Abstract
Environmental considerations have been the cause of increasing research and development of waterborne polymer systems for many different applications, particularly as coatings for several kinds of substrates. In this work, waterborne polyurethanes (WPU) based on block copolymers of ethylene glycol and propylene glycol (EG-b-PG), with 25% of EG segments, poly(propylene glycol), dimethylolpropionic acid, isophorone diisocyanate, and hydrazine, as chain extender, were obtained in the absence of organic solvent. Thermal stability, by thermogravimetry, and mechanical properties of cast films obtained from the aqueous dispersions were evaluated. The degradation process started above 200 °C for all samples. It was verified that the block copolymer improved the thermal resistance but diminished the mechanical resistance of the WPU materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Waterborne polyurethanes (WPU) have been increasingly employed in a wide range of applications due to environmental considerations since these materials do not contribute to the increasing amount of solvent emissions to atmosphere [1]. Nowadays, a great interest on the research and development of WPU has been rising in many industrial segments, especially in footwear, because those formulations offer an efficient alternative to solvent-based adhesives [2]. Polyurethane (PU) chains can also be tailor-made and, as aqueous systems, can present similar features related to conventional solvent-borne coatings and many technological advantages, such as low viscosity and good applicability as coatings for different types of substrates [3].
Polyurethanes, in general, are formed by low-polarity flexible segments and rigid domains containing urethane groups, which are polar and capable of hydrogen bonding [4]. The soft or flexible segments impart the elastomeric character to PU chains and are commonly formed by low-molecular weight polyols. The hard or rigid segments, which are in a glass or semicrystalline state, provide dimensional stability by acting as thermally reversible and multifunctional physical crosslinks and also as reinforcing fillers. They are formed by urethane groups originated from diisocyanates and may also contain urea linkages if, for example, a low-molecular weight diamine (chain extender) is used in the synthesis [5].
Conventional PUs are insoluble in water [6]. Thus, to disperse the chains in water some modifications in the polymer chain are necessary, as the introduction of ionic groups and/or hydrophilic segments [7]. Recent studies [2, 8, 9] demonstrated that the PU ionomers properties are influenced by the ionic groups and/or hydrophilic segments content, the hard/soft segment molar ratio, the type of chain extender, among other factors, since they determine the hard and soft segment interactions.
Thermogravimetry (TG) is a suitable method to evaluate the thermal properties of polymers. The thermal stability of PUs and poly(urethane-urea)s has been extensively studied because of the great importance of this class of materials. It was proposed in the literature that the thermal degradation of PU is primarily a depolycondensation process, which starts at about 250 °C, depending on the type of segments attached to urethane linkages. Commonly, mass loss vs. temperature curves present a two-stage profile where the first one is related to the hard segments whereas the second correspond to the flexible ones. In general, poly(urethane-urea)s are more thermally stable than PUs [5, 9, 10].
The objective of this work was to evaluate the degradation profile and mechanical resistance of cast films obtained from PU aqueous dispersions containing anionic groups and hydrophilic segments. The formulations included block copolymers based on ethylene glycol and propylene glycol (EG-b-PG), with EG (hydrophilic) content of 25%, poly(propylene glycol) (PPG), dimethylolpropionic acid (DMPA), and isophorone diisocyanate (IPDI). The chain extender employed was hydrazine (HYD), which led to the formation of poly(urethane-urea)s. The effect of the amount of hydrophilic segments and hard domains in the thermal and mechanical properties were discussed.
Experimental
Reagents
The following reagents were used as received and the information supplied by the manufacturers were: block copolymers based on ethylene glycol and propylene glycol (EG-b-PG) Polyglycol 149 (Mn = 2350 g/mol; EG content = 25%; hydroxyl number = 47.7 mg/g), Dow Química; dimethylolpropionic acid (DMPA), Aldrich; hydrazine (HYD), Bayer; isophorone diisocyanate (IPDI), Hülls; poly(propylene glycol) (PPG) Voranol 2110 (Mn = 1300 g/mol, hydroxyl number = 106.50 mg/g), Dow Química; and triethylamine (TEA), Vetec.
Synthesis
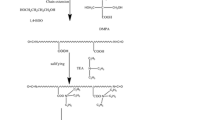
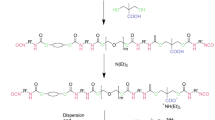
Prepolymers were prepared in bulk at 90 ± 10° C by reacting polyols (copolymer and/or PPG), DMPA, and IPDI for about 20 min. The DMPA carboxylic groups were neutralized by reacting with TEA at 30 °C; afterward, the viscous mixture was dispersed in water, under high agitation rate. The last stage is the chain extension reaction with hydrazine at 35 °C (Fig. 1).
In PU formulations obtained, the ratio between the number of equivalent-grams of isocyanate groups and hydroxyl (PPG, EG-b-PG, and DMPA) total compounds (NCO/OH) was varied as 1.7, 2.0, and 2.5. The amount of chain extender employed was proportional to the respective ratio, in order to attain the reaction of all NCO end groups by forming urea linkages. Hydrophilic segments content was varied in terms of equivalent-gram ratio between the polyols [PPG/(EG-b-PG)]. The equivalent-gram ratio between polyols and DMPA were kept constant in all formulations (1:1). Aqueous dispersions with nearly 40% of solid content were obtained.
Film preparation
Cast films were prepared by adding 20 mL of the aqueous dispersions on leveled Teflon surfaces and allowing them to dry at room temperature and atmospheric pressure for 7 days. The dispersions were also cast on glass, wood, and aluminum surfaces in order to observe their adhesion behavior on those substrates.
TG analysis
Thermogravimetric experiments were performed in a TA Instruments TG 50 Analyser. Film samples (2–4 mg) were placed in a platinum sample pan and heated from 10 to 700 °C under N2 atmosphere (100 mL/min), at a heating rate of 10 °C/min. Aluminum, nickel, and perkalloy were used as standards. During the heating period, the weight loss temperature dependence (TG curves) and the correspondent derivative curves (DTG) were recorded.
Mechanical analysis
The standard tensile test performed was an adaptation of ASTM-D 638-91 method. Tensile test bars (5 × 70 mm2) were cut from PU films, with thickness varying from 0.2 to 0.8 mm. A tensile test machine (INSTRON 5565), equipped with a 100 N load cell and pneumatic grips, was used at crosshead speed of 500 mm/min [3, 5, 9].
Results and discussion
The introduction of the block copolymer with hydrophilic segments (EG) was meant in order to make easier the dispersion of the viscous bulk and also to increase the stability of the solid particles in the aqueous medium. Surprisingly, however, it was observed that the incorporation of high quantities of the copolymer did not produce the desired effects. By increasing the copolymer content, the difficulty in dispersing the organic bulk also increased. The resultant products containing high amounts of hydrophilic groups were gels. The hydrophilic segments improved the swelling behavior enlarging the particle size.
Films characteristics
The insertion of EG-b-PG segments in PU chains led to the formation of transparent and homogeneous films. The formulations having NCO/OH ratio = 1.7 (the lowest content of urethane and urea groups) produced tacky films. The samples obtained employing NCO/OH ratio = 2.0 produced flexible films with low tack. On the other hand, the highest NCO/OH ratio = 2.5 led to rigid films without tack. All the aqueous dispersions were cast on glass, wood, and aluminum surfaces, and formed homogeneous and bright coatings.
Thermogravimetric analysis
Polyurethanes are segmented copolymers, consisting of alternating soft and hard segments [11, 12]. The thermal stability of these polymers is generally not high, starting generally above 200 °C, and the degradation mechanism is very complex due to the variety of products formed in the process. Usually, at a low heating rate, the degradation process results in differential weight loss curves (DTG) with several peaks, which are indicative of the complexity of the degradation process. Those peaks correspond to the temperatures at maximum rate of weight loss of the corresponding step [5, 13].
In the present study, the onset degradation temperatures (Tonset) of the polyols (Table 1) [13] and PUs (Table 2) were defined as the initial temperature of degradation, corresponding to the intercept of the tangent drawn at the inflection point of the decomposition step with the horizontal zero line of the TG curve. Table 1 shows the TG parameters obtained for the polyols.
The onsets observed in the degradation process for WPUs are listed in Table 2. It can be observed that, in general, the presence of low contents of block copolymer in the formulation improved the thermal resistance of the WPU samples and an increase in urethane and urea linkages (higher NCO/OH ratios) resulted in a decrease of the thermal stability [5]. Taking as an example, NCO/OH ratio = 2.5, the sample in which no copolymer was added showed a Tonset of 212 °C, while the sample synthesized with 30% of copolymer presented a Tonset of 234 °C. The PUs prepared employing NCO/OH = 2.0 were the most stable ones produced in this work.
TG curves for some formulations are presented in Figs. 2 and 3 and the corresponding DTG curves are depicted in Figs. 4 and 5. TG curves show two discrete stages of degradation, with very close profiles. In general, PUs present a two or three degradation-step profile, which is clearly distinguished in DTG curves. The initial thermal decomposition occurs in the hard segments (urethane and urea linkages). The subsequent stages of degradation occur in the soft segments formed by the polyols, the most resistant ones as related in literature [5, 13–15].
Figure 2 shows the influence of the block copolymer with 25% of hydrophilic segments (EG) on thermal stability at NCO/OH ratio = 2.5. The first step, which is very discrete, occurs in the rigid segments, and starts at 220 °C. The evolution of the degradation shows that the introduction of the block copolymer tends to improve the thermal resistance of the WPUs. PEG segments present linear chains, i.e., without any pendant group, which facilitates packing and, consequently, significant interactions between chains occur. On the other hand, by reducing the copolymer content an increase in PPG homopolymer content is observed. These chains, and also PG segments present on the block copolymer, contain tertiary carbons, which are more thermally unstable, reducing thermal stability when compared to EG segments. Madorsky and Straus [14] showed that the thermal stability of PEG is higher than PPG because the carbon–carbon bond strength diminishes with the substitution of alkyl group for hydrogen. In other study, Wang and Hsieh [15] reported that thermal stability increased in the order PPG < PEG. Weight loss started at lower temperatures and was faster in PPG as compared to PEG.
Figure 3 shows the degradation profile of PUs, keeping constant the amount of copolymer (20%) and increasing the NCO/OH ratio. It was observed that the thermal stability of WPUs showed a slight tendency to decrease (as expected) as the rigid segments content increased [5, 13].
Figures 4 and 5 present the DTG curves for some WPUs samples. The maximum values in the curves correspond to the temperature of the highest rate of weight loss in the respective step of degradation. The curves show that there are different subtle stages of degradation, which are not detected visually in TG curves.
Figure 4 presents DTG curves similar to the TG curves depicted in Fig. 2, showing clearly two steps of degradation. The first maximum, around 250 °C, represents the evolution of urethane and urea groups degradation. The second one shows the progress of flexible segments degradation exhibiting a maximum around 360 °C (Table 2) [5, 13]. It can be observed that the degradation of hard segments shows some discrete differences in all samples. The profile of the curves suggests that the presence of the block copolymer exerted a discrete influence on the degradation steps of both hard and soft segments, being observed higher thermal stabilities in the samples containing copolymer EG-b-PG.
Figure 5 presents DTG curves similar to the TG curves shown in Fig. 3. The temperature at maximum rate of weight loss for the sample with the lowest content of hard segments (NCO/OH = 1.7) is about 384 °C. As NCO/OH ratio increased, these temperatures decreased to nearly 360 °C (Table 2). This behavior shows that the urethane and urea groups degradation step also affected the degradation progress of flexible segments, showing a relationship and a mutual influence between the degradation process of hard and soft segments.
Mechanical evaluation
The mechanical properties of the PUs are directly related to the amount of hard segment domains [16]. Chattopadhyay and Raju [17] showed that the hard segments structure and its content in the material are very important parameters and largely affect the morphology, thermal, and mechanical behavior as well as the performance of segmented PUs coatings. Wang and Cooper [18] observed that the mechanical properties of polyether PUs depend primarily on the hard segment content.
While the rigid segments improve the mechanical resistance, the flexible segments impart the elastomeric properties to the chains [19]. The chemical composition and molecular weight distribution (MWD) of the incorporated soft block influence the macroscopic properties of the resulting materials [17, 20].
Table 3 shows the data obtained in the mechanical analysis of cast films. In general, it can be observed that the incorporation of EG-b-PG segments in the PU chains showed a tendency to decreasing both the elongation and the tensile strength at break. These results suggest that the introduction of EG-b-PG led to a decrease in the mechanical resistance, but diminishing also its elasticity. The incorporation of the copolymer on the material did improve neither mechanical resistance nor elasticity. A decrease in the elongation at break, as EG-b-PG segments were incorporated, suggests that EG segments should provide some organization within soft segments by reducing their flexibility [21–23].
However, the highest NCO/OH ratio (2.5) produced a material with higher tensile strength at break. This behavior can be attributed to the increase of rigid segments in the PU resulting, consequently, in a higher degree of hydrogen-bonding interactions between the chains [16]. Apparently, a synergistic effect among the hard and hydrophilic part of the soft segments imparted more rigidity to the materials.
By comparing the results of mechanical and thermal properties (Tables 2 and 3), it is possible to observe that NCO/OH = 1.7 produced the poorest films, concerning those properties, and highly unstable dispersions in terms of sedimentation. On the other hand, the NCO/OH = 2.5 produced the most stable dispersions but their cast films were very brittle resulting in a decrease of elongation at break and also in the mechanical resistance. However, formulations produced at an intermediate (2.0) NCO/OH ratio resulted in soft films with good mechanical and thermal properties. This behavior should be attributed to an adequate balance between the rigid (urethane and urea groups) and the soft (polyols) segments.
Conclusion
The method of synthesis employed in this work produced efficiently WPUs based on polyether block copolymer (EG-b-PG) and led to the formation of transparent and homogeneous cast films with good adherence on glass, wood, and aluminum. The tack on the surface of the films decreased as the rigid segments content increased. Thermal degradation of the materials occurred in two stages, the first one being very discrete and attributed to the hard segments degradation and the second one to the soft segments decomposition. The thermal resistance was improved with the introduction of the block copolymer (EG-b-PG) and decreased with the increase of the urethane and urea linkages. The DTG curves showed that the hard segment and copolymer contents affected the thermal profile of the samples as a whole, the fact that was not perceptible in TG curves, showing a relationship and mutual influence between degradation process of hard and soft segments. An increase in rigid segments amount provoked an increase in the mechanical resistance and a decrease in elongation at break. This behavior was probably due to higher quantities of urethane and urea linkages on the PU backbone and, consequently, to a higher degree of hydrogen-bonding interactions among the PU chains. However, the incorporation of hydrophilic segments promoted a decrease in both the tensile strength and the elongation at break, probably due to the increase in the rigidity as a whole. Formulations synthesized with NCO/OH = 2.0 produced materials with the best properties.
References
Ebrahimi MV, Barikani M, Mohaghegh SMS (2006) Iran Polym J 15:323
Pérez-Limiñana MA, Arán-Aís F, Torró-Palau AM, Orgilés-Braceló AC, Martín-Martínez JM (2005) Int J Adhes Adhes 25:507
Coutinho FMB, