Abstract
Introduction
There is an ongoing debate about the potential of patch augmentation to improve biomechanical stability and healing associated with rotator cuff repair. The biomechanical properties of three different patch-augmented rotator cuff repair techniques were assessed in vitro and compared with a standard repair. Dermal collagen patch augmentation may increase the primary stability and strength of the repaired tendon in vitro, depending on the technique used for patch application.
Methods and materials
Forty cadaveric sheep shoulders with dissected infraspinatus tendons were randomized into four groups (n = 10/group) for tendon repair using a knotless double-row suture anchor technique. A xenologous dermal extracellular matrix patch was used for augmentation in the three test groups using an “integrated”, “cover”, or “hybrid” technique. Tendons were preconditioned, cyclically loaded from 10 to 30 N at 1 Hz, and then loaded monotonically to failure. Biomechanical properties and the mode of failure were evaluated.
Results
Patch augmentation significantly increased the maximum load at failure by 61 % in the “cover” technique test group (225.8 N) and 51 % in the “hybrid” technique test group (211.4 N) compared with the non-augmented control group (140.2 N) (P ≤ 0.015). For the test group with “integrated” patch augmentation, the load at failure was 28 % lower (101.6 N) compared with the control group (P = 0.043). There was no significant difference in initial and linear stiffness among the four experimental groups. The most common mode of failure was tendon pullout. No anchor dislocation, patch disruption or knot breakage was observed.
Conclusion
Additional patch augmentation with a collagen patch influences the biomechanical properties of a rotator cuff repair in a cadaveric sheep model. Primary repair stability can be significantly improved depending on the augmentation technique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The total number of surgical rotator cuff repairs as well as the proportion performed arthroscopically has dramatically increased. In the US, a 600 % rise in repair procedures was reported between 1996 and 2006 [1]. This is mainly attributed to tendon degeneration associated with the aging population [2, 3]. Aging is one limiting factor for complete tendon reintegration, which leads to an increased proportion of patients with cuff retear and suboptimal postoperative functional outcome [4–6]. The reported failure rates after a rotator cuff repair range from 4.7 to 94 % [4, 5, 7–10]. In light of these figures, every shoulder surgeon is increasingly confronted with difficult scenarios including: (1) decreased tendon and muscle quality in an aging, yet physically very active patient population at the time of primary surgery; (2) rotator cuff re-tears with decreased tendon quality and large tear size after primary surgery; and (3) new modes of repair failure (e.g., medial cuff failure) resulting in very large tendon defects [11, 12]. These observations highlight the clinical need for new repair strategies, especially those enhancing tendon strength, such as patch augmentation.
Whether patch augmentation of the rotator cuff repair can improve primary biomechanical stability and further enhance the biological healing potential is a matter of ongoing debate [13–18]. Patches in use are extracellular matrices in the form of xenografts, allografts or synthetic structures [19–21]. While several in vitro studies showed that patch augmentation can enhance the biomechanical properties of the repair, only a few human in vivo studies have been published so far [14, 17, 22, 23]. Uncertainty regarding which patch material to use and how to technically perform the augmentation may prevent many surgeons from applying a patch.
The purpose of this study was to describe three arthroscopically manageable techniques for rotator cuff repair with patch augmentation and compare their biomechanical properties with a standard double-row repair. We hypothesized that using a dermal collagen patch for augmentation may increase the primary stability and strength of the repaired tendon in vitro, depending on the technique used for patch application.
Materials and methods
Forty fresh frozen sheep cadaver shoulders (aged 7–8 months) were thawed to room temperature before surgical exposure of the rotator cuff. After the infraspinatus tendon was sharply dissected from its bony insertion, all specimens were randomized into four groups (i.e., n = 10/group) for reinsertion of the tendon according to either one of the three different test augmented repair fixation techniques or the standard control repair fixation method. Visual inspection of the tendons from all cadaver shoulders showed no signs of damage or degeneration as expected for juvenile animals. The average length of the dissection cut at the bony insertion was 2.1 cm [standard deviation (SD) = 0.3]. The average patch square size was 3.9 cm2 (SD = 0.4).
