumors, or secondary ABCs included in this series. The study group consisted of symptomatic small (<50 % cortical involvement) or moderate–large (≥50 % cortical involvement) lesions of the femoral neck and pertrochanteric region, with or without an associated pathological fracture. Incidental small lesions managed only with observation were also not the subject of this study. Ethical Committee approval and patient consent were obtained.
Data were collected on: age and gender of the patients; weight, height and body mass index (BMI) of children; duration and type of symptoms; if present, previous treatment; radiological and histopathological findings; surgical procedure; functional and radiological results; complications including local recurrence; and the rate of reoperation.
The study group consisted of 38 boys and 24 girls, and the median age at surgery was 9 years (range 5–17 years). The mean weight, height, and BMI of children were 36 ± 11.9 kg, 140.6 ± 15 cm, and 17.6 ± 2 kg/m2, respectively. The definitive histopathological diagnoses of the solitary lesions were SBCs (31), ABCs (27), and NOFs (4). The overall health status was good for each patient in this series. The median duration of follow-up was 45 months (range 25–89 months).
All children were symptomatic; the most common symptoms were pain and limp, followed by inability to walk. The majority had enlarging defects with radiologically evident actual micro- (20 patients; 32.25 %) or displaced (28 patients; 45.2 %) fractures. The median duration of symptoms prior to presentation was 5 days (range 0–20 days). Thirty (48.4 %) children presented directly to our institution, and the remaining 32 (51.6 %) were referred from other institutions, with the aforementioned clinical and radiological findings.
Five children with SBC (2) or ABC (3) had previous interventions in other institutions, including curettage and grafting (4) or steroid injections (1). They referred to our institution with recurrence and/or a pathological fracture, an average of 19 months (range 8–40 months) after the first intervention. Whole study group, including new patients and previously treated cases, shared common presenting symptoms.
A surgical treatment strategy based on skeletal maturity of patient, localization and initial diagnosis of lesion, and amount of bone loss in the femoral neck and lateral proximal femur was developed retrospectively. Figure 1 shows different types of lesions (with the number of patients in each type) and recommended surgical procedures. Skeletal maturity was determined based on the presence of radiolucency at the level of the growth plate as seen on plain radiographs. An initial diagnosis was reached based on preoperative imaging studies and intraoperative findings, including macroscopic appearance of the lesion and frozen section. And finally, percentage width ratios (absolute lesion width/absolute proximal femoral transverse width at the level of the tumor) were calculated on anteroposterior (AP) and lateral radiographs to determine the amount of bone loss [15].
The authors’ classification system for benign bone lesions of the proximal femur in children with corresponding surgical procedures. In Type 1, there is a symptomatic small (<50 % cortical involvement) lesion without a pathological fracture in the proximal femur. Curettage and grafting can be performed regardless of skeletal maturity and localization of the lesion. In Types 2, 3, and 4, moderat–large lesions (≥50 % cortical involvement) involve the proximal femur with impending or actual fractures. Following curettage and grafting, internal fixation is recommended for these lesions. Type 2-pertrochanteric and Type 3-neck lesions occur in skeletally immature patients. In Types 2A and 3A, simple bone cysts (SBCs) and some extensive aneurysmal bone cysts (ABCs) are present in pertrochanteric and neck region, respectively. These lesions are managed by intramedullary fixation, regardless of amount of bone loss in femoral neck or lateral proximal femur (lateral buttress). In Type 2B, a moderately sized ABC or nonossifying fibroma (NOF) is present at the base of the femoral neck; there is enough bone in the femoral neck and lateral proximal femur to allow fixation with cannulated screws. In Type 2C, a large ABC or NOF is present at the base of the femoral neck. Although there is enough bone in the femoral neck, there is loss of lateral buttress, so a pediatric hip screw and a side plate should be considered rather than cannulated screws. In Type 3B, a large lesion is present in the femoral neck, so there is not enough bone beneath the physis to accept screws. Parallel pins across the physis can be used in combination with a spica cast. In Type 4, the physis is closing or closed. Type 4A includes SBCs in any location and extensive ABCs involving the neck and pertrochanteric regions. These lesions can be managed by intramedullary fixation. A sliding hip screw and a side plate are another option for ABCs without a subtrochanteric extension. The lateral buttress is present in Type 4B hips, so cannulated screws (or a hip screw and a side plate) can be used for ABCs and NOFs in this group. In all types, we recommend a representative biopsy specimen for frozen section. A walking spica cast immobilization following surgery is also recommended, except for adolescents who are managed by a hip screw and a side plate
The radiological studies included plain radiographs and magnetic resonance imaging (MRI) in all, and computerized tomography (CT) in some patients (Figs. 2, 3, 4). The radiographic examination of the cystic lesions revealed a lytic lesion with expansion and thinning of the cortex. A septated appearance was associated with most of ABCs and occasional cases of SBCs. There was no bony expansion in NOFs, instead a sclerotic rim was observed around the lesions. Plain radiographs showed displaced fractures, and however, they were unable to demonstrate the majority of microfractures. Radiographically, the lesions were classified as Stage 1-latent (4 patients; 6.4 %), Stage 2-active (39 patients; 63 %), or Stage 3-aggressive (19 patients; 30.6 %) [16]. Growth plate was open in 54 (87 %) and closed in 8 (13 %) patients.
