Abstract
Waterborne polyurethane-acrylate (WPUA) composite emulsions were prepared by the physical blending method, graft emulsion polymerization method, and interpenetrating network (IPN)-based method using polytetramethylene glycol (PTMG), isophoronediisocyanate (IPDI), and acrylic monomers (methyl methacrylate (MMA) and butylacrylate (BA)) as the main raw materials. The WPUA polymers were characterized based on their water resistance, morphologies, thermal, spectroscopic and mechanical properties. Graft WPUA film had the highest tensile strength and the best thermal stability, indicating that the strength of the chemical bond between PU and PA plays an important role in modulating the performances of the WPUA films. The results showed that the graft WPUA has excellent integrated properties and thus a potential application in the coating industry.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Waterborne polyurethane (WPU) and polyacrylate (PA) have been commercialized for many years, but each has its own disadvantages. WPU has low water resistance and weather resistance, whereas PA has poor abrasion resistance and solvent resistance [1,2,3,4,5]. However, the combination of PU with PA improves the performances of the resulting composite. It is well known that polyurethane-acrylate (PUA) composite materials have good abrasion resistance, corrosion resistance, water resistance, and weather resistance. Their preparation protocol is cost-effective, requires low-energy consumption, and involves no solvent pollution. PUA is widely used in the coating industry, textile industry, construction industry, and several other fields and is known as the “third-generation waterborne polyurethane” [6, 7].
In recent decades, the study of waterborne polyurethane-acrylate composite emulsions has gained increasing attention from a large number of researchers. Several methods have been suggested for the preparation of the PUA composites. There are three most commonly used techniques for incorporating the acrylic polymers into polyurethane dispersions. These are the physical blending method, emulsion polymerization method, and the interpenetrating polymer network (IPN)-based method [8,9,10,11]. The physical blending method involves the independent preparation of the polyurethane and polyacrylate emulsions, followed by their simple mechanical mixing at a certain proportion. This is the simplest way to compound PU and PA. This composite emulsion retains the properties of both PU and PA to a certain extent, but the poor compatibility between PU and PA gives the film a poor appearance and makes it susceptible to cracks (because of the simple blending of the two emulsions) [12]. In the emulsion polymerization, end-capping of the polyurethane prepolymer with hydroxy vinyl monomers (such as hydroxyl acrylates) introduces a double bond into the molecular structure of polyurethane, which is then copolymerized with acrylate monomers. The hydrophobic PA monomer is more likely to be present inside the nucleus, whereas the hydrophilic PU monomer will be preferably in contact with the water present outside the particle. The emulsion polymerization significantly improves the water resistance, weather resistance, and transparency and is thus widely used in the synthesis of water-borne coatings [13,14,15]. IPN is an important compound polymer, wherein at least one of the components is a network crosslinking structure and is not involved in chemical bonding with the other networks. For the formation of the IPN composite, PU prepolymer is prepared initially and the PUA composite emulsion is synthesized using the PU prepolymer as the seed. Polyurethane and acrylate formed separate microfacies, with physical crosslinking at the interface between them, i.e., there is a three-dimensional “mechanical entanglement” at the interface between them. The existence of “mechanical entanglement” in the IPN-based materials significantly improves their compatibility compared with those prepared by physical blending [16,17,18,19,20]. Yuan et al. [21, 22] claimed they used a combination method of physical and chemical method to obtain polymer particles with core-shell structure. Mehravar et al. [23, 24] synthesized polyurethane prepolymer containing carboxyl group directly with (meth) acrylic monomer as solvent and obtained polyurethane/acrylic waterborne hybrid without solvent and surfactant. In addition, the effects of functional monomer types and monomer polymerization mode on polymer characteristics and final properties of the films were investigated [25, 26].
