Abstract
Background
Previous studies established a role for the wearable cardioverter defibrillator (WCD) to effectively and safely bridge temporary risk for sudden cardiac death (SCD) in patients with advanced heart failure. The prognostic relevance of the WCD remains controversial.
Objectives
The authors investigated adherence to, as well as the safety and effectiveness of, WCD use in a real-world cohort of patients at high risk for SCD.
Material and methods
All consecutive patients (n = 83) receiving a WCD at a German tertiary care hospital between April 2012 and December 2019 were retrospectively included in this analysis. Patient characteristics were collected at the time of the index hospitalization. Using the Zoll® lifeVest® (ZOLL Medical Corporation, Chelmsford, MA, USA) network database, two separate investigators evaluated adherence to the WCD as well as arrhythmic events during WCD wear time.
Results
During 3680 wearing days (mean WCD wear time, 44 days) with a median daily wear time of 23.1 h, three arrhythmic events of relevance (sustained ventricular tachycardia, VT) occurred, one of which was sufficiently terminated by WCD shock. Another patient died from sudden cardiac death while pausing his WCD. Right bundle branch block correlated significantly with sustained VT occurrence (r = 0.3315; 95% CI −0.1265 to 0.3014; p = 0.0022). In 30 patients (36.1%) a cardioverter/defibrillator was implanted.
Conclusion
In a real-life clinical setting, the use of WCD in patients at high risk for sudden cardiac death is effective and safe and adherence to the device is high. The event rate for VA was lower than in comparable patient cohorts. Adherence remains a crucial issue as one patient in the present series died while not wearing the device.
Zusammenfassung
Hintergrund
Frühere Studien belegen die Bedeutung des tragbaren Kardioverter/Defibrillators (WCD) für die wirksame und sichere Überbrückung eines temporären Risikos des plötzlichen Herztods (SCD) bei Patienten mit fortgeschrittener Herzinsuffizienz. Der prognostische Stellenwert einer solchen Therapie ist jedoch weiterhin umstritten.
Ziel der Arbeit
Wir untersuchten Adhärenz, Sicherheit und Effektivität der Kardioverter/Defibrillator-Weste im kardiologischen Versorgungsalltag einer Kohorte von Patienten mit hohem SCD-Risiko.
Material und Methoden
Alle 83 zwischen April 2012 und Dezember 2019 in einer deutschen Klinik der Tertiärversorgung mit einem WCD versorgten Patienten wurden retrospektiv in diese Analyse eingeschlossen. Die Basischarakteristika wurden im Rahmen der Indexhospitalisierung erhoben. Die Auswertung von WCD-Adhärenz und arrhythmogenen Ereignissen während der WCD-Tragezeit erfolgte durch zwei unabhängige Untersucher anhand der Daten des LifeVest® Network der Fa. Zoll® (ZOLL Medical Corporation, Chelmsford, MA, USA).
Ergebnisse
Während 3680 Tragetagen (mittlere WCD-Tragedauer 44 Tage) mit einer medianen täglichen Tragedauer von 23,1 h traten drei anhaltende ventrikuläre Tachykardien (VT) über 30 s Dauer auf. Davon konnte eine erfolgreich durch einen WCD-Schock terminiert werden. Ein anderer Patient erlag einem SCD, als er die Weste gerade nicht trug. Ein Rechtsschenkelblock korrelierte signifikant mit dem Auftreten einer anhaltenden VT (r = 0,3315; 95 %-Konfidenzintervall −0,1265 bis 0,3014; p = 0,0022). Bei 30 Patienten (36,1 %) wurde ein Kardioverter/Defibrillator implantiert.
Schlussfolgerung
Die Anwendung des WCD in einem Hochrisikokollektiv für SCD unter klinischen Alltagsbedingungen ist sicher und effektiv, die Adhärenz der Patienten ist hoch. Die Rate an Arrhythmieereignissen war geringer als in vergleichbaren Patientenkollektiven. Die Adhärenz bleibt ein entscheidendes Problem, da ein Patient bei fehlender individueller Compliance einem SCD erlag.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with myocardial infarction or newly diagnosed advanced heart failure (i.e. left ventricular ejection fraction, LVEF < 35%) are at increased risk of sudden cardiac death (SCD) by arrhythmic events [1]. Immediate implantation of a cardioverter defibrillator (ICD) for primary prevention is not beneficial [2, 3] as treatment of the underlying cardiomyopathy (e.g. cardiac revascularization) and pharmacological heart failure therapy significantly improves left ventricular function in about one third of patients [4,5,6]. Therefore, current guidelines recommend at least 3 months of maximum tolerated doses of guideline-directed medical therapy or a 6-week waiting period in the case of ischemic etiology before reevaluating LVEF as a surrogate of arrhythmic risk and possible definitive implantable cardioverter defibrillator (ICD) implantation [7, 8].