A 9-year-old boy with a simple bone cyst of the proximal femur. Plain radiographs show an expansile lytic lesion involving the pertrochanteric region (Type 2A lesion). A septated appearance is seen (a, b).The cyst fluid is high signal intensity on T2-weighted MRI. A moderate medullary and periosseous edema demonstrating a microfracture is a predominant feature (c–e). The patient underwent biopsy with frozen section, curettage and grafting, and internal fixation with titanium elastic nails. Titanium elastic nails provided a continuous intramedullary decompression. Periodic follow-up radiographs at 24 months demonstrate complete radiographic healing with nearly 100 % opacification of the cyst cavity and cortical thickening (f–g)
An 11-year-old boy with displaced pathological fracture of the proximal femur due to a simple bone cyst (type 2A lesion) (a–c). Gross reduction of the bony fragments was provided after the diagnosis of a benign lesion/simple bone cyst was confirmed by frozen section. Then, curettage, bone grafting, and internal fixation with titanium elastic nails were performed (d, e). The follow-up radiographs at 9 months show fracture healing, in addition to complete opacification of the cyst cavity with progressive cortical thickening (f, g)
A 16-year-old girl with a displaced pathological fracture of the proximal femur through an aneurysmal bone cyst (Type 4A lesion) (a, b). The patient underwent extended curettage and grafting, followed by open reduction and internal fixation with a sliding hip screw and a side plate (c, d). The follow-up radiographs at 46-months demonstrate complete radiographic healing of the lesion with persistent shortening of the femoral neck (e, f)
Microfractures were mainly demonstrated by CT and MRI. CT scans were available in 39 (63 %) patients (all with a pathological fracture; 18 micro- and 21 displaced) and showed cortical disruption in all. On MRI, a moderate to severe medullary and periosseous edema was consistently observed in patients with an associated micro- or displaced fracture. In addition, MRI demonstrated internal septations in majority and fluid–fluid levels in some of ABCs. Multiple septations within the lesion occasionally were observed in SBCs with a previous pathological fracture or surgical intervention. MRI was also helpful in demonstrating extraosseous extension of ABCs.
Macroscopically, the majority of SBCs were seen as a unicameral lesion containing a clear yellow or, more commonly, pinky/red fluid. Occasionally, simple cysts with a previous fracture or intervention had septations. ABCs usually had a septated, multicameral appearance with a hemorrhagic fluid or blood-filled tissue inside. NOFs were yellow to brownish solid lesions. Microscopically, the pathological diagnosis for each patient was confirmed as SBC, primary ABC, or NOF.
Surgical technique
Surgical procedures consisted of curettage, bone grafting, and internal fixation when required, at the same surgical setting as the incisional biopsy and frozen section (Table 1). The procedures were performed by providing an experienced musculoskeletal pathologist available for interpretation of the frozen section specimen at the time of biopsy. Sixty (96.8 %) patients had one staged surgery, despite in four initial diagnoses did not correspond definitive diagnosis (SBC versus ABC). There were only 2 (3.2 %) ABCs with a very aggressive appearance on imaging studies that required a closed biopsy procedure prior to definitive surgery.
Under an image intensifier, the patient was placed in supine position on the radiolucent operative table. Lateral approach over the trochanter was used. Through the fracture or a small cortical window, a representative biopsy specimen was obtained for frozen section. The definitive surgical procedure was performed after the diagnosis of the frozen section was established. A headlamp and a surgical loupe were used when required.