There are only a few reports on the effect of the preparation methods on the properties of waterborne polyurethane acrylate (WPUA). Hence, in this work, waterborne polyurethane-acrylate composite emulsions were prepared using all the three methods discussed above; the internal structures of the composite emulsions were found to vary according to the method used. The effects of different preparation methods on WPUA composite emulsions and the films were investigated by means of dynamic light scattering (DLS), Fourier-transform infrared (FT-IR) spectroscopy, dynamic mechanical analysis (DMA), thermogravimetric analysis (TGA), scanning electron microscope (SEM), etc.
Experimental section
Materials and reagents
Polytetramethylene ether glycol (PTMG, Mn = 2000 g/mol, Bayer) and 1,4-butanediol (1,4-BDO, Aldrich Chemical Co.) were dehydrated under a vacuum oven at 70 °C for 2 h. Methyl methacrylate (MMA, Aldrich Chemical Co.) and butylacrylate (BA, Aldrich Chemical Co.) were used after distillation under reduced pressure. Isophorone diisocyanate (IPDI) was purchased from Bayer. Dimethylolpropionic acid (DMPA), dibutyltin dilaurate (DBTDL), hydroxyethyl methacrylate (HEMA), trimethylolpropane (TMP), actone, potassium persulfate (KPS), divinylbenzene (DVB) and sodium dodecyl sulfate (SDS) were obtained from Aldrich Chemical Co. N,N-Dimethylacetamide (DMAC) and triethylamine (TEA) were purchased from Sinopharm Chemial Reagent Co. Ltd. The materials were used as received without further purification unless otherwise mentioned. Unless otherwise specified, all chemicals and reagents were of analytical grade. Deionized water (DDI) was used in the experiment.
Synthesis
The preparation of WPU/PA physical blends (WPUA1)
Basic formula for WPU latex is given in Table 1. The stoichiometric PTMG and 1,4-BDO were dried at 70 °C for 2 h in a vacuum oven in order to remove moisture and were then pulled into a three-necked glass reaction kettle of 500 ml equipped with a mechanical stirrer, reflux condenser, a nitrogen inlet and thermostatic oil bath. DMPA, DMAC, IPDI and DBTDL were then added into the reactor, and the reaction was carried out at 80 °C for 1.5 h. Then, the reactor was cooled below 40 °C, and freshly dried acetone was added to bring down the viscosity of the reaction. After 30 min, the polyurethane was neutralized by triethylamine (TEA), and then dispersed in water with a stirred rate of 1200 r/min for 30 min. Finally, the acetone was removed under vacuum to obtain the polyurethane dispersion in water.
The PA latex polymerization was conducted in a 500ml three-neck glass flask under nitrogen at 75 °C and stirred at a speed of 200 rpm. First, the water and SDS were added to the glass flask and stirred under nitrogen. After 10 min, the MMA and BA monomers were added in a continuous feeding way to the flask. The monomer recipes used for synthesis of PA are shown in Table 1.
WPU/PA physical blends were prepared by simply mixing WPU with PA at weight ratios of 30/70 and are designated as WPUA1.
The preparation of WPUA composite emulsion by graft emulsion polymerization (WPUA2)
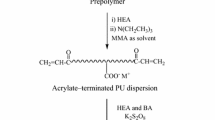
Graft WPUA composite emulsion which was designated as WPUA2 was prepared in advance as described in a previous work [15]. For graft WPUA composite emulsion, HEMA was added to end-cap the prepolymer. The same processes were employed as for WPU and PA latex preparation except that, acetone, used just before WPU prepolymer dispersion, was replaced by one third of the PA monomers (MMA and BA).
The preparation of WPUA composite emulsion by interpenetrating polymer network method (WPUA3)
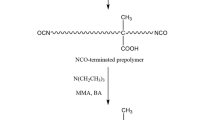
For IPN WPUA composite emulsion, the same processes were employed as for core-shell WPUA latex preparation except that, HEMA, used as end-capping agent, was not added. At the same time, TMP was introduced as a crosslinking agent of WPU, which makes WPU have network structure. The SDS, MMA, BA, and DVB were added to the glass flask and stirred for 24 h under room temperature. Finally, KPS was added and the reaction was proceeded at 75 °C for 3 h. IPN PUA composite emulsion was prepared and is designated as WPUA3.