Repair fixation techniques
For all four experimental groups, the tendon was reattached according to the SpeedBridge™ technique (Arthrex, Naples, FL) using four PEEK SwiveLock® anchor screws (4.75 × 15 mm) and two FiberTape® braided polybend sutures (2 mm). The control group was tested without any modification of the SpeedBridge™ technique (Fig. 1a). For the three test groups, each repair fixation was further enhanced by augmentation with a 5 × 5 cm xenologous dermal DX Reinforcement Matrix patch (Arthrex, Naples, FL); the hydrated patch was cut to fit the desired size and applied using one of three various techniques (Fig. 1b–d).
Illustrated coronal detail (upper) and frontal-oblique (lower) views of the patch application techniques in a human shoulder. a SpeedBridge™ technique only = control group. b “Integrated” technique. c “Cover” technique. d “Hybrid” technique. (Note: the patch suture techniques are illustrated for a human supraspinatus. Testing was performed using a sheep infraspinatus model)
Technique “integrated”: Two SwiveLock® anchors were preloaded with FiberTape® sutures and inserted as the medial row. Each of the four suture strands was independently shuttled through the tendon and then through the patch. Thereafter, the sutures were crossloaded to the tips of the lateral row SwiveLock® anchors in SpeedBridge™ formation, tensioned and secured by insertion of the anchors resulting in the integration of the patch between the sutures and the tendon (Fig. 1b).
Technique “cover”: For this group, the patch was applied to the tendon independent of the SpeedBridge™ procedure. Cut to partially cover the completed repair, the medial corners of the patch were stitched [using standard #2 FiberWire® (Arthrex, Naples, FL)] to the tendon just medial of the medial anchor row. The patch was then tensioned over the lateral row with two sutures at its lateral corners and secured by another two SwiveLock® anchors. Half of the patches in this group were secured to the tendon with three additional stitches at the anterior, medial, and posterior edges of the patch (Figs. 1c, 2).
Technique “hybrid”: As a combination of the “integrated” and “cover” techniques, the patch was secured to the tendon and anchor after completion of the SpeedBridge™ procedure. At the medial row, one strand of the preloaded, SwiveLock® anchor-dependent FiberWire®, was shuttled through the tendon and patch with the other strand shuttled through the tendon only. After tying together, the strands fixed the patch to the tendon medially. Lateral tensioning of the patch was performed in a similar manner to that done in the “cover” technique group (Fig. 1d).
Biomechanical testing
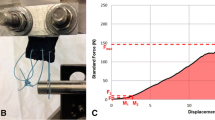
The humeral bones were fixed in polymethyl methacrylate and placed in a custom made steel fixture rigidly fixed to the frame of an ElectroPuls™ E10000 All-Electric Test instrument (Instron, Norwood, MA) (Fig. 3). The fixture allowed precise angle adjustment to ensure that the loading direction was in line with the anatomic neutral direction of the specimen. The medial end of the infraspinatus tendon was attached to a nylon strap secured by six FiberWire® mattress sutures, and the nylon strap was rigidly clamped to the actuator of the testing machine. In pilot experiments, the rigidity of the nylon strap and the integrity of the suture connection to the tendon were verified by video analyzing the deformation of a speckled pattern applied to the tendon and strap (Fig. 4). The tendons were preconditioned with a load of 20 N for 2 min to eliminate creep. Construct length, defined as the free length between the suture attachment to the nylon strap and tendon insertion to the bone, was measured at 20 N preload with a digital caliper (±0.01 mm). Cycling preconditioning was then performed between 10 and 30 N at 1 Hz for 10 cycles to stabilize the viscous response. Analysis of the hysteresis curve verified that a stable response was achieved after ten preconditioning cycles. Subsequently, tendon load and actuator displacement were recorded at 20 Hz during quasi-static load to failure, a monotonic increase in displacement at a constant displacement rate of 1 mm/s.
