Abstract
Background
In recent years, the popularity of unicompartmental knee arthroplasty (UKA) has increased. However, the effect of femoral component positioning in UKA continues to invite a considerable debate. The purpose of this study involved assessing the biomechanical effect of mal-alignment in femoral components in UKA under dynamic loading conditions using a computational simulation.
Methods
A validated finite element model was used to evaluate contact stresses in polyethylene (PE) inserts and lateral compartment and force on collateral ligament in the femoral component ranging from 9° of varus to 9° of valgus.
Results
The results indicated that contact stress on the PE insert increased with increases in the valgus femoral alignment when compared to the neutral position while contact stress on the lateral compartment increased with increases in the varus femoral alignment. The forces on medial and lateral collateral ligaments increased with increases in valgus femoral alignments when compared to the neutral position. However, there was no change in popliteofibular and anterior lateral ligaments with respect to the malpositioning of femoral component.
Conclusion
The results of the study confirm the importance of conservation in post-operative accuracy of the femoral component since the valgus and varus femoral malalignments affect the collateral ligament and lateral compartment, respectively. Our results suggest that surgeons should avoid valgus malalignment in the femoral component and especially malalignment exceeding 9°, which may induce higher medial collateral ligament forces.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unicompartmental knee arthroplasty (UKA) is widely used to treat single-compartment osteoarthritis (OA) leading to good intermediate and long-term outcomes [1, 2]. Medial UKA involves performing resurfacing of the medial compartment in isolated medial compartment degenerative joint disease [3].
The potential advantages of UKA include a more normal gait, reduced perioperative morbidity, better range of motion, a less invasive procedure, preservation of bone stock, and faster rehabilitation [4,5,6]. Despite the fore-mentioned advantages of UKA relative to total knee arthroplasty (TKA), a recent study reported that UKA leads to a higher risk of revision when compared to TKA [7]. Additionally, UKA requires a technically demanding procedure and precise component positioning [8,9,10].
Patient factors are associated as potential factors that affect UKA survivorship. However, component malpositioning is correlated with revision rates [11, 12]. As reported by previous studies, inaccurate alignment or technical errors of prosthetic components can lead to point contact and increased stress concentration that are identified as main contributors of early polyethylene (PE) wear [13, 14]. Additionally, the fore-mentioned types of malalignment periprosthetic fractures, failures of the device, residual pain, subsidence, and OA progression in the lateral compartments are caused by an altered stress pattern in the bone/cartilage [15,16,17,18]. However, biomechanical studies examining the malpositioning have largely focused on varus and valgus alignment in the tibial component with respect to the antero-medial aspect of proximal tibia pain [19,20,21,22]. The studies do not consider main postoperative problems including medial knee pain that is related to bone overload, components malpositioning and soft tissue tensioning, and OA progression in the lateral compartment. Innocenti et al. examined load sharing and ligament strains in balanced, overstuffed, and understuffed UKA [23].
Kwon et al. [24] used computational simulation and reported that joint line preservation in UKA implantation is a key factor for postoperative outcomes in the lateral compartment. However, to the best of the authors’ knowledge, extant studies have not evaluated contact stress on the PE insert and lateral compartment and force on collateral ligaments in femoral component with varus and valgus malalignment. It is impractical to apply experimental measurements to directly investigate the biomechanical effect on femoral component malalignment. However, these limitations can be resolved with finite element (FE) analysis [24].
The purpose of this study involved evaluating the biomechanical effects on different varus/valgus alignment positions of femoral components in UKA. Specifically, contact stress on the PE insert and lateral compartment and force on collateral ligaments were investigated using a validated FE model for fixed-bearing UKA by comparing differently aligned configurations under a gait cycle condition. We hypothesized that the coronal alignment of UKA femoral component is an important factor and that the PE insert, lateral compartment and collateral ligament could be negatively affected by malalignment.