The synthesis route of WPUA composite emulsion prepared by three methods is shown in Fig. 1.
Preparation of WPUA films
The films were made by casting the emulsion on a Teflon disk dried at 25 °C for a week. The resulting films were placed in a vacuum oven at 50 °C for 48 h before test.
Characterization
Dynamic light scattering
The particle size and distribution of emulsion were measured with dynamic light-scattering (Brookhaven 90 Plus Laser Particle Analyzer). The latex was diluted with deionized water at room temperature. Each sample was measured five times at 10 min.
Transmission electron microscopy
Some of the particles were placed on a copper grid, and the morphology of the latexes was examined using transmission electron microscope (JEOL-1011). The grid containing sample was placed in the 3% phosphotungstic acid (PTA) water solution for 5 min before observation.
Fourier-transform infrared spectroscopy
FT-IR spectra were acquired by attenuated total reflectance (ATR) mode with a Fourier-transform infrared spectrometer (IS50, Nicolet). Spectra were recorded over the wavenumber range of 650 to 4000 cm−1 with a resolution of 8 cm−1 using a thin film of the polymer.
Contact angle test
The contact angle of the film was tested by the contact Angle Goniometer (DSA30, Kruss Company). Two liquids, water and ethylene glycol, were placed on the surface of the film using a microsyringe. Three measurements were made for each sample.
Water resistance measurement
The films with size of 20 × 20 mm were put into water at room temperature for 48 h, then, the surface of latex film was sipped up rapidly and quantified. The water absorption W was calculated as follows:
where W1 and Wt are the mass of film before being put into the water and the mass of the film at time t, respectively.
Dynamic mechanical analysis
Dynamic mechanical analysis was performed on DMA 242 dynamic mechanical analyzer at a frequency of 1 Hz. Small rectangular bar specimens of 10 × 10 × 2 mm3 were tested in the tensioning mode at a temperature varied from − 100 to 100 °C with a heating rate of 3 °C/min.
Tensile test
The tensile measurements of the films were investigated using a universal testing machine (Instron 3365 tensile tester) with a cross-head speed of 50 mm/min at 25 °C, according to ASTM D638. Each sample was tested three times and the optimum value was obtained.
Thermogravimetric analysis
TGA experiments were carried out with a Pyris 1 TGA thermogravimetric analyzer (Perkin Elmer). Film samples (3–4 mg) were placed in a platinum pan and heated under nitrogen atmosphere, at a heating rate of 10 °C/min from 30 to 600 °C.
Scaning electron microscope
Samples were gold coated in Denton vaccum apparatus before observation. The morphology of the fractured surfaces of WPUA films was studied by SEM to study the compatibility between PU and PA phases.
Results and discussion
Particle size analysis
Figure 2 shows the particle size and particle size distribution of the WPU, PA, and WPUA latexes tested by DLS. Figures 2a and 2b show that the average particle sizes of WPU and PA are 66 and 84.9 nm, respectively. The average particle size of WPUA1 obtained by the simple mixing of the two was 78.1 nm (Fig. 2c), which is between those of WPU and PA. The average particle size of the graft WPUA2 was 218.6 nm (Fig. 2d). This is because that, in the synthesis of WPUA2, HEMA was used as end-capping agent and introduced the double bond into the end of PU pre-polymer. However, the double bond in HEMA is hydrophobic. In the emulsification process, the hydrophilic carboxyl groups were not enough to wrap all the hydrophobic particles in the aqueous solution. In order to maintain the stability of particles in the system, some small particles must coalesce or coagulate to form larger particles. Moreover, some PA monomers of IPN particles did not enter the interior of PU for polymerization but were nucleated independently, resulting in the number of particles increases and the size of particles decreases accordingly. Figure 2e shows that the average particle size of WPUA3 was 156 nm. WPUA3 was obtained by free radical copolymerization, wherein the acrylate monomer was incorporated into the WPU network, using WPU as the seed emulsion. The increase in particle size is due to the swelling of the acrylate monomers into WPU prepolymer. It is evident that the particle size distributions of all the WPUA emulsions are broader than that of the pure WPU emulsion. The size of the WPUA emulsion obtained by graft emulsion polymerization is the largest, and that obtained by physical blending is the smallest. The particles in PUA2 (Fig. 2d) and PUA3 (Fig. 2e) exhibit a bimodal distribution because some of the PA monomers did not enter the interior of PU for polymerization but were secondary nucleated for free radical polymerization.