Previous studies established the wearable cardioverter defibrillator (WCD) as a bridging tool during the vulnerable interval to LVEF restitution or definitive ICD-implantation by sufficiently detecting and terminating ventricular arrhythmias (VA) [9,10,11,12,13]. According to current guidelines, WCD use has a IIa [14, 15] or IIb indication [8, 29] in patients at high risk for SCD by arrhythmic events. However, the prognostic role of the WCD remains controversial and a lack of profound evidence has led to uncertainty in decision-making regarding its use in clinical practice. Several studies documented inappropriate therapies and high rates of inappropriate alarming [16,17,18]. Occurrence of life-threatening ventricular tachycardia (VT)/ventricular fibrillation (VF) during WCD use was low (2.6%) in a meta-analysis [19], and the only prospective randomized trial on WCD therapy did not show a reduction in sudden arrhythmic deaths in patients with acute myocardial infarction and a LVEF of 35% or less [20]. In this trial, device adherence was unexpectedly low, representing a finding that was discussed as a major limiting factor of the study. Therefore, the authors investigated adherence, safety and effectiveness of WCD use in a real-world cohort of patients at high risk for sudden cardiac death.
Methods
Patient population
All consecutive patients (n = 83) receiving a WCD (LifeVest®, ZOLL Medical Corporation, Chelmsford, MA, USA) at a German tertiary care hospital between April 2012 and December 2019 were retrospectively included in this investigator-initiated registry. Patient characteristics were collected at the time of index hospitalization. The study protocol conforms to the 1975 Declaration of Helsinki (in its most recently amended version), and the local ethics committee approved the study. Patients with prior myocardial infarction, advanced heart failure with LVEF ≤ 35%, ventricular arrhythmic events, myocarditis or ICD explantation due to infection or complications were included. Allocation to WCD fitting remained at the discretion of the treating physician with regard to the patient’s individual risk of VA (i.e. VF/sustained VT). The WCD wearing period was pre-set to 3 months, but could be adapted if substantial changes in arrhythmic risk (e.g. LVEF improvement) occurred earlier or if further medical therapy optimization was possible under prolonged WCD protection.
WCD programming
WCD programming was adapted to individual patient characteristics regarding prior arrhythmic events. In most patients the VT zone was programmed at 150 bpm with a response time of 60 s and the VF zone at 200 bpm with a response time of 25 s. In younger and more active patients the VT threshold was programmed higher. The first shock energy was set to 150 J. Follow-up visits with reevaluation of LVEF were scheduled 3 months after the initial hospitalization. Patients with previously explanted ICDs were followed-up according to clinical indications. ICD implantation was performed according to current guideline recommendations [8, 21] if LVEF remained ≤ 35% despite optimal medical therapy or if arrhythmic risk was unchanged despite increased LVEF (e.g. persistent scar after myocarditis).
Arrhythmic events and adherence
Using the Zoll® LifeVest® network database, two separate investigators (HA, CW) evaluated arrhythmic events during WCD wear time. Arrhythmic events were classified according to the 2015 ESC guidelines on VA and the prevention of SCD [22] as follows: adequate WCD shock for VF or sustained VT (VT ≥ 30 s in duration), sustained VT without shock (inhibited by patient), inadequate shock (without VF or sustained VT), non-sustained VT (three or more consecutive ventricular complexes in duration, terminating spontaneously in < 30 s), supraventricular tachycardia, bradycardia ≤ 30 bpm and inappropriate alarming defined as WCD alarming not due to VT or VF. Similar episodes less than 3 min apart were counted as one. Wear time adherence was defined as median daily wear time as recorded by the LifeVest® network.