Materials and methods
Intact knee model
The right leg of a 36-year-old male subject was used to represent the geometric intact of the knee model. Anatomic structures were reconstructed from the subject’s computed tomography (CT) and magnetic resonance imaging (MRI). Prior to the reconstruction, medical records for the subject indicated neutral lower limb alignment without any anatomical abnormality, previous operations, and arthritis.
The contours of the bony structures (including femur, tibia, fibula, and patella) and soft tissues (including ligaments and menisci) were reconstructed from CT and MRI images, respectively. Previous studies have developed and validated the computational knee joint model [25, 26]. Medical imaging was performed using a 64-channel CT scanner (Somatom sensation 64; Siemens Healthcare, Erlangen, Germany) and a 3-T MRI system (Discovery MR750w; GE Healthcare, Milwaukee, WI, USA). The CT and MRI scans were performed with 0.1 and 0.4 mm slice thickness, respectively.
The reconstructed CT and MRI models were combined with the positional alignment of each model using a commercial software (Rapidform version 2006; 3D Systems Korea Inc., Seoul, Republic of Korea). The bony structures were assumed as rigid bodies using four-node shell elements. The cartilage and menisci were modeled as isotropic and transversely isotropic, respectively, with linear elastic material properties using eight-node hexahedral elements [26,27,28] (Fig. 1). All the major ligaments were defined as hyperelastic rubber-like materials that represent nonlinear stress–strain relations [29, 30]. The interfaces between the cartilage and the bones were modeled as fully bonded. The contacts between the femoral cartilage and meniscus, the meniscus and tibial cartilage, and the femoral cartilage and tibial cartilage were modeled for both the medial and lateral sides, thereby resulting in six contact pairs [25]. Convergence was defined as a relative change of < 5% between two adjacent meshes. The average element size of the simulated cartilage and menisci corresponded to 0.8 mm.
FE models with varus/vagus of the femoral component for UKA
A fixed bearing UKA (Zimmer, Inc., Warsaw, IN, USA) was virtually implanted in the medial compartment of the developed intact knee model. The bone models were imported and appropriately positioned, trimmed, and meshed with rigid elements using surgical techniques [31].
Based on the dimension of femur and tibia, devices with sizes six and five were selected for the femoral component and tibial baseplate, respectively. The devices were then aligned with the mechanical axis and positioned at the medial edge of the tibia. The neutrally aligned tibial baseplate was defined as a square (0°) inclination in the coronal plane with a 5° posterior slope. A rotating axis was defined as a line parallel to the lateral edge of the tibial baseplate that passes through the center of the femoral component peg. A neutral femoral component distal cut that is perpendicular to the mechanical axis of the femur and parallel to the tibial cut was reproduced.
Varus and valgus alignments of 3°, 6°, and 9° were achieved by an equivalent repositioning of the femoral component from the mechanical axis (Fig. 2). With respect to the implanted model, a 1 mm cement gap was simulated between the component and bone.
The PE insert and the femoral and tibial components were modeled as elastoplastic and as linear elastic isotropic materials, respectively [20, 22, 32]. The materials of the femoral component, PE insert, and tibial component corresponded to a cobalt chromium alloy (CoCr), ultra high molecular weight polyethylene (UHMWPE), and a titanium alloy (Ti6Al4V), respectively (Table 1). The femoral component came into contact with the PE insert. The selected coefficient of friction between the PE and metal corresponded to 0.04 [32].
Loading and boundary conditions
The FE investigation included two types of loading conditions corresponding to loads used in the experimental part of the study for UKA model validation and model predictions for gait cycle loading scenarios. The intact model was validated in a previous study, and the UKA model was validated by comparing it with models in previous experimental studies [25, 26, 33].
The validation of the UKA model was performed with flexion angles of 0°, 30°, 60°, and 90° using passive flexion simulation. Additionally, anterior and posterior drawer loads of 130 N were separately applied to the tibia at the knee center in a manner similar to that in a previous experimental study [25]. Gait cycle loading was applied as a second loading to compare the biomechanical effect of the varus/valgus malalignment femoral component. The contact stresses on the PE insert, lateral compartment, and force on collateral ligaments were predicted using the model under gait-cycle loading conditions (ISO 14,243) [34]. The computational analysis was performed with force controls to both the tibiofemoral and patellofemoral joint motions with respect to the compressive load applied to the femoral component.