When the diagnosis of a benign lesion was confirmed (or at least a malignancy was ruled out), the lateral cortical window was enlarged to allow adequate tumor removal. Gross reduction of the bony fragments was achieved in displaced fractures, before enlarging the cortical window. Microfractures usually did not require a reduction. When needed, femoral neck lesions were approached anteriorly, with a limited capsulotomy, to improve exposure.
The lesions were curetted under direct vision. If the initial diagnosis was an ABC, by high-speed burring and cauterization, the surgical margins were extended beyond the reactive zone of the lesion. If there was no bony envelope or a calcified periosteum encircling an ABC, then that part of the cyst was carefully curetted without using a burr. In skeletally immature patients with femoral neck involvement, great care was taken for not to damage the physis by these procedures. No local adjuvant (phenol and alcohol) was used in this series.
After the curettage was completed, the defect was filled by bone graft, and the surgical procedure was ended in a standard fashion in Type 1 lesions. Type 2, 3, and 4 lesions required internal fixation before grafting. The position and alignment were checked with an image intensifier, and different types of implants were applied for different types of lesions (Fig. 1).
Two or three TENs (2.5–4.0 mm in thickness) were inserted retrograde through the oval window(s) opened on lateral and medial cortices of the distal femur. They were inserted proximal into distal femoral physis, and in patients with an open growth plate, and did not pass the proximal femoral physeal line (Figs. 2, 3). The preferred position for sliding hip screw was inferior or center to the femoral neck and head (in skeletally mature patients) (Fig. 4). If cannulated screws were preferred for fixation, two screws were used. Great care was taken to place the screw(s) away from the growth plate in skeletally immature patients. Fluoroscopic image intensifier determined the correct position and alignment.
The average volume of the defect after tumor removal was about 90 cc (range 30–210 cc). Cancellous allograft cubes were used alone (55 patients; 88.7 %) or in combination (7 patients; 11.3 %) with morselized cancellous autograft taken from the iliac crest. The wide cortical window allowed easy application of the grafts.
Postoperative patient management
Except for older children who were managed by a sliding hip screw and a side plate, a unilateral walking spica cast allowing free ankle motion was applied for 6 weeks postoperatively with toe touch weight bearing with crutches. All patients were seen at 3 and 6 weeks postoperatively for evaluation of fracture alignment and fracture healing, respectively. After removal of the cast at 6 weeks, patients progressed from partial weight bearing to full weight bearing in 6 weeks. They attended a physical therapy program for 1–2 months to increase range of motion of the hip joint and strengthening of the affected lower extremity. Physical therapy program was lengthened when required.
Clinical and radiological follow-up
Clinical and radiological follow-up was performed by the two orthopedic surgeons (X.X., X.X) conducted the study. The patients were followed by 3-month intervals in the first year, by 6 month intervals in the second year and then annually. Functional evaluation was done with Musculoskeletal Tumor Society (MSTS) scoring [17]; preoperative and postoperative annual follow-up MSTS scores were obtained. The parameters for lower limb, including pain, function, emotional acceptance, supports, walking, and gait, were evaluated for each child. The difference between preoperative and postoperative last follow-up MSTS scores was analyzed statistically. Cyst healing was determined radiographically, and different types of responses to treatment were recorded (Table 2).
Early or late postoperative complications, including infection, wound problems, nonunion, refracture, failure of metallic implant, partial or complete injury to growth plate (due to surgical procedure or tumor itself), varus malunion, or avascular necrosis of the femoral head, were investigated. The rate of reoperation for clinical or radiological healing, recurrence, or complications was searched.
Statistical analysis
The data obtained were processed in Statistical Package for the Social Sciences (SPSS) program (release 11.0; SPSS Incorporation, Chicago, IL, USA). The Wilcoxon signed-rank test was used for nonparametric comparison of individual patient’s preoperative and postoperative functional scores.
Results
Complete clinical recovery was achieved in 56 (90.3 %) patients between 4 and 8 months postoperatively; full weight-bearing and mobilization, without pain and limping, was possible. A residual limping persisted till the end of 12 months in 6 (9.7 %) patients. Limping regressed by the help of long-term physical therapy in two patients. Three patients, with mild shortening of the involved extremity secondary to varus malunion of the proximal femur (2) or shortening of the femoral neck (1), required conservative treatment to manage limping. One child with partial healing on radiographs experienced pain, in addition to persistent limping, and required a reoperation at postoperative 12th months (the following paragraphs in Results explain these conditions in detail).