TEM observation
The morphology of WPUA emulsion particles was observed by TEM and is shown in Fig. 3. The size of the particles observed by TEM (Fig. 3) is in good agreement with the size obtained from the particle size distribution analysis (Fig. 2). It is generally accepted that the darker region is PU, and the lighter region is PA. This is because of the higher polarity of PU, as compared with that of PA, which results in a higher electron cloud density around the PU chains [27, 28]. However, WPUA3 shows a network-like structure that is not a regular sphere. Although most of the PA can enter the PU for polymerization after 20 h of mechanical stirring, some free PA monomers continue to react with the internally generated long chains and form an interpenetrating network structure. The network-like feature observed in WPUA3 can be ascribed to this phenomenon.
FT-IR analysis
Figure S1 (Electronic supplementary material (ESM)) represents the FT-IR spectra of WPU, PA, and WPUA films. The characteristic bands at approximately 3322, 1700, 1532, and 1107 cm−1 are indicative of the N–H stretching, C=O stretching (urethane and ester groups), N–H bending and C–O–C stretching (ether groups), respectively [29,30,31]. The absence of the characteristic stretching band of free NCO group from 2205 to 2270 cm−1 in polyurethane indicates that all the NCO groups have reacted to form urethane [32, 33]. The PUA spectrum exhibits the stretching vibration of –OC4H9 group at 841 cm−1. The absorption intensity of the N–H bond in the WPUA1 and WPUA3 films is much higher than that of the WPUA2 film. According to the lower hydrogen bond, the higher the phase uniformity of the film, the ordered degree of the hard segments in the WPUA2 is significantly reduced due to the graft reaction of the polyacrylate molecule. However, the ordered degrees of hard segments in the WPUA1 and WPUA3 films have little changes. The absence of absorption bands for C=C groups at 1640 and 1667 cm−1 indicates the completion of the reaction between PU and PA. Thus, the FT-IR analysis showed that WPUA composite emulsions were successfully prepared by physical blending, graft emulsion polymerization and network interpenetration.
Contact angle and surface-free energy of the films
The surface contact angle reflects the hydrophobicity of WPUA films. The surface energy for the films was deduced indirectly from their contact angles with two different liquids, water and ethylene glycol, and was calculated as follows [34, 35]:
where θ1 and θ2 are the contact angles of water and ethylene glycol on the surface of the films; γs, \( {\gamma}_s^d \), and \( {\gamma}_s^p \) are the surface energy, dispersion and polar components of the films, respectively; γ1, \( {\gamma}_1^d \), and \( {\gamma}_1^p \) are the surface energy, dispersion, and polar components of water, respectively (\( {\gamma}_1^d \) = 21.8 mJ/m2, \( {\gamma}_1^p \) = 51.0 mJ/m2); γ2, \( {\gamma}_2^d \), and \( {\gamma}_2^p \) are the surface energy, dispersion and polar components of ethylene glycol, respectively (\( {\gamma}_2^d \) = 29.3 mJ/m2, \( {\gamma}_2^p \) = 19.0 mJ/m2).
