Abstract
Introduction
We assessed the efficacy of fibrin sealant (FS) and tranexamic acid (TXA) administered topically in patients with a hip fracture treated with prosthetic replacement.
Materials and methods
Parallel, multicentre, open label, randomised, clinical trial. We compared three interventions to reduce blood loss: (1) 10 ml of FS, (2) 1 g of topical TXA, both administered at the end of the surgery, and (3) usual haemostasis (control group). The main outcome was blood loss collected in drains. Other secondary variables were total blood loss, hidden blood loss, transfusion rate, average hospital stay, complications, adverse events, and mortality.
Results
A total of 158 patients were included, 56 in the FS group, 52 in the TXA group, and 50 in the control group. The total amount of blood collected in drains was lower in the TXA group (148.6 ml, SD 122.7 in TXA; 168.2 ml, SD 137.4 in FS; and 201.5 ml, SD 166.5 in control group) without achieving statistical significance (p = 0.178). The transfusion rate was lower in the TXA group (32.7%), compared with FS group (42.9%) and control group (44.0%), without statistical significance (p = 0.341). There were no complications or adverse effects related to the evaluated interventions.
Conclusions
The use of TXA and FS administered topically prior to surgical closure in patients with a sub-capital femoral fracture undergoing arthroplasty did not significantly reduce either postoperative blood loss or transfusion rate, compared with a group that only received usual haemostasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proximal femoral fractures are usually caused by high-energy trauma in young adults, but by a low-energy trauma in the elderly [1]. This condition occurs more often in the elderly, presenting one of the highest incidences of morbidity and mortality. Specifically, mortality incidence ranges from 18 to 33% per year [2]. Age, sex, dementia, and frailty are associated with increased mortality [2].
Despite the advances in surgical techniques and materials in the treatment of femoral fractures, the blood loss causes anaemia and increases perioperative morbidity and mortality [3,4,5]. Pharmacological and non-pharmacological strategies should be applied to reduce bleeding and to avoid transfusions. One strategy is the intraoperative administration of topical or intravenous haemostatic agents. Tranexamic acid (TXA) inhibits fibrinolysis by blocking the lysine-binding sites on plasminogen, and facilitates the coagulation process [6]. Fibrin Sealant (FS) is a biological adhesive which initiates the final stages of coagulation. It is derived from the transformation of fibrinogen in fibrin by thrombin [7]. Several clinical trials and meta-analyses have studied the efficacy of FS and topical TXA in patients undergoing elective prosthetic hip and knee surgery [8,9,10,11,12,13], but the research in hip fractures is scarce. At the time we designed our study, in patients with proximal femoral fracture, only two clinical trials of the use of intravenous TXA had been reported and no studies about topical administration [14, 15]. One of them found a diminution of blood loss and transfusion in the TXA group, but found an increased risk of cardiovascular adverse events [15]. For these reasons, we conducted a randomised controlled trial with the hypothesis that FS and TXA administered topically (with a lower systemic absorption) would reduce postoperative blood loss, and minimize the adverse events in patients undergoing prosthetic replacement for proximal femoral fracture.
Materials and methods
This study is a multicentre, open label, three-arm, randomised, parallel-group clinical trial. It is registered at ClinicalTrials.gov (number NCT02150720).
The protocol was approved by the local Research Ethics Committees of seven participating university hospitals and the Spanish National Agency of Medicines and Health Products. The study was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained before surgery from all included participants.
Patient population
We recruited consecutive patients from February 2013 to March 2015. Inclusion criteria were adult patients, aged 18 or older, with unilateral sub-capital femoral fracture, requiring (total or partial) hip replacement, who agreed to participate and signed the consent form or the legal representative. Older patients and patients with dementia were included.
Exclusion criteria were known allergy to FS or TXA, multiple fractures, use of intraoperative blood salvage, history of coagulopathy or thromboembolic events like history of thromboembolic events like cerebral vascular accident, myocardial infarction or angina, deep vein thrombosis, pulmonary embolism, peripheral arterial vasculopathy, patients with thrombogenic arrhythmias, patients with cardiovascular stents, prothrombotic alteration in coagulation; patients treated with anticoagulant (anticumarin, direct anticoagulants), antiplatelets (including AAS 100 mg) or contraceptive or estrogens and, patients who refused to participate.