A proportional–integral–derivative controller was incorporated into the computational model to allow for the control of the quadriceps in a manner similar to that in the experiment [35]. Furthermore, anterior–posterior (AP) load and internal–external (IE) rotation were applied to the PE insert, and varus-valgus rotation in the medial–lateral was controlled by the ankle joint followed by quadriceps force attached to the patellar button [34,35,36,37]. The FE model was analyzed using ABAQUS software (version 6.11; Simulia, Providence, RI, USA).
Results
Validation of UKA model
Anterior tibial translations in the anterior drawer test at 130 N corresponded to 6.1, 9.9, 8.7, and 8.5 mm, and posterior tibial translations in the posterior drawer test at 130 N corresponded to 5.8, 4.3, 3.8, and 4.9 mm at 0°, 30°, 60° and 90° of knee flexion, respectively, in UKA model (Fig. 3). The findings indicated that the results from the simulation and those from a previous experimental study agreed well within the ranges of values under anterior and posterior drawer loadings.
Comparison of contact stress on the PE insert and lateral compartment in varus and valgus femoral components
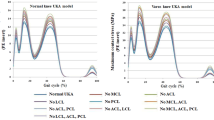
Figure 4 shows the contact stress on the PE inserts in the neutral position, and the varus/valgus UKA FE models during the gait cycle condition. Contact stress on the PE insert increased in the valgus UKA model when compared to that in the neutral position, and the highest increase was observed in the 9° valgus model. The contact stresses on the PE insert increased by 8, 19, and 27% in 3°, 6°, and 9° valgus models, respectively, when compared to the neutral position UKA model in stance phase under a gait cycle. However, differences were not observed in the swing phase.
Similar to valgus UKA model, contact stress on PE insert in varus UKA model also increased and its maximum value was found in 9° varus UKA model. However, the amount of increase was less than valgus condition. The contact stress on PE insert increased by 5, 12 and 17%, respectively, in 3°, 6° and 9° degree varus UKA models compared to the neutral position UKA model. Furthermore, in a manner similar to the valgus mode, a difference was only observed in the stance phase under the gait cycle.
Contact stress distributions in PE insert with respect to varus, valgus and neutral positions in UKA model are shown in Fig. 5. The contact point between the femoral component and the superior surface of PE insert changes with respect to the alignment. In a varus UKA model, the contact point is positioned in the lateral side of PE insert. On the other hand, in a valgus alignment, it is positioned closer to the medial edge.
Figure 6 shows the contact stress on lateral compartment in the neutral position and the varus/valgus UKA FE models during the gait cycle condition. Contact stress on the lateral compartment in the varus model increased when compared to that in the neutral position. In contrast, contact stress on the lateral compartment in the valgus model decreased when compared to that in the neutral position. In a manner similar to the PE insert, the difference was also only observed in the stance phase. The contact stress increased by 5, 8, and 13% in varus 3°, 6°, and 9° models, and it decreased by 3, 6, and 8% in valgus 3°, 6°, and 9° models compared to neutral position.
Comparison of collateral ligament forces in varus and valgus femoral components
Figure 7 shows the ligament forces on the medial collateral ligament (MCL), lateral collateral ligament (LCL), popliteofibular (PFL), and anterior lateral ligament (ALL) in the neutral position, and the varus/valgus UKA FE models during the gait cycle condition. The force on the MCL exhibited complex patterns. The forces on the MCL increased during the stance and swing phases in the varus and valgus FE UKA models, respectively, under the gait cycle when compared to those in the neutral position UKA FE model. The forces on the MCL increased by 28 and 61% during stance and swing phases, respectively, in varus 9° and valgus 9° FE UKA models under the gait cycle when compared to those in the neutral position UKA FE model. However, the force on LCL increased in the valgus UKA FE model and decreased in the varus UKA FE model when compared to those in the neutral position UKA FE model. The forces on the LCL increased by 9% and decreased by 11% in valgus 9° and varus 9° FE UKA models, respectively. The forces on PFL and ALL were not influenced by the varus and valgus malalignment when compared to those in the neutral position UKA. However, the force exerted on PFL decreased by 8% during stance phase in valgus 9° UKA model compared to those in the neutral position UKA.