The water contact angles of the films and their surface free energies are presented in Fig. 4 and Table 2, respectively. Figure 4 shows the contrasting surface polarity of the three WPUA films. The surface of WPUA1 film has the greatest hydrophilic, while the hydrophobic of WPUA2 and WPUA3 films is improved. This may be attributed to the flexibility of the chain, which is an important factor in controlling the contact angle. The rigid chain makes it difficult for the ionic groups to reach the surface of the particle. A higher content of ions on the particle surface results in better hydrophilicity and a smaller contact angle. WPUA3 has the highest contact angle due to its interpenetrating network structure, rigid arrangement of the chain segments, and poor flexibility of the chains. The surface energy follows the opposite order.
Water absorption rate of the films
The water contact angle is a measure of the wettability of the surface of the films. We also studied the water resistance of the films by water absorption analysis. The water absorption rate reflects the water resistance of the films and is a significant parameter for its application in many fields. Figure 5 shows the water absorption of the films. The water absorption saturates after being soaked for 48 h. WPUA1 prepared by physical blending has the maximum water absorption rate. It can be attributed to the fact that there are no chemical bonds formed in physical blending, thus making it easier for water to diffuse into the molecules. WPUA3 film shows the lowest water absorption rate, this can be attributed to the interpenetrating network structure, which makes the intramolecular structure more compact and does not allow water molecules to enter the interior of the particles easily. This is in good agreement with the results obtained from the water contact angle measurement. In this study, high contact angle, low surface free energy, and low water absorption rate were used as the primary criteria to estimate the water resistance of the films.
Dynamic mechanical analysis
In DMA, the damping properties of the vibrations can be determined by the width and height of the peak of the mechanical loss tangent (tan δ). In order to meet the criterion for good damping materials, tan δ of the material should be higher than 0.3 at 60 °C (at least) [15, 36].
Figure 6 shows the curve of the storage modulus changing with temperature. It can be found that only one glass transition in WPUA2, which conforms to the rule of storage modulus changing with temperature. When the temperature is lower than − 30 °C, the storage modulus does not change significantly with the increase of temperature. When the temperature rises from − 30 to 40 °C, the storage modulus decreases significantly with the increase of temperature, and the glass transition occurs at this time. As the temperature increases, the storage modulus decreases slowly. At the same time, the material enters the rubber state, indicating that the two phases in WPUA2 have a good compatibility. However, the storage modulus in WPUA1 and WPUA3 does not change significantly with temperature, which is due to the poor compatibility between the two phases.
Figure 7 shows the damping properties of WPU, PA and WPUA films. Because the soft and hard segments of PU are chemically dissimilar, they are incompatible and separate into different phases. The peak at − 75 °C in the tan δ curve of WPU can be attributed to the glass transition temperature (Tg) of the soft segments, while the Tg of the hard segments cannot be observed because of the viscous flow of samples at higher temperature. Tan δ of the WPUA1 film shows two peaks, one at about − 60 °C corresponding to the Tg of PU and the other at − 10 °C corresponding to the Tg of PA. This is probably because the internal molecular chain structure of the chains forming WPUA does not change during physical blending. The Poor compatibility and weak interaction between PU and PA result in a low damping performance. The two Tgs in WPUA3 were closer to each other as compared with the Tgs of PU and PA, which means that IPN can improve the compatibility of PU and PA. However, a single tan δ peak is obtained in WPUA2, which implies that the formation of chemical bond between PU and PA allows them to behave as a single entity. WPUA2 shows high damping ((tan δ)max = 1.2, which is much higher than 0.3—the minimum required for efficient damping) over a much broader range of temperature (−11 to 64 °C). The high damping performance is because of the intermolecular forces and good compatibility between PU and PA, which restrict its segmental motion to some extent.
Tensile behavior of the films
Figure 8 shows the stress-strain curves of WPUA films prepared by the three different methods. The WPUA1 shows the lowest tensile strength and elongation at break, which are 3.9 Mpa and 372%, respectively. The tensile strength and elongation at break of WPUA2 are 10.2 Mpa and 457%, respectively. The tensile strength and elongation at break of WPUA3 are 9.9 Mpa and 467%, respectively. The tensile strength and the elongation at break of WPUA2 and WPUA3 were significantly better than WPUA1. The best results were obtained for WPUA3 due to the crosslinking between both phases PU and PA. The good compatibility of PU and PA and the “synergistic effect” between them increases the strength of the material, thereby resulting in a good tensile strength or elongation at break. It is evident that the tensile strength and elongation at break of the films formed using the graft polymerization method and IPN-based method are superior to those formed by the physical blending method.