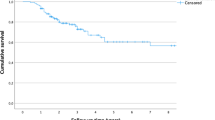
The functional scores increased significantly in the postoperative period; the mean preoperative and postoperative last follow-up MSTS scores were measured as 13.3 % (range 10–23 %) and 96.6 % (range 90–100 %), respectively (p < 0.0001) Fig. 5).
The pathological fractures were healed in 10 weeks on average (range 8–12 weeks). None of the patients required an additional procedure for stabilization of the fracture. All lesions responded well to surgical treatment with good radiographic incorporation of the bone graft. Fifty-seven (92 %) patients demonstrated complete or significant partial radiographic healing between 5 and 7 months that maintained throughout follow-up (Figs. 2, 3, 4). There were only 5 (8 %) patients with less than 80 % (60–80 %) obliteration of the cystic defect (4 SBCs, 1 ABC). In four of them, a progressive cortical thickening was observed, and these asymptomatic children did not require a reoperation to eradicate the lesions. The other child with a pertrochanteric ABC underwent repeat extended curettage, grafting, and additional internal fixation at the 12th month when he experienced significant pain on weight bearing in addition to persistent limping (last follow-up MSTS score before reoperation was used for this child). Local recurrence was not observed in this series.
There were 5 (8 %) early or late complications, only 1 (1.6 %) of which required a reoperation. One patient had a superficial wound infection which was managed by antibiotherapy. Another patient had skin necrosis which required a wound revision under general anesthesia. A mild (10°–15°) varus deformity of the proximal femur was observed in two patients with pertrochanteric SBCs. In another child with an aggressive ABC of the femoral neck and trochanteric region, shortening of the femoral neck was persisted postoperatively (Fig. 4). All three conditions led mild (<2 cm) limb length discrepancy and limping and, however, did not cause a significant functional problem requiring an additional operation. Using a shoe lift to compensate length discrepancy, limping was disappeared completely in children with malunion and significantly in the child with femoral neck shortening. None of the patients in this series had growth plate injury or avascular necrosis of the femoral head.
We did not observe failure of metallic implant in none of the patients. Implant removal was performed in 17 younger children, at least 2 years after the original procedure.
Discussion
The proximal femur is a common site for benign bone tumors in children. The specific anatomic location makes the management of these lesions unique. The small, silent lesions usually do not require treatment other than close observation. On the other hand, moderate–large tumors with progressive growth and pathological fracture frequently require surgery.
The risk of fracture and deformation is a potential problem when an active or aggressive benign tumor involves the proximal femur, an anatomic site that undergoes heavy mechanical loading. The majority of these lesions are cysts or cyst like with bony defects, very often extending from the subtrochanteric area to the femoral neck. The bone cortex in the fractured area is usually thinned and very often resembles a crushed eggshell. Surgical treatment is often indicated, and should include a reliable initial diagnosis, effective curettage, optimal filling of the resulting defect, neutralization of forces across the proximal femur to allow bone healing, and prevention of possible complications [1].
The preoperative imaging studies, particularly of plain radiographs and MRI, frequently support the diagnosis of bone cysts and NOFs in children. Except for rare cases of aggressive ABCs mimicking a malignant tumor, intraoperative findings consisting of macroscopic appearance of the lesion and frozen section are enough to make decision and proceed with definitive surgical treatment. Thus, a separate biopsy procedure usually is not required.
A large cortical window is required for effective curettage and complete excision of the tumor. In locally aggressive lesions such as ABCs, surgical margins should be extended beyond the reactive zone of the tumor by use of a high-speed burr and cautery [18–20]. Chemical adjuvants can be used [21–24], and however, their contribution to local recurrence is still questionable. Finally, cancellous bone grafting should be preferred to fill the defects, because this allows for bony healing and remodeling, which are important for long-term heavy mechanical loading [25].
The persistence of the lesion and refractures are the main indications for surgical treatment of SBCs. Current treatment options include compression, curettage, and injection of steroids, bone marrow aspirate, demineralized bone matrix, and bone substitutes. By providing a continuous drainage between the medullary canal and the cyst cavity, elastic nails can reduce intracystic pressure and stimulate healing [26–28]. Elastic nails can also stabilize the pathological fractures [12, 29–32]. However, healing with a residual cyst is a frequent finding following isolated use of intramedullary nailing [33–36]. This disadvantage can be eliminated by mechanical destruction of the membrane covering the inner wall of the cyst, and bone formation can be stimulated by grafting.