Randomisation
Patients were randomised to receive topical FS (Evicel®, Omrix Biopharmaceuticals N.V, Diegem, Belgium), topical TXA (Amchafibrin®, Rottafarm SL, Valencia, Spain), or routine haemostasis only.
A table of random numbers was computer-generated and stratified by centre. Random allocation was made before surgery, via phone call to the coordinating centre. The treatment allocation sequence was concealed to clinical investigators.
Group 1. Evicel® 5.0 ml was administered following the manufacturer’s instructions; 5 ml of fibrinogen solution (50–90 mg/ml) was combined with 5 ml of human thrombin (800–1200 UI/ml), and the 10 ml product was applied using a special syringe that was supplied with the product.
Group 2. The TXA group received a single 1 g dose of TXA in a 10 ml solution topically by syringe-spray. We chose this dose based on our experience in a previous study with topical TXA in knee arthroplasty that showed a significant reduction in blood loss compared with routine haemostasis [9].
In both groups, routine haemostasis was applied and after the prosthesis was inserted, the entire surgical site was thoroughly rinsed and meticulously dried. FS and TXA were applied on the surrounding soft tissues and the exposed surfaces of the femur.
Group 3. The control group only received routine haemostasis consisting only in the electro-coagulation of all bleeding points and vessels.
Operative and postoperative procedures
Surgery was performed by senior orthopaedic surgeons who decided the type of prosthesis, cemented or non-cemented. The used anaesthetic technique was intra-spinal with controlled hypotension. An intravenous prophylactic antibiotic was administered during anaesthetic induction, lasting for 24 h after surgery. Electro-coagulation of blood vessels was performed during surgery in all patients (routine haemostasis). One number eight vacuum drain was inserted before closing the wound, and was removed 24–48 h after surgery. We clamped the drain during 1 h preventing the loss of topical medications through the drain and favouring its effect. In the control group the drain was also clamped during 1 h after surgery.
All patients received low-molecular-weight heparin for 30 days to prevent thromboembolic complications. All participating hospitals followed the same blood-transfusion protocol. The transfusion was indicated when haemoglobin (Hb) was ≤ 7 g/dl and patients were ≤ 65 years old without history of cardiovascular disease; Hb was ≤ 8 g/dl and patients > 65 years or with history of cardiovascular or respiratory disease or an increase in O2 consumption; Hb ≤ 9–10 g/dl and patients with history of angina or myocardial infarction or left ventricular insufficiency. The decision of transfusing allogeneic blood was made by the anaesthesiologist during surgery or by the ward physician during the postoperative period. Patients remained seated from hour 24 to 48 after surgery. Progressively, with the support of physical therapists, they started walking with load and ongoing support with crutches and/or walkers.
Outcomes
The primary outcome was the blood collected postoperatively in the vacuum drains. Secondary outcomes were total blood loss and hidden blood loss (blood lost during surgery or retained in the wound or in adjacent tissues), the rate of perioperative blood transfusion, the preoperative and postoperative haemoglobin levels, the units of transfused blood, the rate of surgical infections, the length of hospital stay, the rate of venous thrombosis, treatment-related adverse events, and mortality.
The data of blood loss collected in drains were extracted from the daily nursing records. Total blood loss and hidden blood loss were calculated based on haemoglobin balance according to equations described by Nadler et al. [16].
The intensity of adverse events was classified as mild (not interfering with the patients’ normal activities), moderate (interfering with normal activities), and severe (preventing normal activities).
Patients were followed up during the postoperative days until hospital discharge and at day 30 (± 15 days after hospital discharge). One year after surgery we examined the patients’ medical records or contacted by telephone with them to assess the mortality rate.
Statistical analysis
Based on a previous clinical trial [13], we assumed 230 ml less blood in drains in the experimental groups than in the control group, a 15% drop-out rate, an alpha risk of 0.05, and a beta risk of 0.2. The number of patients required for each group was 55, that is, 165 in total.
The main analysis was by Intention to Treat (ITT), and, secondarily, Per Protocol (PP). For categorical data we calculated frequency count. For quantitative data, when appropriate, we calculated mean and standard deviation (SD), or mean difference and 95% confidence interval (CI). Pearson’s Chi square tests were used for categorical data. Blood loss was analysed using one-way ANOVA. Statistical significance was set at p ≤ 0.05.