Thermogravimetric analysis
Thermogravimetric apparatus was used to estimate the thermal stability of the WPUA films. The curves of TGA and differential weight loss (DTG) are shown in Figs. S2 and S3 (ESM), respectively. There is barely any weight loss before 200 °C for any sample. Two rapid decomposition phases can be observed in Fig. S3. The weight loss in the temperature range of 220–330 °C is due to the decomposition of the hard segments (urethane and urea bonds). The weight loss from 330 to 400 °C was associated with the decomposition of the soft segments and PA components [37,38,39,40,41,42]. The two degradation temperatures in WPUA2 are the closest, which indicates that this sample has the best compatibility. In other words, WPUA2 had a much higher thermal stability than WPUA1 and WPUA3. This can be attributed to the synergistic effect of PU and PA polymer.
SEM analysis of the films
The morphology of WPUA films was also investigated by SEM. Figure 9 shows the freeze-fractured SEM micrographs. The darker region in the figure is PU as the continuous phase, and the brighter region is PA as the dispersed phase. The morphology of the sample also changes due to different preparation methods. As shown in the figure, all the samples showed different degrees of phase separation. The phase separation phenomenon, ascribable to the incompatibility of PU and PA, can be distinctly observed in Fig. 9a. The phase separation degree of WPUA2 (Fig. 9b) with graft structure and WPUA3 (Fig. 9c) with IPN structure was significantly lower than that of WPUA1, indicating an excellent compatibility between the two compounds.
Conclusion
WPUA hybrid latexes have been synthesized using three different methods—the physical blending method, graft emulsion polymerization method, and IPN-based method. The effect of the different methods on the properties of WPUA composite emulsions and its latex films was studied. FT-IR spectra confirmed that WPUA composite emulsion was successfully prepared. TEM and particle size analysis show that the particles are spherical, and the size of the graft PUA composite is the largest. WPUA2 and WPUA3 were more hydrophobic than WPUA1, resulting in their water contact angle increasing from 77° to 87°. In particular, the graft WPUA film showed an excellent mechanical property and water resistance, indicating that the chemical bond between PU and PA played an important role in regulating the performances of WPUA films. The SEM images clearly depict the phase separation phenomenon in WPUA1. This is due to the incompatibility of PU and PA. It can be concluded that graft polymerization is an extremely efficient method for obtaining high-performance WPUA composite emulsions. The graft WPUA2 composite film has the desired mechanical properties, water resistance and thermal stability, and therefore, it is a potential candidate for coating applications.