When a pathologic fracture occurs in the proximal femur, the fragments may be displaced by the effect of huge muscle pull, leading to deformity and shortening. Surgical treatment should neutralize these forces to stabilize the fracture and maintain length. There are several factors which limit the internal fixation of pathological fractures of the proximal femur in children. The small diameter of the femoral neck and open epiphyseal plate, particularly in young children, should be considered when inserting a fixation device. Also, it is difficult to stabilize extensive lesions of this region, because very little surrounding bone remains for bone healing.
The literature on the treatment of pediatric pathological hip fractures is limited. Malkawi et al. treated 12 subtrochanteric fractures through benign bone tumors by curettage, cancellous bone grafting, and internal fixation [11]. There were three complications including nonunion and stress fracture, both resulted in varus deformity, and collapse of the femoral head. Roposh et al. reported 12 children with SBCs of the proximal femur who were treated with retrograde flexible nailing [13]. Six showed a pathological fracture. Even though there was no recurrence or nonresponder, nine patients healed with residuals. The only major complication was a coxa vara deformity. Vigler et al. performed curettage and grafting with bone or bone substitute, followed by internal or external fixation in seven children with subtrochanteric fractures due to SBCs [14]. The authors achieved good clinical results with high complete radiographic healing rates.
Dormans [2] and Erol [5] previously classified the treatment of benign bone lesions of the proximal femur in children into three types, based on skeletal maturity and amount of bone loss in the femoral neck and lateral proximal femur. In this study, based upon retrospective analysis of 62 children, this classification was modified, and localization and initial diagnosis of the lesion were also considered as important determining criterias.
The management of patients in this series mainly included frozen section, intralesional excision, allogenic cancellous bone grafting, and internal fixation. The framework of the proximal femur, which was lost its structural integrity (due to the tumor itself and the wide cortical window), was reconstructed to restore hip joint function. Different types of internal fixation methods provided good support during bone healing, and prevented refracture and possible long-term complications in majority of the patients. The reconstruction was usually protected by a walking spica cast during the healing period, because young children constituted the vast majority of the study group.
In this study, 60 (96.8 %) patients had single-staged surgery consisting of biopsy with a frozen section prior to eradication of the lesions. Imaging studies and intraoperative findings demonstrated an initial diagnosis, which was consistent with the definitive diagnosis in 58 (93.5 %) patients. Even though a simple curettage was enough for SBCs and NOFs, an extended curettage with use of a mechanical burr and cauterization was routinely performed to eradicate ABCs. The combination of open mechanical destruction of the cyst membrane and continuous intramedullary decompression of the cyst cavity by elastic nails was effective in the management of SBCs. This individualized approach provided high radiographic healing rates, and eliminated the need for additional surgery to eradicate the lesions.
The resultant defects were filled with cancellous allograft cubes in 55 (88.7 %) procedures. Cancellous allograft and autograft combination was required only in 7 (11.3 %) procedures performed for extensive lesions in adolescents. Even though autografts have a higher rate of complete or partial healing than allografts in large defects [25], limited autograft sources are frequently insufficient to fill large defects in children. Also, allografts obviate the need for autograft harvesting, with its associated morbidity. Good graft incorporation with bony healing and remodeling was achieved throughout this series. Even though bone graft substitutes (beta-tricalcium phosphate, CaSO4, CaSO4/CaPO4 composites, and so on) were reported as effective alternatives to autologous bone grafts [37–39], they were not routinely available in the country that the study was conducted.
Titanium elastic nails (34 patients; 54.8 %) and a sliding hip screw and a side plate (15 patients; 24.1 %) constituted 79 % of the internal fixation devices used in this series. In addition to intramedullary decompression and stabilization of SBCs, TENs were helpful to stabilize extensive pertrochanteric ABCs, which would require a very long side plate otherwise. Pediatric or adult type of hip screw and a side plate was used safely in patients with ABCs and NOFs. The lesions involving the femoral neck in immature patients were fixed by K-wires crossing the physeal line. Even though stabilization with K-wires had a relative safety, probably the use of postoperative spica casting prevented varus malunion in these patients. The recommended internal fixation options of this protocol, when combined with a walking spica cast in majority, were successful in terms of fracture stabilization and long-term structural integrity.