The initial failure point was defined as the first inflection point in the load–displacement curve, and the relative stretch at initial failure was calculated (i.e., construct extension normalized to original construct length). The initial stiffness (i.e., slope of the load displacement curve between 40 and 60 N) and the linear stiffness (i.e., slope of the load–displacement curve between 40 N and load at 90 % of the first failure point) were determined using a self-written script (MATLAB, Mathworks, Natick, MA). The yield load, or functional limit, was defined as the load at which stiffness dropped to 50 % of the initial stiffness. The maximum force achieved during load to failure was noted, and the mode of failure was documented by digital video recording (commercial Sony video camcorder, without any specific modifications) of the procedure.
Statistical analysis
Data were evaluated using R version 2.14.1 [R Foundation for Statistical Computing, Vienna, Austria (http://www.r-project.org/foundation/)]. The mean and SD were determined for each value. Differences among the experimental groups were analyzed using the Kruskal–Wallis rank sum test followed by pairwise Wilcoxon tests.
Results
The mean maximum force for the control group was 140.2 N (Table 1; Fig. 5). Maximum force significantly differed among the test groups as well as compared with the control (Kruskal–Wallis rank sum; P = 0.001); maximum force for patch augmentation using the “cover” and “hybrid” techniques exceeded values for the control by 61 % (225.8 N; P < 0.001) and 51 % (211.4 N; P = 0.015), respectively. The mean maximum force for the “integrated” technique was 101.6 N, indicating a construct that was significantly weaker than the other augmentation techniques (P < 0.001) as well as the control group (P = 0.043). Intragroup analysis of the “cover” technique revealed no difference when the patch was medially secured to the tendon by either two or five stitches (P = 0.536).
Box plots representing the maximum force (right) and functional limit (left) data for the standard double-row fixation (control) group and three different patch augmentation techniques. The ends of each rectangle correspond to the upper and lower quartiles of the data values. The line drawn through the rectangle corresponds to the median value. The whiskers, starting at the ends of the rectangle (or points representing extreme values), indicate minimum and maximum values
Stretch at initial failure also differed among the experimental groups (P < 0.001) (Table 1). The “cover” and “hybrid” techniques showed a significantly greater change in length before displacement (73.2 and 67.4 %, respectively) compared with the “integrated” technique (24.5 %) (P < 0.001). In comparison to the control group with a stretch of 41.0 %, only the “hybrid” technique showed a significantly higher stretch at initial failure (P = 0.028); no statistical difference was observed between the “cover” technique and the control groups (P = 0.061). Intragroup analysis of the “cover” technique also revealed no significant difference in the stretch for constructs with two or five stitches (P = 0.428).
There were no significant differences among all the shoulder constructs regarding initial and linear stiffness (Kruskal–Wallis rank sum; P = 0.060 and P = 0.140, respectively). However, there were significant differences in the yield load or functional limit among the experimental groups (Kruskal–Wallis rank sum; P = 0.001). The mean functional limit of the “hybrid” technique was 189.9 N and 68 % higher than that reported for the control group (112.8 N) (P = 0.004) (Table 1; Fig. 5). The “cover” technique achieved a mean functional limit of 116.8 N, which was similar to that reported for the control group (112.8 N) (P = 0.863). On the other hand, the mean functional limit for the “integrated” technique group was 74.5 N, indicating a significantly weaker construct compared with that in the control group with no patch augmentation (P < 0.001).
The mode of failure was similar for constructs in the control and “integrated” technique groups, that is, all tendons pulled/slipped out of the SpeedBridge™ repair. In addition, no patch displacement was observed for the “integrated” technique group. For all ten “hybrid” technique constructs, pullout of the tendon was observed without any displacement of the patches or breakage of lateral patch sutures. For two “hybrid” technique constructs, one of the medial patch knots cuts out of the patch, and for another, both knots cut out of the patch. The mode of failure observed in the “cover” technique group differed from that observed in the other experimental groups. For all five patches secured to the tendon with two medial stitches, the stitches cut through the patch and were displaced together with the pulled out tendon; the patch remained fixed to the lateral anchors. For the other five “cover” technique patches secured to the tendon with five medial stitches, the stitches were displaced together with the pulled out tendon and patch due to the cut-out of the lateral patch stitches; this mode of failure was only recorded in this subgroup of cadaver constructs. Therefore, tendon-bone attachment was disrupted in all 30 patch augmented constructs, but medial displacement of the patch was only observed in five specimens. Overall, no suture anchor dislocation or knot breakage was observed in any experimental group. Of 95 suture stitches used to secure the patch to the tendon in the three augmentation test groups, 14 cut through the patch during pullout. There were also no signs of a disrupted patch, except at the areas of suture cut out, in any test construct.
Discussion
Age related tendon degeneration is considered a main cause for rotator cuff tears [2, 3]. Hence, the aging population may explain why the risk of rotator cuff repair failure remains high, despite advanced surgical techniques [4, 5, 7–9]. The quest for improved biomechanical stability and optimal biologic conditions in a degenerative surrounding has led to: (1) the introduction of very stable “transosseous equivalent” repair techniques; (2) more focus on the important tendon to bone contact area; and (3) minimizing the iatrogenic impact on the tissue by means of minimal-invasive, arthroscopic procedures [6, 24]. In addition, the concept of patch augmentation for tendon repair has developed with the aim to further strengthen the repair construct [13, 15, 19, 21, 25].
We hypothesized that the use of a xenologous dermal matrix patch increases the primary stability and strength of the repaired tendon in vitro. Our results confirm a significant 50–60 % increase in the maximum force at failure of a standard rotator cuff fixation construct with patch augmentation using either the “cover” or the “hybrid” technique. Similar findings have been reported by Barber et al. and Omae et al. with significantly increased time-zero failure loads using a human dermal allograft for supraspinatus tendon augmentation [22, 23]. McCarron et al. also reported a 56–76 % increase in ultimate load for a woven poly-L-lactide graft [17]. However, the overall outcomes from the aforementioned studies should be interpreted and compared with caution, due to large differences in the test models, patch types, and patch fixation techniques used. To the best of our knowledge, the influence of the fixation technique for patch augmentation on the strength and stability of tendon fixation has not yet been investigated. The techniques described for augmentation in this study are real world options coming out of the operating room. In contrast to most of the current literature, these techniques are arthroscopically applicable in daily routine [26].
In all our specimens, the dominant mode of failure was observed at the tendon-suture interface. This corresponds with previously published data that have highlighted this interface as the weakest link [22, 27–29]. None of the three patch application techniques we used prevented this failure mode. Our data from the “integrated” technique test group suggest that applying the patch without additional fixation between the tendon or anchor and the patch may even weaken the repair. While the reasons for this weakening effect remain unclear, the supportive patch augmentation effects using the applied “cover” and “hybrid” techniques might be explained by the self-reinforcing effects that have recently been described for another double-row repair implant [30]. Furthermore, McCarron et al. reported a bridging effect of the patch between the tuberosity and the tendon, while the tendon-bone attachment was already disrupted [17]. We observed this effect in 15 of the total number of specimens (i.e., 40). One could speculate that a remaining bridge between the tendon and the bone, covering the defect, could be better than a complete disruption. Such a construct has been described, whereby a tendon defect is simply filled with a patch without the tendon to bone reconstruction [31].
The three additional medial tendon-patch sutures used for five specimens in the “cover” technique test group did not alter the measured biomechanical properties of the construct, but resulted in a cut out of the two lateral fixation sutures. This mode of failure was only observed in this subgroup, and because of the dislocation of the patch medially still attached to the tendon, no bridge effect resulted. As the maximum achieved forces were not significantly higher than the “cover” technique constructs with two sutures only, the additional sutures seem to add no further benefit to the reconstruction while losing the potential benefits of the bridging effect.