Discussion
The main findings of this study indicated the prevalence of different trends with respect to the PE insert, lateral compartment, and collateral ligament with femoral component malalignment. The contact stress on PE insert increased with valgus femoral component compared to varus condition. However, the contact stress on lateral compartment increased and decreased with varus and valgus femoral component conditions, respectively, compared to neutral position. In addition, forces on MCL and LCL increased in valgus femoral component.
Precise restoration of mechanical and component positioning were reported as major factors in improving the component longevity and clinical outcomes of UKA [38]. However, there is an absence of general agreement with respect to the optimal position of the femoral component. In addition, many computational researches have been previously studied with respect to biomechanical effect in different varus and valgus components [19,20,21, 39,40,41]. However, most of them were focused on varus and valgus conditions in the btibial component [19,20,21, 39,40,41].
Extant research has reported several complications following the UKA such as rupture of the medial or lateral collateral ligaments, wear of PE inserts, degenerative changes in the lateral compartment, and fractures of the medial proximal tibia [11,12,13,14,15,16,17,18]. Thus, UKA requires a demanding procedure in terms of special experience with early failure risk [8,9,10]. Previous studies have widely investigated and reported on post-operative alignment in medial UKA for varus OA knees. However, there is a paucity of studies examining the risk factors of post-operative malalignment [42,43,44]. In addition, previous studies reported on pre-operative valgus stress angle measurement and coronal knee alignment as important predictors for post-operative alignment [44, 45]. As mentioned above, UKA is characterized by several complications, and these adverse effects are significant factors that contribute to post-operative malalignment. However, to the best of the authors’ knowledge, studies to date have not evaluated the contact stress on the PE insert, lateral compartment, and forces on the exerted collateral ligaments in a malalignment condition.
The advantage of computational simulation using a single subject is that the effects of component alignment within the identical subject can be determined with the exception of variables such as weight, height, bony geometry, differences in ethnicities and sex, ligament properties, and component size [46]. Additionally, most in-vitro biomechanical studies involved evaluations using aged cadaveric subjects with loosening between the specimen and device as well as some attenuation of the tissue itself that can occur by successive loading in mechanical testing [47].
Complication trends were observed in contact stresses on PE insert and lateral compartment. Contact stresses on the PE insert increased in the valgus and varus FE UKA models, but contact stress on lateral compartment increased only in varus FE UKA model.
This phenomenon could be explained by the difference in stiffness between PE insert and lateral compartment of the knee with UKA. In the lateral compartment on both the tibia and femur displayed an elastic modulus of 15 MPa, while the PE insert exhibited an elastic modulus of 685 MPa. Consequently, there is difference of more than one order of magnitude in the Young’s Modulus between the medial and lateral compartments, and the materials in each compartment deform based on their elastic modulus [21].
This trend that contact stresses on PE insert and lateral compartment increased and decreased, respectively, was frequently shown in valgus UKA model and it could be caused by the transfer of loading. Furthermore, the contact stresses and patterns on the PE insert and lateral compartment showed good agreements with those obtained in a previous study [48, 49]. However, the increase rate of contact stress on lateral compartment was lower than that in the PE insert. Furthermore, in the neutral FE UKA model, UKA was virtually implanted in a perfect anatomic position achieved by replacing the original cartilage and joint line as performed in a previous study [24]. The result of this study indicated support for the induction of overcorrection of alignment by highlighting a few of the erroneous varus and valgus alignments due to the change in stiffness [50].