Results
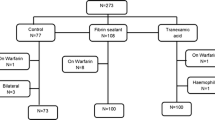
Four hundred and eighteen patients were screened, 257 (61%) of which were excluded, mainly because they did not meet the inclusion criteria. A total of 161 patients were thus randomised and homogeneously allocated to one of the three treatment groups: 56 to FS (group 1), 53 to TXA (group 2), and 52 to control (group 3). Three patients retired the consent to participate in the study and 14 patients did not receive the intervention. Additionally, a patient was excluded because he underwent an open reduction and an internal fixation surgery. Therefore, the number of assessable patients by ITT was 56 in the FS group, 52 in the TXA group, and 50 in the control group; the number of patients included PP was 143, 45 in the FS group, 48 in the TXA group, and 50 in the control group (see flow diagram in Fig. 1). During the 12 month follow-up, 21 (13%) patients died (five in TXA, seven in FS and nine in control group).
Patients’ mean age was 83.8 (SD 8.5) years; 122 (77.2%) patients were women and 36 (22.8%), men. The mean BMI was 24.6 (SD 3.9). Treatment groups showed no differences in their baseline characteristics or co-morbidities (Table 1). The co-morbidities with higher rates were hypertension and previous surgery. Seventeen patients presented dementia. The most frequent previous surgeries were general (25 patients), orthopaedic (21 patients) and cataract (14 patients) surgeries. Most patients had an American Society of Anesthesiologists (ASA) score of II–III. Ninety-two percent of prosthesis were hemi-arthroplasties. There were no significant differences between the treatment groups regarding preoperative anaemia, time from admission to surgery, duration of surgery, type of prosthesis, and prosthesis cementation (Table 1).
The blood loss collected by the drains was lower in the TXA group than in the other groups, but had no statistically significant difference: 148.6 (SD 122.7) ml in the TXA group, 168.2 (SD 137.4) ml in the FS group and 201.5 (SD 166.5) ml (p = 0.178) (Table 2). The PP analysis showed similar results.
Total blood loss was 1192.9 (SD 785.7) ml in the TXA group, 1620.4 (SD 1042.7) ml in the FS group and, 1557.2 (SD 847.1) ml in the control group (p = 0.063). Total blood loss in PP analysis was significantly lower in the TXA group: 1177.9 (SD 733.6) ml in the TXA group, 1557.2 (SD 847.1) ml in the control group and 1653.8 (SD 1085.0) ml in the FS group (p = 0.048). The hidden blood loss was lower in the TXA group than in the other groups, in both ITT (Table 2) and PP analyses, but had no statistically significant difference.
The mean overall Hb levels of patients prior to surgery were 12.3 g/dl (SD 1. 7) with no differences between groups. The overall pre-transfusional Hb was 8.4 g/dl (SD 1.1). Sixty-three patients (39.9%) needed postoperative transfusion. The TXA group received fewer transfusions, but with no statistically significant differences between groups (p = 0.431). The total number of units of transfused red blood cells was 122. Likewise, the group with TXA received the lowest number of blood units, but with no statistically significant differences (p = 0.722) (Table 3). The overall mean hospital stay of study patients was 11.5 (SD 11.1) days, with no statistically significant difference between groups (p = 0.969).
There were no adverse events with a certain causal relationship associated to the study interventions. However, there was one event with a possible causal relationship.
One patient in the TXA group suffered a stroke secondary to an arrhythmia in the immediate postoperative period associated with massive pulmonary thromboembolism and exitus. Another six patients died during the first 30 postoperative days (two intra-operatively, two of cardiorespiratory arrest, one due to a hospital pneumonia, and one of fever of unknown origin after discharge). Additionally, 14 patients died from 30 postoperative days to the end of 12 month follow-up. Therefore, the overall mortality rate one year after surgery was 13% (21 patients; five in TXA, seven in FS and nine in control group).
Thirty-three patients presented medical complications with no differences between treatment groups. The most frequent medical complication was urinary tract infection in nine patients. There were two cases of early prosthetic infection unrelated to the treatments. Fifteen patients presented surgery-related complications; 12 of these were severe. Seven patients (4.5%) required a re-intervention, six patients due to prosthesis dislocation and one patient because a prosthesis infection. Four patients were of TXA group and three to control group.