The initial and linear stiffness of the different repair constructs showed no significant differences. This suggests that no repair type with or without patch augmentation is more rigid than the other at submaximal loads. The “cover” and “hybrid” techniques showed, respectively, a tendency toward or significantly more stretch before initial failure, compared with the “integrated” technique and the control groups. These differences in stretch at initial failure suggest a greater margin of safety offered by patch augmentation during physiological and hyperphysiological loading. In addition, the load at which the stiffness dropped to 50 % of the initial stiffness (i.e., the functional limit or yield load) was higher for the “hybrid” technique, which might support the self-reinforcing theory described earlier.
No differences were observed in the initial and linear stiffness of the constructs compared with the control group. Based on pilot experiments, the force limits for evaluation of these stiffness values were chosen to represent the regions on the load–displacement curve that demonstrate the well-described “toe-region”; in this instance, stiffness increases exponentially as individual collagen fiber bundles are straightened, and the subsequent linear region in which force is directly proportional to stretch. The lack of difference in stiffness values implies that the overall compliance of the construct is determined primarily by the properties of the residual tendon tissue; it is likely that the reconstruction itself has a higher overall stiffness. Therefore, the reconstruction can directly influence the strength of the overall construct without substantially altering (increasing or decreasing) construct stability.
The limitations of this study are mainly related to the used test model. The results recorded in vitro with one loading direction using ovine specimens cannot be directly transferred to the complex in vivo situation in humans, though the scientific value of sheep shoulder models has previously been described [32]. In an in vitro test-setting, the ovine infraspinatus tendon is a suitable substitute for the human supraspinatus tendon because of its similarities regarding size, shape, and thickness, as well as its biomechanical and histological properties. On the medial side of the tendon, a nylon strap was fixed to transmit the load between the fatigue testing instrument and the bone [22]. As previously suggested by Barber et al., this could lead to the elongation of the nylon–tendon interface and bias the load to failure values in all or only single specimens [22]. However, our own compliance analysis of the strap/tendon interface suggests that there is negligible deformation at this junction up to the point of initial failure. As the tendons were dissected artificially in healthy, age-correlated specimens, the tendon thickness and quality are not comparable to the degenerative situation found in a majority of our patients; conclusions regarding tendon healing can also not be drawn based on our time-zero data only. So far, only a very few human in vivo studies have been published with small cohorts and short-term follow-up time periods, suggesting that further work is required to define which procedures should be used in the future [14, 17, 22, 23, 33]. The force transmission along the tendon was carefully applied by respecting the natural anatomy of the sheep infraspinatus footprint. This does not simulate the multiple forces transmitted to the tendon during human glenohumeral movements, e.g., during postoperative rehabilitation. The costs for patch and additional anchor implants have also to be considered as well as the prolonged time of surgery [26].
The use of young sheep cadavers of similar age eliminates a potential bias caused by tendon degeneration or differences in gender or size. One surgeon, with clinical experience of more than 500 rotator cuff repairs, performed the artificial dissection of the infraspinatus tendon, followed by standardized tendon reinsertion to reduce technical variations to a minimal level. The SpeedBridge™ technique can provide a superior initial strength [30, 34, 35]. No dislocation or breakage of anchors or sutures was observed. The size of the patch used in this study was large enough to cover every individual reconstruction area. As this animal model is smaller than the human shoulder, the graft size has to be considered. To avoid mismatching in daily practice, the relevant anatomic landmarks can be evaluated on preoperative MRI scans. The application of the patch in any of the described ways can be conducted entirely in an arthroscopic manner, without additional equipment, except for cannulas used for easier handling [26, 36]. None of the patch augmentation procedures were performed using an open or mini-open technique; therefore, it is not known whether these options might be a possible alternative. The “cover” technique is the standard technique used in the author’s clinic for patch augmentation. Clinical studies have been initiated to evaluate the influences of patch augmentation in vivo. Other authors recently reported promising, though still controversial, clinical results with varying implants and augmentation techniques [37–40].
Conclusion
Additional patch augmentation with a collagen patch influences the biomechanical properties of a double-row rotator cuff repair in cadaveric sheep shoulders. Depending on the technique of patch application, the maximum sustainable load can be significantly improved. This might be of benefit for patients, especially in the case of severe tendon degeneration or failed repair.
References
Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL (2012) National trends in rotator cuff repair. J Bone Joint Surg Am 94:227–233
Kannus P, Józsa L (1991) Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am 73:1507–1525
Tashjian RZ, Hollins AM, Kim HM, Teefey SA, Middleton WD, Steger-May K, Galatz LM, Yamaguchi K (2010) Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med 38:2435–2442
Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG (2005) Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am 87(6):1229–1240
Lafosse L, Brozska R, Toussaint B, Gobezie R (2007) The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am 89:1533–1541
Sugaya H, Maeda K, Matsuki K, Moriishi J (2005) Functional and structural outcome after arthroscopic full-thickness rotator cuff repair: single-row versus dual-row fixation. Arthroscopy 21:1307–1316
Duquin TR, Buyea C, Bisson LJ (2010) Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med 38:835–841
Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K (2004) The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 86:219–224
Gerber C, Fuchs B, Hodler J (2000) The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am 82:505–515
Mihata T, Watanabe C, Fukunishi K, Ohue M, Tsujimura T, Fujiwara K, Kinoshita M (2011) Functional and structural outcomes of single-row versus double-row versus combined double-row and suture-bridge repair for rotator cuff tears. Am J Sports Med 39:2091–2098
Gerhardt C, Hug K, Pauly S, Marnitz T, Scheibel M (2012) Arthroscopic single-row modified mason-allen repair versus double-row suture bridge reconstruction for supraspinatus tendon tears: a matched-pair analysis. Am J Sports Med 40:2777–2785
Rhee YG, Cho NS, Parke CS (2012) Arthroscopic rotator cuff repair using modified Mason-Allen medial row stitch: knotless versus knot-tying suture bridge technique. Am J Sports Med 40:2440–2447
Aurora A, McCarron JA, van den Bogert AJ, Gatica JE, Iannotti JP, Derwin KA (2012) The biomechanical role of scaffolds in augmented rotator cuff tendon repairs. J Shoulder Elbow Surg 21:1064–1071
Barber FA, Burns JP, Deutsch A, Labbé MR, Litchfield RB (2012) A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy 28(1):8–15
Coons DA, Alan Barber F (2006) Tendon graft substitutes-rotator cuff patches. Sports Med Arthrosc 14:185–190
Mazzocca AD, Trainer G, McCarthy MB, Obopilwe E, Arciero RA (2012) Biologic enhancement of a common arthroscopic suture. Arthroscopy 28:390–396
McCarron JA, Milks RA, Chen X, Iannotti JP, Derwin KA (2010) Improved time-zero biomechanical properties using poly-L-lactic acid graft augmentation in a cadaveric rotator cuff repair model. J Shoulder Elbow Surg 19:688–696
Nho SJ, Delos D, Yadav H, Pensak M, Romeo AA, Warren RF, MacGillivray JD (2010) Biomechanical and biologic augmentation for the treatment of massive rotator cuff tears. Am J Sports Med 38:619–629
Aurora A, McCarron J, Iannotti JP, Derwin K (2007) Commercially available extracellular matrix materials for rotator cuff repairs: state of the art and future trends. J Shoulder Elbow Surg 16:S171–S178
Barber FA, Aziz-Jacobo J (2009) Biomechanical testing of commercially available soft-tissue augmentation materials. Arthroscopy 25:1233–1239
Cheung EV, Silverio L, Sperling JW (2010) Strategies in biologic augmentation of rotator cuff repair: a review. Clin Orthop Relat Res 468:1476–1484