An interesting result was also observed with respect to a collateral ligament in the valgus 9° UKA FE and neutral position models. The forces on the MCL and LCL increased by 61 and 9%, respectively, in the valgus 9° UKA FE model when compared to those in the neutral position. However, the force on the MCL increased albeit slightly during the stance phase in the varus UKA FE model when compared to that in the neutral position UKA FE model. With respect to the medial UKA implantation, the valgus deformity mainly induced a stress increase on the MCL as a consequence of the difference in stiffness [21, 51]. The study indicated that the forces on collateral ligament were different in the varus and valgus UKA FE models according to the conditions. An overcorrection can cause ligament overstretching on one side and relaxation on the opposite side. Specifically, the stretched ligament side can induce stiffness or pain [21]. A previous study defined the surrounding tension as a relative position of the components and the tension of the surrounding soft tissues that impact the results [32]. Heyse et al. reported that overstuffing in UKA evidently led to considerable tension in the valgus knee joint and higher strains in the MCL [50]. The findings of the study confirmed the conclusions obtained in the study by Heyse et al. in which a strain increase was not observed with respect to the MCL across all motor tasks and over the entire flexion range [50].
Intraoperative balancing should be considered in this condition to prevent tension on the MCL. However, most surgeons only focus on knee balancing with full extension and 90° of flexion [50]. In addition, we found that force on LCL with valgus femoral component increased as also shown in Heyse et al’s study [50]. The implantation of an UKA to the medial side influence the distribution of load transfer in knee joint. In other words, MCL force increase keeps the knee from tilting too much into valgus during loading, thus preventing overload on the lateral side. In addition, decreased contact stress on lateral compartment in valgus model supported it. Therefore, more loads pass throughout the medial compartment even in optimum balancing condition. It may lead to clinical problems such as loosening of the tibial component or fractures of the medial tibial plateau or the underlying bone [52]. Pain is a frequent revision reason in UKA [53]. The results in the present study indicate that coronal alignment of the femoral component is important. Specifically, it is important to avoid valgus femoral malalignment that may cause wear on the PE insert and pain in the MCL. However, ± 3 varus and valgus malalignments could alter due to a lower degree of biomechanical change.
It is important to highlight several strengths of the present study. First, in contrast to previous UKA studies, the FE model in the study included the tibia as well as the femur and related soft tissues [20, 22, 39]. Second, in contrast to the current biomechanical UKA model, the present study included the application of gait cycle loading as opposed to a simple vertical static loading condition [19,20,21,22,23, 39]. Third, the current study validated the intact model and performed kinematic validation on the UKA FE model.
Nevertheless, several limitations should also be noted. First, the bony structures were assumed as rigid. In reality, bone is composed of cortical and cancellous tissues. However, the main purpose of the study did not involve evaluating the effects of different prostheses on bone. Additionally, this assumption exerted a minimal influence on the study since the stiffness of bone exceeds that of the relevant soft tissues [27]. Second, the lateral compartment was considered as an elastic material, and the effects of anisotropy and viscoelasticity were not considered. Third, potential malalignments of the tibial component were not included in this analysis. This could be evaluated in a future study that investigates the effect of mixed femoral/tibial component malalignment of the medial compartment. Finally, only the gait cycle was simulated, and more demanding activities (such as sitting on and standing from a chair, ascending and descending stairs, or squatting) should be included in a future study.
In conclusion, contact stresses on PE insert, lateral compartment, and collateral ligament were investigated with respect to femoral malalignment under the gait cycle daily activity condition using a computation simulation. The results suggest that surgeons should avoid valgus malalignment in the femoral component and especially malalignment exceeding 9°, which may induce higher MCL forces.
References
Price AJ, Svard U (2011) A second decade lifetable survival analysis of the Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res 469(1):174–179
Seng CS, Ho DC, Chong HC, Chia SL, Chin PL, Lo NN, Yeo SJ (2017) Outcomes and survivorship of unicondylar knee arthroplasty in patients with severe deformity. Knee Surg Sports Traumatol Arthrosc 25(3):639–644
Yildirim G, Davignon R, Scholl L, Schmidig G, Carroll KM, Pearle AD (2015) Advantages of a cementless unicompartmental knee arthroplasty approach. Op Techn Orthop 25(2):150–154
Robertsson O, Borgquist L, Knutson K, Lewold S, Lidgren L (1999) Use of unicompartmental instead of tricompartmental prostheses for unicompartmental arthrosis in the knee is a cost-effective alternative. 15,437 primary tricompartmental prostheses were compared with 10,624 primary medial or lateral unicompartmental prostheses. Acta Orthop Scand 70(2):170–175
Engh GA (2002) Orthopaedic crossfire–can we justify unicondylar arthroplasty as a temporizing procedure? in the affirmative. J Arthroplasty 17(4 Suppl 1):54–55
Sah AP, Scott RD (2007) Lateral unicompartmental knee arthroplasty through a medial approach. Study with an average five-year follow-up. J Bone Jt Surg Am 89(9):1948–1954
Niinimaki T, Eskelinen A, Makela K, Ohtonen P, Puhto AP, Remes V (2014) Unicompartmental knee arthroplasty survivorship is lower than TKA survivorship: a 27-year Finnish registry study. Clin Orthop Relat Res 472(5):1496–1501
Sculco TP (2002) Orthopaedic crossfire–can we justify unicondylar arthroplasty as a temporizing procedure? in opposition. J Arthroplasty 17(4 Suppl 1):56–58
Zambianchi F, Digennaro V, Giorgini A, Grandi G, Fiacchi F, Mugnai R, Catani F (2015) Surgeon’s experience influences UKA survivorship: a comparative study between all-poly and metal back designs. Knee Surg Sports Traumatol Arthrosc 23(7):2074–2080
Liow MH, Tsai TY, Dimitriou D, Li G, Kwon YM (2016) Does 3-dimensional in vivo component rotation affect clinical outcomes in unicompartmental knee arthroplasty? J Arthroplasty 31(10):2167–2172
Berend KR, Lombardi AV Jr, Mallory TH, Adams JB, Groseth KL (2005) Early failure of minimally invasive unicompartmental knee arthroplasty is associated with obesity. Clin Orthop Relat Res 440:60–66
Kuipers BM, Kollen BJ, Bots PC, Burger BJ, van Raay JJ, Tulp NJ, Verheyen CC (2010) Factors associated with reduced early survival in the Oxford phase III medial unicompartment knee replacement. Knee 17(1):48–52
McAuley JP, Engh GA, Ammeen DJ (2001) Revision of failed unicompartmental knee arthroplasty. Clin Orthop Relat Res 392:279–282
Weinstein JN, Andriacchi TP, Galante J (1986) Factors influencing walking and stairclimbing following unicompartmental knee arthroplasty. J Arthroplasty 1(2):109–115
Lindstrand A, Stenstrom A, Lewold S (1992) Multicenter study of unicompartmental knee revision. PCA, Marmor, and St Georg compared in 3777 cases of arthrosis. Acta Orthop Scand 63(3):256–259
Palmer SH, Morrison PJ, Ross AC (1998) Early catastrophic tibial component wear after unicompartmental knee arthroplasty. Clin Orthop Relat Res 350:143–148
Hamilton WG, Ammeen D, Engh CA Jr, Engh GA (2010) Learning curve with minimally invasive unicompartmental knee arthroplasty. J Arthroplasty 25(5):735–740
Berger RA, Meneghini RM, Jacobs JJ, Sheinkop MB, Della Valle CJ, Rosenberg AG, Galante JO (2005) Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Jt Surg Am 87(5):999–1006
Zhu GD, Guo WS, Zhang QD, Liu ZH, Cheng LM (2015) Finite element analysis of mobile-bearing unicompartmental knee arthroplasty: the influence of tibial component coronal alignment. Chin Med J (Engl) 128(21):2873–2878