References
Du S, Wang Y, Zhang C, Deng X, Luo X, Fu Y, Liu Y (2018) Self-antibacterial UV-curable waterborne polyurethane with pendant amine and modified by guanidinoacetic acid. J Mater Sci 53:215–229
Turri S, Levi M, Trombetta T (2004) Process design of fluorinated polyurethane-urea anionomer aqueous dispersions. Macromol Symp 218:29–38
Asif A, Hu L, Shi W (2009) Synthesis, rheological, and thermal properties of waterborne hyperbranched polyurethane acrylate dispersions for UV curable coatings. Colloid Polym Sci 287:1041–1049
Lee BS, Chun BC, Chung YC, Sul KI, Cho JW (2001) Structure and thermomechanical properties of polyurethane block copolymers with shape memory effect. Macromolecules 34:6431–6437
Wang C, Li X, Du B, Li P, Lai X, Niu Y (2014) Preparation and properties of a novel waterborne fluorinated polyurethane–acrylate hybrid emulsion. Colloid Polym Sci 292:579–587
Ma L, Song L, Li F, Wang H, Liu B (2017) Preparation and properties of poly (propylene carbonate)-based waterborne polyurethane-acrylate composite emulsion. Colloid Polym Sci 295:2299–2307
Athawale VD, Kulkarni MA (2009) Preparation and properties of urethane/acrylate composite by emulsion polymerization technique. Prog Org Coat 65:392–400
Alvarez GA, Fuensanta M, Orozco VH, Giraldo LF, Martín-Martínez JM (2018) Hybrid waterborne polyurethane/acrylate dispersion synthesized with bisphenol A-glicidylmethacrylate (Bis-GMA) grafting agent. Prog Org Coat 118:30–39
Chai SL, Jin MM, Tan HM (2008) Comparative study between core–shell and interpenetrating network structure polyurethane/polyacrylate composite emulsions. Eur Polym J 44:3306–3313
Yang C, Castelvetro V, Zhang Y, Hu C (2012) Facile hydrophobic modification of hybrid poly (urethane-urea) methacrylate aqueous dispersions and films through blending with novel waterborne fluorinated acrylic copolymers. Colloid Polym Sci 290:1–506
Imane B, Marie-Anne D, Hervé D (2017) Preparation of porous polyurethanes by emulsion-templated step growth polymerization. Polymer 132:243–251
Okamoto Y, Hasegawa Y, Yoshino F (1996) Urethane/ acrylic composite polymer emulsions. Prog Org Coat 29:175–182
Jingqiang S, Yafeng Z, Jindong Q, Jianzheng K (2004) Core-shell particles with an acrylate polyurethane core as tougheners for epoxy resins. J Mater Sci 39:6383–6384
Jiang M, Zheng ZH, Ding XB, Cheng X, Peng YX (2007) Convenient synthesis of novel fluorinated polyurethane hybrid latexes and core-shell structures via emulsion polymerization process with self-emulsification of polyurethane. Colloid Polym Sci 285:1049–1054
Deng YJ, Zhou C, Zhang MY, Zhang HX (2018) Effects of the reagent ratio on the properties of waterborne polyurethanes acrylate for application in damping coating. Prog Org Coat 122:239–247
Kim BK, Shin JH (2002) Modification of waterborne polyurethane by forming latex interpenetrating polymer networks with acrylate rubber. Colloid Polym Sci 280:716–724
Chen S, Chen L (2003) Structure and properties of polyurethane/polyacrylate latex interpenetrating networks hybrid emulsions. Colloid Polym Sci 282:14–20
Hourston DJ, Schäfer FU (1996) Poly (ether urethane)/poly (ethyl methacrylate) interpenetrating polymer networks: morphology, phase continuity and mechanical properties as a function of composition. Polymer 37:3521–3530
Sperling LH (1997) Polymeric multicomponent materials: an introduction. Wiley-Interscience
Wang Y, Qiu F, Xu B, Xu J, Jiang Y, Yang D, Li P (2013) Preparation, mechanical properties and surface morphologies of waterborne fluorinated polyurethane-acrylate. Prog Org Coat 76:876–883
Yuan CD, Chen GH, Zhang XX, Li ZJ, Liu ZM (2016) Method for preparing aqueous polyacrylate modified polyurethane dispersions. USP 9 234 068 B2
Yuan CD, Chen GH, Zhang XX, Li ZJ, Liu ZM (2011) Method for preparing aqueous polyacrylate modified polyurethane dispersions. CN 201010288586.8
Mehravar S, Ballard N, Veloso A, Tomovska R, Asua JM (2018) Toward a green synthesis of polyurethane/(meth) acrylic dispersions through control of colloidal characteristics. Langmuir 34:11772–11783