Discussion
Based on the main results of our study, FS or topical TXA does not reduce postoperative bleeding and transfusion requirements in patients undergoing hip replacement for sub-capital femoral fracture. Only topical TXA showed a significant reduction in the secondary outcome, total blood loss. Cardiovascular complications, thromboembolism, infection rates or mortality were similar in all compared groups.
Published studies have assessed FS [13, 16,17,18] and topical TXA [19,20,21,22,23] efficacy in primary elective hip arthroplasty, but we only found Emara et al. [24] and Kang et al. [25] studies evaluating topical TXA, and no studies assessing FS in hip fractures.
Emara et al. [24] study differed in regard to our clinical trial because patients were younger (50–60 years old) and the inclusion criteria were more restrictive (patients with preoperative anaemia or associated medical pathology were excluded). Furthermore, the dose of topical TXA used was higher than in our study, 1.5 g diluted in 150 ml of saline solution.
Kang et al. [25] included 160 patients divided in two groups of 80 patients each, undergoing total hip arthroplasty for osteoarthritis or hemi-arthroplasty for femoral fracture. Each of these groups was subdivided in two groups of 40 patients: one group with topical TXA and the other was a control group. The dose used of TXA was also higher than in our trial (3 g diluted in 100 ml of saline solution) and administered in a different way (through surgical drainage after wound closure, leaving drain aspiration closed for 30 min). In Kang et al. study, there was a statistically significant decrease in bleeding and transfusions in patients treated with topical TXA with hip osteoarthritis but not in those with femoral fracture.
We could partially explain our results because of the characteristics of the included population, i.e., advanced age, frailty and associated co-morbidities, a precarious haemostasis, and the hyperfibrinolysis state due to fracture and increased by surgery. Another reason could be related to the drug dose and the dilution of the product in saline solution. Except in one trial [19]—which used 1 g of TXA—, previous studies in hip arthroplasty had most frequently used a 3 g dose; the amount of saline solution in which the TXA was diluted ranged from 50 to 150 ml [20,21,22,23,24,25]. However, we only used 1 g of TXA in a 10 ml solution, possibly insufficient to produce a clinical effect in reducing blood loss.
Furthermore, the FS administered dose in our study was similar to other, but its administration by drip probably did not cover the entire surgical site, as we could expect using an air pressure pump.
Among the strengths of our study there is the fact that it was a prospective, controlled, randomised, multicentre study with an adequate sample size to detect differences between groups. The surgical procedure and transfusion criteria were standardised. Furthermore, we compared the effect of haemostatic treatments to reduce bleeding in a little-studied population group.
Nevertheless, our study presented some limitations. It was not blinded due to difficulties in concealing interventions. Yet, the nursing staff who recorded blood losses was unaware of the study hypothesis. There were difficulties in recruiting patients, which forced us to extend the study period and the number of centres. Despite being a multicentre study, 76% of the included patients belonged to one single centre. Due to the potential prothrombotic effect of TXA, we applied strict exclusion criteria to avoid complications; as a result, 42% of potential patients were excluded, mainly for history or risk of thromboembolic events.
In conclusion, the use of 10 ml FS and 1 g topical TXA in patients undergoing surgery due to a hip fracture did not significantly reduce postoperative blood loss and transfusion requirements compared with usual haemostasis. Further randomised controlled clinical trials are needed to evaluate the efficacy and safety, especially of topical TXA administered at higher doses.