Statistics
Descriptive statistics were performed using excel (Microsoft Corporation, Redmond, USA) and GraphPad Prism9 (GraphPad Software, San Diego, CA, USA). Data are presented as mean ± standard deviation unless otherwise specified. Categorical variables are presented by absolute and relative frequencies. Statistical comparisons between groups were performed using the Pearson chi-square test for categorical variables and paired and unpaired t‑test or Wilcoxon rank sum test or one-way ANOVA for continuous variables where appropriate. Significance tests were two-tailed with p < 0.05 considered significant. Results are shown as effect size with 95% confidence interval.
Results
Patient population
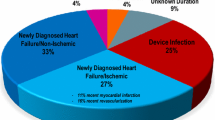
Between 2012 and 2019, 83 patients received a WCD (78% male). Mean age at index hospitalization was 60 (SD 13) years. Median LVEF at baseline was 28% (12–69%). Guideline-directed heart failure medication containing beta-blocker, ACE-I/AT1-A/ARNI and mineralocorticoid receptor antagonist (MRA) was established in 86.7%. Baseline characteristics and comorbidities are shown in Table 1. The indication for WCD use was dilated cardiomyopathy (DCM) in over half of the patients (51.8%), ischemic cardiomyopathy (ICM) in one third (32.5%) and myocarditis or device complication (i.e. infection, lead problems) in 7% each (Fig. 1). One patient presenting with sustained VT received WCD as a bridge to decision following theophylline intoxication. Median LVEF at the end of WCD use improved by 12 to 40% (13–78%). A further two patients died during the follow-up period from non-cardiac causes. A total of 11 patients were lost to follow-up.
Arrhythmic events during wear time and definitive AICD implantation
A total of 25 patients (30%) had ventricular arrhythmic events (VF, sustained-/non-sustained VT) prior to WCD prescription. During WCD wear time three ventricular tachycardias lasting 30 s or more were detected in three patients. Of these, one patient after ICD explantation due to infection received one adequate WCD shock for sustained VT (Fig. 2). One patient inhibited WCD shock by repeatedly pressing the response button before VT was self-terminating after 289 s. The third was readmitted immediately after demission due to sustained VT (patient inhibited shock) and died in combined cardiogenic and hemorrhagic shock in the further course. Detailed information is shown in Table 2.
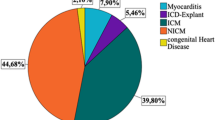
No inadequate therapies were applied but overall 989 inappropriate alarms appeared due to artefacts. An overview of all arrhythmic events is shown in Table 3. To further characterize potential factors that may determine risk for arrhythmogenic events we correlated a panel of risk markers to occurrence of sustained VT/VF. Of all tested variables as shown in Table 4, only right bundle branch block (RBBB) correlated significantly with sustained VT occurrence, with medium effect size (r = 0.3315; 95% CI −0.1265 to 0.3014; p = 0.0022). In 30 patients (36.1%) an ICD was implanted.
Device adherence
The mean WCD usage period was 44 (SD 32) days with a median daily wear time of 23.1 h (0–23.97 h). Daily WCD use was similar regarding sex, age, indication and total days of WCD wear (Table 5). Two patients returned their WCD directly after discharge due to discomfort. One patient with DCM died from sudden cardiac arrest while pausing his WCD. His prior daily wear time was 15.1 h/day.
Discussion
This study investigates the use of the WCD in a real-world setting of patients at high risk of SCD. WCD use was effective and safe. During wear time life threatening arrhythmic events were safely detected and efficiently terminated by the device. No inadequate WCD therapies were observed. The incidence of arrhythmic events was low as only few events of relevance occurred during WCD monitoring. Compared to similar cohorts, patient adherence to the device was high.
SCD due to VT/VF remains a preventable cause of death and the concept of WCD use provides a safe and effective approach for aborting death among patients at high risk of sudden arrhythmic death. Several prospective trials [11, 12] and real-world data [9, 10, 13] in different patient populations (e.g. early post myocardial infarction, non-ischemic cardiomyopathy) consistently proved effectiveness and safety. Accuracy and precision in the detection of ventricular tachyarrhythmia/ventricular fibrillation was high throughout all studies. Successful termination of VT/VF events was achieved in up to 98% with the first shock [23]. Rates of inappropriate shocks differ between patient cohorts and are reported as being up to 5.9% [14]. However, they fall below inappropriate shock rates in ICD patients, which have been shown to be up to 13% [24]. In the present study, three episodes of sustained VT were correctly detected and one (1.2%) of these was effectively treated by the device. This corresponds to a large German cohort of 6043 patients and US data which reported 1.6% of patients treated by WCD in response to VF/VT [7, 13]. However, the small number of relevant VA in our cohort (3.6%) differs from comparable reports describing up to 9.6% of VA [16,17,18, 25]. Whereas in comparable study collectives inappropriate shock treatments occurred in up to 2% [18], there was no inadequate therapy applied in the present cohort despite a relevant number of inappropriate alarms. Regarding indication for WCD, patient age and comorbidities, the authorsʼ collective is comparable to others [16,17,18, 25] and shows even higher rates of arrhythmic events prior to WCD prescription (30%). Therefore, the low rate of arrhythmic events observed during WCD treatment cannot be entirely explained by divergent patient selection or a younger and healthier patient collective.