Mehravar S, Ballard N, Tomovska R et al (2019) The influence of macromolecular structure and composition on mechanical properties of films cast from solvent-free polyurethane/acrylic hybrid dispersions. Macromol Mater Eng 304:1900155
Mehravar S, Ballard N, Agirre A et al (2018) Role of grafting on particle and film morphology and film properties of zero VOC polyurethane/poly (meth) acrylate hybrid dispersions. Macromol Mater Eng 304:1800532
Mehravar S, Roschmann KJ, Arocha PU et al (2019) Correlating microstructure and performance of PU/(meth) acrylic hybrids as hardwood floor coating. Prog Org Coat 131:417–426
Chai SL, Jin MM (2009) Morphology and particle size of nanograde polyurethane/polyacrylate hybrid emulsions. J Appl Polym Sci 114:2030–2035
Dong A, An Y, Feng S, Sun D (1999) Preparation and morphology studies of core-shell type waterborne polyacrylate–polyurethane microspheres. J Colloid Interface Sci 214:118–122
Dong A, Wan T, Feng S, Sun D (1999) IR spectra studies of core-shell type waterborne polyacrylate-polyurethane picroemulsions. J Polym Sci B Polym Phys 37:2642–2650
Xu J, Cai X, Shen F (2016) Preparation and property of UV-curable polyurethane acrylate film filled with cationic surfactant treated graphene. Appl Surf Sci 379:433–439
Lu Y, Larock RC (2007) New hybrid latexes from a soybean oil-based waterborne polyurethane and acrylics via emulsion polymerization. Biomacromolecules 8:3108–3114
Kurimoto Y, Takeda M, Koizumi A, Yamauchi S, Doi S, Tamura Y (2000) Mechanical properties of polyurethane films prepared from liquefied wood with polymeric MDI. Bioresour Technol 74:151–157
Ates B, Koytepe S, Karaaslan MG, Balcioglu S, Gulgen S (2014) Biodegradable non-aromatic adhesive polyurethanes based on disaccharides for medical applications. Int J Adhes Adhes 49:90–96
Yu F, Cao L, Meng Z, Lin N, Liu X (2016) Crosslinked waterborne polyurethane with high waterproof performance. Polym Chem 23:3913–3922
Packham DE (2003) Surface energy, surface topography and adhesion. Int J Adhes Adhes 23:437–448
Urayama K, Takashi Miki TT, Kohjiya S (2004) Damping elastomer based on model irregular networks of end-linked poly (dimethylsiloxane). Chem Mater 16:173–178
Santos CC, Delpech MC, Coutinho FMB (2009) Thermal and mechanical profile of cast films from waterborne polyurethanes based on polyether block copolymers. J Mater Sci 44:1317–1323
Coutinho FMB, Delpech MC, Alves TL, Ferreira AA (2003) Degradation profiles of cast films of polyurethane and poly (urethane-urea) aqueous dispersions based on hydroxyterminated polybutadiene and different diisocyanates. Polym Degrad Stab 81:17–27
Jung DH, Kim EY, Kang YS, Kim BK (2010) High solid and high performance UV cured waterborne polyurethanes. Colloids Surf A: Physicochem Eng Aspects 370:58–63
García-Pacios V, Iwata Y, Colera M, Martín-Martínez JM (2011) Influence of the solids content on the properties of waterborne polyurethane dispersions obtained with polycarbonate of hexanediol. Int J Adhes Adhes 31:787–794
Fu H, Wang Y, Chen W, Zhou W, Xiao J (2015) A novel silanized CoFe2O4/fluorinated waterborne polyurethane pressure sensitive adhesive. Appl Surf Sci 351:1204–1212
Koberstein JT, Galambos AF, Leung LM (1992) Compression-molded polyurethane block copolymers. 1. Microdomain morphology and thermomechanical properties. Macromolecules 25:6195–6204
Acknowledgments
The financial supports of Changchun Provincial Science and Technology Department (No. 18DY014) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 75 kb)
Rights and permissions
About this article
Cite this article
Deng, Y., Zhou, C., Zhang, Q. et al. Structure and performance of waterborne polyurethane-acrylate composite emulsions for industrial coatings: effect of preparation methods. Colloid Polym Sci 298, 139–149 (2020). https://doi.org/10.1007/s00396-019-04583-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-019-04583-6