This study has some limitations. First, it was conducted based on a retrospective database. Second, even though the study population was larger than the previously reported series, a larger number of patients might be required to develop a treatment strategy. Third, the study group was heterogeneous in terms of the type of lesions and surgeries. Therefore, the overall results mainly revealed the success rate of certain surgeries performed for most common types of lesions.
Surgical treatment of pathological fractures through benign lesions of the proximal femur can be a challenge in children. Treatment of the fracture and the lesion has equal priority in this specific location. Long-term structural integrity should also be provided to avoid possible complications. A uniform approach based on sound principles of tumor treatment can be applied to eradicate the lesions. However, stabilization of the framework of the proximal femur should be individualized due to anatomical considerations of this region in children. This treatment protocol, based upon retrospective review of a moderate number of children, provided effective treatment options for different types of lesions in this particular region, with good local tumor control and excellent long-term functional and radiological results.
References
Arkader A, Dormans JP (2010) Pathological fractures associated with tumors and unique conditions of the musculoskeletal system. In: Beaty JH, Skaggs DL, Flynn JM, Waters K (eds) Rockwood and Wilkins’ fractures in children, 7th edn. Lippincott Williams & Wilkins, Philadelphia, pp 120–191
Dormans JP, Pill SG (2002) Fractures through bone cysts: unicameral bone cysts, aneurysmal bone cysts, fibrous cortical defects, and non-ossifying fibromas. Instr Course Lect 51:457–467
Ortiz EJ, Isler MH, Navia JE, Canosa R (2005) Pathological fractures in children. Clin Orthop Relat Res 432:116–126
Erol B, Dormans JP (2005) Musculoskeletal tumors in children. In: Dormans JP (ed) Core knowledge in orthopaedics. Pediatric orthopaedics. Philadelphia: Elsevier Mosby, pp 290–336
Erol B, Pill SG, Guttenberg ME, Meyer JS, Dormans JP (2002) Pathologic hip fracture in a 4-year-old boy. Clin Orthop Relat Res 403:264–273
Saraph V, Linhart WE (2005) Modern treatment of pathological fractures in children. Injury 36:64–74
Shih HN, Cheng CY, Chen YJ, Huang TJ, Hsu RW (1996) Treatment of the femoral neck and trochanteric benign lesions. Clin Orthop Relat Res 328:220–226
Wai EK, Davis AM, Griffin A, Bell RS, Wunder JS (2001) Pathologic fractures of the proximal femur secondary to benign bone tumors. Clin Orthop Relat Res 393:279–286
Abdel-Mota’al MM, Mohamad ASO, Katchy KC, Mallur AA, Ahmad FH, El-Alfy B (2008) Management of unicameral bone cyst of proximal femur: experience of 14 cases and review of literature. Kuwait Med J 40:202–210
Cohen J (1977) Unicameral bone cysts: a current synthesis of reported cases. Orthop Clin North Am 8(4):715–736
Malkawi H, Shannak A, Amr S (1984) Surgical treatment of pathological subtrochanteric fractures due to benign lesions in children and adolescents. J Pediatr Orthop 4(1):63–69
Roposch A, Saraph V, Linhart WE (2000) Flexible intramedullary nailing for the treatment of unicameral bone cysts in long bones. J Bone Joint Surg Am 82-A(10):1447–1453
Roposh A, Saraph V, Linhart WE (2004) Treatment of femoral neck and trochanteric simple bone cysts. Arch Orthop Trauma Surg 124(7):437–442
Vigler M, Weigl D, Schwarz M, Ben-Itzhak I, Salai M, Bar-On E (2006) Subtrochanteric femoral fractures due to simple bone cysts in children. J Pediatr Orthop B 15(6):439–442
Arata MA, Peterson HA, Dahlin DC (1981) Pathological fractures through non-ossifying fibromas. J Bone Joint Surg 63(6):980–988
Enneking WF (1983) Natural history. In: Enneking WF (ed) Musculoskeletal tumor surgery. Churchill Livingstone, New York, pp 1–23
Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ (1993) A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res 286:241–246