References
Bentler SE, Liu L, Obrizan M, Cook EA, Wright KB, Geweke JF, Chrischilles EA, Pavlik CE, Wallace RB, Ohsfeldt RL, Jones MP, Rosenthal GE, Wolinsky FD (2009) The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol 170:1290–1299
Singer BR, McLauchlan GJ, Robinson CM (1998) Epidemiology of fractures in 15000 adults: the influence of age and gender. J Bone Jt Surg Br 80:243–248
Carson JL, Duff A, Poses RM, Berlin JA, Spence RK, Trout R et al (1996) Effect of anaemia and cardiovascular disease on surgical mortality and morbility. Lancet 348:1055–1060
Kirksey M, Lin Chiu Y, Ma Y, Gonzalez Della Valle A, Poultsides L, Gerner P, Memtsoudis SG (2012) Trends in in-hospital major morbidity and mortality after total joint arthroplasty: United States 1998–2008. Anesth Analg 115:321–327
Spahn DR (2010) Anemia and patient blood management in hip and knee surgery. A systematic review of the literature. Anesthesiology 113:482–495
Okamoto S, Hijikata-Okunomiya A, Wanaka K et al (1997) Enzyme controlling medicines: introduction. Semin Thromb Hemost 23:493–501
Brennan M (1991) Fibrin glue. Blood Rev 5:2404
Li J, Li HB, Zhai XC, Qin-Lei, Jiang XQ, Zhang ZH (2016) Topical use of topical fibrin sealant can reduce the need for transfusión, total blood loss and the volumen of drainage in total knee and hip arthroplasty: a systematic review and meta-analysis of 1489 patients. Int J Surg 36:127–137
Aguilera X, Martínez-Zapata MJ, Hinarejos P, Jordán M, Leal J, González JC, Monllau JC, Celaya F, Rodríguez-Arias A, Fernández JA, Pelfort X, Puig-Verdie Ll (2015) Topical and intravenous tranexamic acid reduce blood loss compared to routine hemostasis in total knee arthroplasty: a multicenter, randomized, controlled trial. Arch Orthop Trauma Surg 135:1017–1025
Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM (2014) A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Jt J 96:1005–1015
Wong J, Abrishami A, El Beheiry H, Mahomed N, Davey JR, Gandhi R et al (2010) Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty. J Bone Jt Surg Am 92:2503–2513
Molloy DO, Archbold HA, Ogonda L, McConway J, Wilson RK, Beverland DE (2007) Comparision of topical fibrin spray and tranexamic acid on blood loss after total knee replacement: a prospective, randomized controlled trial. J Bone Jt Surg Br 89:306–309
Mawatari M, Higo T, Tsutsumi Y, Shigematsu M, Hotokebuchi T (2006) Effectiveness of autologous fibrin tissue adhesive in reducing postoperative blood loss during total hip arthroplasty: a prospective randomised study of 100 cases. J Orthopaedic Surg 14:117–121
Sadeghi M, Mehr-Aein A (2007) Does a single bolus dose of tranexamic acid reduce blood loss and transfusion requirements during hip fracture surgery? A prospective study randomized double blind study in 67 patients. Acta Medica Iranica 45:437–442
Zufferey PJ, Miquet M, Quenet S, Martin P, Adam P, Albaladejo P, Mismetti P, Molliex S for the investigators of the tranexamic acid in hip-fracture surgery (THIF) study (2010) Tranexamic acid in hip fracture sugery: a randomized controlled trial. Br J Anaesth 104: 23–30
Nadler SB, Hidalgo JU. Bloch T (1962) Prediction of blood volume in normal human adults. Surgery 51:224–232
Mc Connell JS, Shewale S, Munro NA, Shah K, Deakin AH, Kinninmonth AWG (2011) Reduction of blood loss in primary hip arthroplasty with tranexamic acid or fibrin spray. A randomized controlled trial. Acta Orthop 82:660–663
Randelli F, Banci L, Ragone V, Pasevi M, Randelli G (2013) Effectiveneness of fibrin sealant after cementless total hip replacement: a double blind randomized controlled trial. Int J Immunopathol Pharmacol 26:189–197
Alshryda S, Mason J, Sarda P, Nargol A, Cooke N, Ahmad H, Tang S, Logishetty R, Vaghela M, McPartlin L, Hungin APS (2013) Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement. A randomized controlled trial (TRANX-H). J Bone Joint Surg Am 95:1969–1974
Konig G, Hamlin BR, Waters JH (2013) Topical tranexamic acid reduces blood Loss and transfusions rates in total hip and total knee arthroplasty. J Arthroplasty 28:1473–1476
Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Estendorf DS, Garton AS, Lemke JH (2014) Topical administration of tranexamic acid in primary total hip and knee arthroplasty. J Arthroplasty 29:889–894