In the present study a high proportion of patients received guideline-directed drug therapy according to baseline data (beta-blocker 100%, ACE inhibitor/angiotensin receptor blocker 95%, mineralocorticoid receptor antagonist 87%). In comparable prior studies this ratio goes down to 90.3% for beta-blocker and 32.7% for MRA [16,17,18]. Stringent pharmacological heart failure therapy, in particular beta-Blocker therapy, may have prevented a higher incidence of arrhythmic events in the present cohort. Hypothetically, a high daily wear time as documented in this series may be suggestive of increased adherence to medication, which may have contributed to this finding. Another point is that a relevant proportion of patients experienced rapid recovery of LVEF and therefore WCD wearing was terminated (mean wear time: 44 days, median LVEF improvement 12%). An ICD was implanted in 30 patients (36.1%) since LVEF remained ≤ 35% or due to evidence for unchanged arrhythmic risk despite increased LVEF (e.g. scar after myocarditis). Although rates of definitive ICD implantation appear relatively low compared to other studies (55% [18]), this data should be interpreted with caution as 11 patients (13%) were lost to complete follow-up.
Patient adherence is of crucial importance for effective WCD therapy as it emphasizes the patient’s agreement to actively participate in the treatment concept [14]. In contrast to clinical studies investigating adherence to medication, which is often subject to self-report, adherence to WCD use can be monitored effectively by the treating physician. Registry data show that adherence to WCD use is high. Several case series report daily use of > 90% per day [9, 13, 26]. However, in the VEST trial, the only randomized controlled trial of WCD use, the wear time with the device was lower than anticipated even under trial conditions. Wear time of only 18 h per day and only 12 of 48 participants in the device group wearing the WCD at the time of death contributed to the primary result of the trial. Contrary to expectations, WCD use did not lead to a significantly lower rate of the primary outcome of arrhythmic death [20]. In the present cohort adherence to WCD was among the highest reported [19] at 23.1 h per day overall median wear time. However, although all patients signed informed consent to WCD therapy, individual adherence went as low as zero hours, resulting in one patient dying from SCD.
Nevertheless, one event per 1227 wearing days in contrast to nevertheless one SCD victim due to low adherence raises concerns about the effectiveness of WCD as well as appropriate patient selection to WCD. Patient allocation to WCD therapy is challenging as underlying cardiomyopathy, prior arrhythmic events, LVEF and other parameters mentioned above are only surrogates of further arrhythmic events and SCD. The observed correlation between RBBB and sustained VT occurrence might be biased by the relatively small cohort. The present findings support WCD use in patients with a clear and ongoing AICD indication that are temporarily unprotected, for example as a result of device explantation due to infection [27]. In the light of the low arrhythmic event rates it seems reasonable that other potentially high-risk patients for SCD receive guideline-directed medical therapy particularly including beta-blockers and recurrent echocardiographic LVEF reevaluation during index hospitalization before even considering WCD provision. Patient adherence is crucial for the effectiveness of WCD therapy. Despite relatively strong adherence in the present study, even more efforts (i.e. intensified use of local heart failure networks/planned close follow-ups) are necessary to maintain each individual patient’s adherence as high as possible. Currently, about 5% of patients for whom the WCD is prescribed are ultimately considered unsuitable for appropriate and safe handling of the system or are unwilling to wear the WCD [23, 28]. Having said that, in the context of a particularly motivated high-risk patient, WCD may be considered as a safe and effective strategy for the prevention of SCD.
Study limitations
This study is limited by its monocentric, retrospective, nonrandomized character. Furthermore, patient allocation to WCD was not standardized. Both might have led to selection bias. Assignment to a single cardiac diagnosis resulted in overlap among disease types.