Dormans JP, Hanna BG, Johnston DR, Khurana JS (2004) Surgical treatment and recurrence rate of aneurysmal bone cysts in children. Clin Orthop Relat Res 421:205–211
Gibbs CP Jr, Hefele MC, Peabody TD, Montag AG, Aithal V, Simon MA (1999) Aneurysmal bone cyst of the extremities. Factors related to local recurrence after curettage with a high-speed burr. J Bone Joint Surg Am 81(12):1671–1678
Erol B, Topkar MO, Calıskan E, Erbolukbası R (2015) Surgical treatment of active or aggressive aneurysmal bone cysts in children. J Pediatr Orthop B 24(5):461–468
Biesecker JL, Marcove RC, Huvos AG, Miké V (1970) Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer 26(3):615–625
Marcove RC, Sheth DS, Takemoto S, Healey JH (1995) The treatment of aneurysmal bone cyst. Clin Orthop Relat Res 311:157–163
Ozaki T, Hillmann A, Lindner N, Winkelmann W (1997) Cementation of primary aneurysmal bone cysts. Clin Orthop Relat Res 337:240–248
Schreuder HW, Veth RP, Pruszczynski M, Lemmens JA, Koops HS, Molenaar WM (1997) Aneurysmal bone cysts treated by curettage, cryotherapy and bone grafting. J Bone Joint Surg Br 79(1):20–25
Glancy GL, Brugioni DJ, Eilert RE, Chang FM (1991) Autograft versus allograft for benign lesions in children. Clin Orthop 262:28–33
Bumci I, Vlahović T (2002) Significance of opening the medullar canal in surgical treatment of simple bone cyst. J Pediatr Orthop 22(1):125–129
Givon U, Sher-Lurie N, Schindler A, Ganel A (2004) Titanium elastic nail-a useful instrument for the treatment of simple bone cyst. J Pediatr Orthop 24(3):317–318
Santori F, Ghera S, Castelli V (1988) Treatment of solitary bone cysts with intramedullary nailing. Orthopedics 11(6):873–878
Cha SM, Shin HD, Kim KC, Kang DH (2013) Flexible intramedullary nailing in simple bone cysts of the proximal humerus: prospective study for high-risk cases of pathologic fracture. J Pediatr Orthop B 22(5):475–480
de Sanctis N, Andreacchio A (2006) Elastic stable intramedullary nailing is the best treatment of unicameral bone cysts of the long bones in children? Prospective long-term follow-up study. J Pediatr Orthop 26(4):520–525
Glanzmann MC, Campos L (2007) Flexible intramedullary nailing for unicameral cysts in children’s long bones. J Child Orthop 1(2):97–100
Masquijo JJ, Baroni E, Miscione H (2008) Continuous decompression with intramedullary nailing for the treatment of unicameral bone cysts. J Child Orthop 2(4):279–283
Hou HY, Wu K, Wang CT, Chang SM, Lin WH, Yang RS (2010) Treatment of unicameral bone cyst: a comparative study of selected techniques. J Bone Joint Surg Am 92(4):855–862
Hunt KJ, Bergeson A, Coffin CM, Randall RL (2009) Percutaneous curettage and bone grafting for humeral simple bone cysts. Orthopedics. 32(2):89
Mik G, Arkader A, Manteghi A, Dormans JP (2009) Results of a minimally invasive technique for treatment of unicameral bone cysts. Clin Orthop Relat Res 467(11):2949–2954
Schreuder HW, Conrad EU 3rd, Bruckner JD, Howlett AT, Sorensen LS (1997) Treatment of simple bone cysts in children with curettage and cryosurgery. J Pediatr Orthop 17(6):814–820
Hirata M, Murata H, Takeshita H, Sakabe T, Tsuji Y, Kubo T (2006) Use of purified beta-tricalcium phosphate for filling defects after curettage of benign bone tumors. Int Orthop 30:510–513
Dormans JP, Sankar WN, Moroz L, Erol B (2005) Percutaneous intramedullary decompression, curettage, and grafting with medical-grade calcium sulfate pellets for unicameral bone cysts in children: a new minimally invasive technique. J Pediatr Orthop 25:804–811
Gentile JV, Weinert CR, Sclechter JA (2013) Treatment of unicameral bone cysts in pediatric patients with an injectable regenerative graft: a preliminary report. J Pediatr Orthop 33:254–261
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Erol, B., Topkar, M.O., Aydemir, A.N. et al. A treatment strategy for proximal femoral benign bone lesions in children and recommended surgical procedures: retrospective analysis of 62 patients. Arch Orthop Trauma Surg 136, 1051–1061 (2016). https://doi.org/10.1007/s00402-016-2486-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-016-2486-9