Wei W, Wei B (2014) Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty 29:113–116
Yue C, Kang P, Yang P, Xie J, Pei F (2014) Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty 29:2452–2456
Emara WM, Moez KK, Elkhouly AH (2014) Topical versus intravenous tranexamic acid as a blood conservation intervention for reduction of postoperative bleeding in hemiarthroplasty. Anaesth Essays Res 8:48–53
Kang JS, Moon KH, Kim BS, Yang SJ (2016) Topical administration of tranexamic acid in hip arthroplasty. Int Orthop 41:259–263
Acknowledgements
We thank patients who agreed to participate; secretaries, nurses and in general, the staff of Clinical Epidemiology and Publica Health Department-Iberoamerican Cochrane Centre and Tramatology Department of participant centres. To Andrea Cervera for editing the manuscript. Dr. Jordan used this study to develop his PhD at the Universitat Autonoma de Barcelona (Spain). Dr. Mª José Martinez Zapata is funded by a Miguel Servet research contract from the Instituto de Salud Carlos III (CP15/00116). This project was funded by the Ministry of Health and Social Policy, Spain. Directorate General of Pharmacy and Health Products. “Projects for the translation of the advanced therapeutic application of human medicines, orphans and advanced therapies”. This study also received funding from European Regional Development Fund (FEDER; “A way of making Europe”). Number of project: EC11-341. TRANEXFER Group is also composed by: Julio De Caso: Orthopedic and Traumatology Service. Hospital de la Santa Creu I Sant Pau, Barcelona, Spain, e-mail: JCaso@santpau.cat. Ion Carrera: Orthopedic and Traumatology Service. Hospital de la Santa Creu I Sant Pau, Barcelona, Spain, e-mail: ICarrera@santpau.cat. Angie Millán: Orthopedic and Traumatology Service. Hospital de la Santa Creu I Sant Pau, Barcelona, Spain, e-mail: AMillan@santpau.cat. Mª Carmen Pulido: Orthopedic and Traumatology Service. Hospital de la Santa Creu I Sant Pau, Barcelona, Spain, e-mail: MPulido@santpau.cat. Marius Valera: Orthopedic and Traumatology Service. Hospital de la Santa Creu I Sant Pau, Barcelona, Spain, e-mail: MValera@santpau.cat. Xavier Crusi: Orthopedic and Traumatology Service. Hospital de la Santa Creu I Sant Pau, Barcelona, Spain, e-mail: XCrusi@santpau.cat. José Antonio Fernández Núñez: Anesthesiology Service. Hospital de la Santa Creu I Sant Pau, Barcelona, Spain, e-mail: JFernandezN@santpau.cat. Anna Canalias Bage: Orthopedic and Traumatology Service. Hospital Universitari Mútua Terrassa, Barcelona, Spain, e-mail: anna.canalias@gmail.com. Anna Alavedra: Orthopedic and Traumatology Service. Consorci Hospitalari Parc Taulí, Sabadell, Barcelona, Spain, e-mail: aalavedra@tauli.cat. Margarita Novellas: Anesthesiology Service. Hospital Universitari Terrassa, Barcelona, Spain, e-mail: mnovellas@mutuaterrassa.cat. Francesc Anglès Crespo: Orthopedic and Traumatology Service. Hospital Universitari Mútua de Terrassa, Barcelona, Spain, e-mail: fangles@mutuaterrassa.es. Gerard Urrutia: Public Health and Clinical Epidemiology Service-Iberoamerican Cochrane Centre. IIB Sant Pau. CIBERESP, Barcelona, Spain, e-mail: gurrutia@santpau.cat. Esther Cànovas: Iberoamerican Cochrane Centre-IIB Sant Pau, Barcelona, Spain, e-mail: ecanovas@santpau.cat.
Funding
This project was funded by the Ministry of Health and Social Policy, Spain. Directorate General of Pharmacy and Health Products. “Projects for the translation of the advanced therapeutic application of human medicines, orphans and advanced therapies”. This study also received funding from European Regional Development Fund (FEDER; “A way of making Europe”). Number of project: EC11-341.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national Research Ethics Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
TRANEXFER Group memebers list are given in “Acknowledgement” section.
Rights and permissions
About this article
Cite this article
Jordan, M., Aguilera, X., González, J.C. et al. Prevention of postoperative bleeding in hip fractures treated with prosthetic replacement: efficacy and safety of fibrin sealant and tranexamic acid. A randomised controlled clinical trial (TRANEXFER study). Arch Orthop Trauma Surg 139, 597–604 (2019). https://doi.org/10.1007/s00402-018-3089-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-018-3089-4