Conclusion
In a real-life clinical setting, the use of WCD in patients at high risk for SCD is effective and safe, and adherence to the device is generally high (Fig. 3). Adherence remains a crucial issue as one patient in the present series died while not wearing the device. In the authorsʼ cohort the event rate for VA was lower than expected.
Practical conclusion
-
The present findings support WCD use in patients with an ongoing ICD indication temporarily unprotected as a result of device explantation (e.g. due to infection).
-
High-risk-patients for SCD should receive LVEF reevaluation during index hospitalization prior to WCD allocation as arrhythmic event rates appear lower with contemporary medical therapy.
-
As adherence remains crucial for the effectiveness of WCD therapy, every effort should be made to promote each individual patient’s adherence.
Abbreviations
- ARNI:
-
Angiotensin receptor neprilysin inhibitor
- BMI:
-
Body mass index
- CABG:
-
Coronary artery bypass graft
- CAD:
-
Coronary artery disease
- DCM:
-
Dilated cardiomyopathy
- ICD:
-
Implantable cardioverter defibrillator
- ICM:
-
Ischemic cardiomyopathy
- LBBB:
-
Left bundle branch block
- LVEF:
-
Left ventricular ejection fraction
- MRA:
-
Mineralocorticoid receptor antagonist
- RBBB:
-
Right bundle branch block
- SCD:
-
Sudden cardiac death
- VA:
-
Ventricular arrhythmias
- VF:
-
Ventricular fibrillation
- VT:
-
Ventricular tachycardia
- WCD:
-
Wearable cardioverter defibrillator
References
Solomon SD, Zelenkofske S, McMurray JJ et al (2005) Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med 352(25):2581–2588
Hohnloser SH, Kuck KH, Dorian P et al (2004) Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med 351:2481–2488
Steinbeck G, Andresen D, Seidl K et al (2009) IRIS Investigators. Defibrillator implantation early after myocardial infarction. N Engl J Med 361:1427–1436
Binkley PF, Lesinski A, Ferguson JP et al (2008) Recovery of normal ventricular function in patients with dilated cardiomyopathy: predictors of an increasingly prevalent clinical event. Am Heart J 155:69–74
Teeter WA, Thibodeau JT, Rao K et al (2012) The natural history of new-onset heart failure with a severely depressed left ventricular ejection fraction: implications for timing of implantable cardioverter-defibrillator implantation. Am Heart J 164:358–364
Savarese G, Vedin O, D’Amario D et al (2019) Prevalence and prognostic implications of longitudinal ejection fraction change in heart failure. J Am Coll Cardiol 7:306–317
Epstein AE, DiMarco JP, Ellenbogen KA et al (2008) ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. J Am Coll Cardiol 51:e1–62
Priori SG, Blomstrom-Lundqvist C, Mazzanti A et al (2015) 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace 17:1601–1687
Chung MK, Szymkiewicz SJ, Shao M et al (2010) Aggregate national experience with the wearable cardioverter-defibrillator: event rates, compliance, and survival. J Am Coll Cardiol 56:194–203
Epstein AE, Abraham WT, Bianco NR et al (2013) Wearable cardioverter-defibrillator use in patients perceived to be at high risk early post-myocardial infarction. J Am Coll Cardiol 62:2000–2007
Feldman AM, Klein H, Tchou P et al (2004) Use of a wearable defibrillator in terminating tachyarrhythmias in patients at high risk for sudden death: results of the WEARIT/BIROAD. Pacing Clin Electrophysiol 27:4–9
Kutyifa V, Arthur JM, Klein H et al (2015) Use of the wearable cardioverter defibrillator in high-risk cardiac patients: data from the prospective registry of patients using the wearable cardioverter defibrillator (WEARIT-II registry). Circulation. https://doi.org/10.1161/CIRCULATIONAHA.115.015677
Wäßnig NK, Günther M, Quick S et al (2016) Experience with the wearable cardioverter-defibrillator in patients at high risk for sudden cardiac death. Circulation 134:635–643
Piccini JP Sr, Allen LA, Kudenchuk PJ et al (2016) American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing. Wearable cardioverter-defibrillator therapy for the prevention of sudden cardiac death: a science advisory from the American Heart Association. Circulation 133(17):1715–1727
Al-Khatib SM, Stevenson WG, Ackerman MJ et al (2018) 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation 138(13):e210-e271