Barber FA, Herbert MA, Boothby MH (2008) Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy 24:20–24
Omae H, Steinmann SP, Zhao C, Zobitz ME, Wongtriratanachai P, Sperling JW, An KN (2012) Biomechanical effect of rotator cuff augmentation with an acellular dermal matrix graft: a cadaver study. Clin Biomech 27(8):789–792
Park MC, Elattrache NS, Ahmad CS, Tibone JE (2006) “Transosseous-equivalent” rotator cuff repair technique. Arthroscopy 22:1360 e1–e5
Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP (2006) Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. J Bone Joint Surg Am 88:2665–2672
Flury M (2012) Arthroscopic rotator cuff repair with patch augmentation. Oper Orthop Traumatol 24:486–494
Beitzel K, Chowaniec DM, McCarthy MB, Cote MP, Russell RP, Obopilwe E, Imhoff AB, Arciero RA, Mazzocca AD (2012) Stability of double-row rotator cuff repair is not adversely affected by scaffold interposition between tendon and bone. Am J Sports Med 40:1148–1154
Coons DA, Barber FA, Herbert MA (2006) Triple-loaded single-anchor stitch configurations: an analysis of cyclically loaded suture-tendon interface security. Arthroscopy 22:1154–1158
Nho SJ, Yadav H, Pensak M, Dodson CC, Good CR, MacGillivray JD (2007) Biomechanical fixation in arthroscopic rotator cuff repair. Arthroscopy 23:94–102
Burkhart SS, Adams CR, Burkhart SS, Schoolfield JD (2009) A biomechanical comparison of 2 techniques of footprint reconstruction for rotator cuff repair: the SwiveLock-FiberChain construct versus standard double-row repair. Arthroscopy 25:274–281
Gupta AK, Hug K, Boggess B, Gavigan M, Toth AP (2013) Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med 41:872–879
Gerber C, Schneeberger AG, Perren SM, Nyffeler RW (1999) Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am 81:1281–1290
Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ (2008) Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy 24(403–409):e1
Anderl W, Heuberer PR, Laky B, Kriegleder B, Reihsner R, Eberhardsteiner J (2012) Superiority of bridging techniques with medial fixation on initial strength. Knee Surg Sports Traumatol Arthrosc 20:2559–2566
Lo IK, Burkhart SS (2003) Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy 19:1035–1042
Cho CH, Lee SM, Lee YK, Shin HK (2014) Mini-open suture bridge repair with porcine dermal patch augmentation for massive rotator cuff tear: surgical technique and preliminary results. Clin Orthop Surg 6(3):329–335
Ciampi P, Scotti C, Nonis A, Vitali M, Di Serio C, Peretti GM, Fraschini G (2014) The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears: a 3-year follow-up study. Am J Sports Med 42(5):1169–1175
Giannotti S, Ghilardi M, Dell’osso G, Magistrelli L, Bugelli G, Di Rollo F, Ricci G, Calabrese R, Siciliano G, Guido G (2014) Study of the porcine dermal collagen repair patch in morpho-functional recovery of the rotator cuff after minimum follow-up of 2.5 years. Surg Technol Int 24:348–352
Proctor CS (2014) Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J Shoulder Elbow Surg 23(10):1508–1513
Sears BW, Choo A, Yu A, Greis A, Lazarus M (2015) Clinical outcomes in patients undergoing revision rotator cuff repair with extracellular matrix augmentation. Orthopedics 38(4):e292–e296
Acknowledgments
The authors would like also to thank M. Wilhelmi, PhD for the preparation and editing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, C., Spreiter, G., Audigé, L. et al. Patch-augmented rotator cuff repair: influence of the patch fixation technique on primary biomechanical stability. Arch Orthop Trauma Surg 136, 609–616 (2016). https://doi.org/10.1007/s00402-016-2436-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-016-2436-6