Inoue S, Akagi M, Asada S, Mori S, Zaima H, Hashida M (2016) The valgus inclination of the tibial component increases the risk of medial tibial condylar fractures in unicompartmental knee arthroplasty. J Arthroplasty 31(9):2025–2030
Innocenti B, Pianigiani S, Ramundo G, Thienpont E (2016) Biomechanical effects of different varus and valgus alignments in medial unicompartmental knee arthroplasty. J Arthroplasty 31(12):2685–2691
Pegg EC, Walter J, Mellon SJ, Pandit HG, Murray DW, D’Lima DD, Fregly BJ, Gill HS (2013) Evaluation of factors affecting tibial bone strain after unicompartmental knee replacement. J Orthop Res 31(5):821–828
Innocenti B, Bilgen OF, Labey L, van Lenthe GH, Sloten JV, Catani F (2014) Load sharing and ligament strains in balanced, overstuffed and understuffed UKA. A validated finite element analysis. J Arthroplasty 29(7):1491–1498
Kwon OR, Kang KT, Son J, Suh DS, Baek C, Koh YG (2017) Importance of joint line preservation in unicompartmental knee arthroplasty: Finite element analysis. J Orthop Res 35(2):347–352
Kim YS, Kang KT, Son J, Kwon OR, Choi YJ, Jo SB, Choi YW, Koh YG (2015) Graft extrusion related to the position of allograft in lateral meniscal allograft transplantation: biomechanical comparison between parapatellar and transpatellar approaches using finite element analysis. Arthroscopy 31(12):2380–2391
Kang KT, Kim SH, Son J, Lee YH, Kim S, Chun HJ (2017) Probabilistic evaluation of the material properties of the in vivo subject-specific articular surface using a computational model. J Biomed Mater Res B Appl Biomater 105(6):1390–1400
Peña E, Calvo B, Martinez MA, Palanca D, Doblaré M (2006) Why lateral meniscectomy is more dangerous than medial meniscectomy. A finite element study. J Orthop Res 24(5):1001–1010
Haut Donahue TL, Hull M, Rashid MM, Jacobs CR (2003) How the stiffness of meniscal attachments and meniscal material properties affect tibio-femoral contact pressure computed using a validated finite element model of the human knee joint. J Biomech 36(1):19–34
Mesfar W, Shirazi-Adl A (2005) Biomechanics of the knee joint in flexion under various quadriceps forces. Knee 12(6):424–434
Takeda Y, Xerogeanes JW, Livesay GA, Fu FH, Woo SL (1994) Biomechanical function of the human anterior cruciate ligament. Arthroscopy 10(2):140–147
Zimmer, Inc Zimmer unicompartmental high flex knee: intramedullary, spacer block option and extramedullary minimally invasive surgical techniques
Godest AC, Beaugonin M, Haug E, Taylor M, Gregson PJ (2002) Simulation of a knee joint replacement during a gait cycle using explicit finite element analysis. J Biomech 35(2):267–275
Suggs JF, Li G, Park SE, Steffensmeier S, Rubash HE, Freiberg AA (2004) Function of the anterior cruciate ligament after unicompartmental knee arthroplasty: an in vitro robotic study. J Arthroplasty 19(2):224–229
ISO 14,243-1 (2002) Implants for surgery. Wear of total knee joint prostheses. Loading and displacement parameters for wear-testing machines with load control and corresponding environmental conditions for test
Kang KT, Koh YG, Jung M, Nam JH, Son J, Lee YH, Kim SJ, Kim SH (2017) The effects of posterior cruciate ligament deficiency on posterolateral corner structures under gait- and squat-loading conditions: A computational knee model. Bone Jt Res 6(1):31–42
Kutzner I, Heinlein B, Graichen F, Bender A, Rohlmann A, Halder A, Beier A, Bergmann G (2010) Loading of the knee joint during activities of daily living measured in vivo in five subjects. J Biomech 43(11):2164–2173
Halloran JP, Clary CW, Maletsky LP, Taylor M, Petrella AJ, Rullkoetter PJ (2010) Verification of predicted knee replacement kinematics during simulated gait in the Kansas knee simulator. J Biomech Eng 132(8):081010
Keene G, Simpson D, Kalairajah Y (2006) Limb alignment in computer-assisted minimally-invasive unicompartmental knee replacement. J Bone Jt Surg Br 88(1):44–48
Iesaka K, Tsumura H, Sonoda H, Sawatari T, Takasita M, Torisu T (2002) The effects of tibial component inclination on bone stress after unicompartmental knee arthroplasty. J Biomech 35(7):969–974
Sawatari T, Tsumura H, Iesaka K, Furushiro Y, Torisu T (2005) Three-dimensional finite element analysis of unicompartmental knee arthroplasty–the influence of tibial component inclination. J Orthop Res 23(3):549–554
Simpson DJ, Price AJ, Gulati A, Murray DW, Gill HS (2009) Elevated proximal tibial strains following unicompartmental knee replacement–a possible cause of pain. Med Eng Phys 31(7):752–757
Ridgeway SR, McAuley JP, Ammeen DJ, Engh GA (2002) The effect of alignment of the knee on the outcome of unicompartmental knee replacement. J Bone Jt Surg Br 84(3):351–355
Gulati A, Chau R, Simpson DJ, Dodd CA, Gill HS, Murray DW (2009) Influence of component alignment on outcome for unicompartmental knee replacement. Knee 16(3):196–199
Mullaji AB, Shetty GM, Kanna R (2011) Postoperative limb alignment and its determinants after minimally invasive Oxford medial unicompartmental knee arthroplasty. J Arthroplasty 26(6):919–925
Tashiro Y, Matsuda S, Okazaki K, Mizu-Uchi H, Kuwashima U, Iwamoto Y (2014) The coronal alignment after medial unicompartmental knee arthroplasty can be predicted: usefulness of full-length valgus stress radiography for evaluating correctability. Knee Surg Sports Traumatol Arthrosc 22(12):3142–3149
Thompson JA, Hast MW, Granger JF, Piazza SJ, Siston RA (2011) Biomechanical effects of total knee arthroplasty component malrotation: a computational simulation. J Orthop Res 29(7):969–975
Chun YM, Kim SJ, Kim HS (2008) Evaluation of the mechanical properties of posterolateral structures and supporting posterolateral instability of the knee. J Orthop Res 26(10):1371–1376
Mononen ME, Jurvelin JS, Korhonen RK (2013) Effects of radial tears and partial meniscectomy of lateral meniscus on the knee joint mechanics during the stance phase of the gait cycle-A 3D finite element study. J Orthop Res 31(8):1208–1217
Kwon OR, Kang KT, Son J, Kwon SK, Jo SB, Suh DS, Choi YJ, Kim HJ, Koh YG (2014) Biomechanical comparison of fixed- and mobile-bearing for unicomparmental knee arthroplasty using finite element analysis. J Orthop Res 32(2):338–345
Heyse TJ, El-Zayat BF, De Corte R, Scheys L, Chevalier Y, Fuchs-Winkelmann S, Labey L (2016) Balancing UKA: overstuffing leads to high medial collateral ligament strains. Knee Surg Sports Traumatol Arthrosc 24(10):3218–3228
Au AG, Raso VJ, Liggins AB, Otto DD, Amirfazli A (2005) A three-dimensional finite element stress analysis for tunnel placement and buttons in anterior cruciate ligament reconstructions. J Biomech 38(4):827–832
Palumbo BT, Henderson ER, Edwards PK, Burris RB, Gutierrez S, Raterman SJ (2011) Initial experience of the Journey-Deuce bicompartmental knee prosthesis: a review of 36 cases. J Arthroplasty 26(6 Suppl):40–45
Knutson K, Robertsson O (2010) The swedish knee arthroplasty register (www.knee.se). Acta Orthop 81(1):5–7
Funding
There is no funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kang, KT., Son, J., Baek, C. et al. Femoral component alignment in unicompartmental knee arthroplasty leads to biomechanical change in contact stress and collateral ligament force in knee joint. Arch Orthop Trauma Surg 138, 563–572 (2018). https://doi.org/10.1007/s00402-018-2884-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-018-2884-2