Beiert T, Malotki R, Kraemer N et al (2017) A real world wearable cardioverter defibrillator experience—Very high appropriate shock rate in ischemic cardiomyopathy patients at a European single-center. J Electrocardiol 50:603–609
Röger S, Rosenkraimer SL, Hohneck A et al (2018) Therapy optimization in patients with heart failure: the role of the wearable cardioverter-defibrillator in a real-world setting. BMC Cardiovasc Disord 18:52
Erath JW, Vamos M, Sirat AS, Hohnloser SH (2017) The wearable cardioverter-defibrillator in a real-world clinical setting: experience in 102 consecutive patients. Clin Res Cardiol 106(4):300–306
Nguyen E, Weeda ER, Kohn CG et al (2018) Wearable cardioverter-defibrillators for the prevention of sudden cardiac death: a meta-analysis. J Innov Cardiac Rhythm Manag 9:3151–3162
Olgin JE, Pletcher MJ, Vittinghoff E et al (2018) Wearable cardioverter-defibrillator after myocardial infarction. N Engl J Med 379(13):1205–1215
Ponikowski P, Voors AA, Anker SD et al (2016) ESC Scientific Document Group, 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37(27):2129–2200
Priori SG, Blomstrom-Lundqvist C, Mazzanti A et al (2015) ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death—web addenda. Eur Heart J. https://doi.org/10.1093/eurheartj/ehv316
Reek S, Burri H, Roberts PR et al (2017) The wearable cardioverter-defibrillator: current technology and evolving indications. Europace 19:335–345
van Rees JB, Borleffs CJ, de Bie MK et al (2011) Inappropriate implantable cardioverter-defibrillator shocks: incidence, predictors, and impact on mortality. J Am Coll Cardiol 57:556–562
Rosenkaimer SL, El-Battrawy I, Dreher TC et al (2020) The wearable cardioverter-defibrillator: experience in 153 patients and a long-term follow-up. J Clin Med 9:893
Garcia R, Combes N, Defaye P et al (2021) Wearable cardioverter-defibrillator in patients with a transient risk of sudden cardiac death: the WEARIT-France cohort study. Europace 23(1):73–81
Deneke T, Bosch R, Eckardt L et al (2019) Der tragbare Kardioverter/Defibrillator (WCD) – Indikationen und Einsatz. Kardiologe 13:292–304
Klein HU, Goldenberg I, Moss AJ (2013) Risk stratification for implantable cardioverter defibrillator therapy: the role of the wearable cardioverter-defibrillator. Eur Heart J 34:2230–2242
McDonagh TA, Metra M, Adamo M et al (2021) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. https://doi.org/10.1093/eurheartj/ehab368
Funding
The author(s) received no financial support for the research, authorship and/or publication of this article.
Author information
Authors and Affiliations
Contributions
Christian Weth: Conceptualization, Methodology, Data curation, Investigation, Formal analysis, Roles/Writing of the original draft, Visualization, Project administration Hasan Abuazab: Data curation, Investigation Sebastian Ewen: Formal analysis, Validation, Resources Michael Böhm: Methodology, Supervision Christian Ukena: Supervision Florian Custodis: Conceptualization, Methodology, Resources, Validation, Writing of the review and editing, Project administration.
Corresponding author
Ethics declarations
Conflict of interest
C. Weth, H. Abuazab, C. Ukena and F. Custodis declare that they have no competing interests. S. Ewen received speaker or consultant honorarium from Medtronic, Recor, Bayer, Daiichi Sankyo, Boehringer Ingelheim, Novartis, AstraZeneca, Akcea Therapeutics and Bristol Myers Squibb Pfizer Alliance. M. Böhm is supported by the Deutsche Forschungsgemeinschaft (German Research Foundation; TTR 219, project number 322900939) and reports personal fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cytokinetics, Medtronic, Novartis, Servier and Vifor.
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated in each case.
Additional information

Scan QR code & read article online
Rights and permissions
About this article
Cite this article
Weth, C., Abuazab, H., Ewen, S. et al. Real-world experience with the wearable cardioverter defibrillator: clinical effectiveness and wear-time adherence in patients at high risk for sudden cardiac death. Herzschr Elektrophys 33, 55–62 (2022). https://doi.org/10.1007/s00399-021-00816-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00399-021-00816-w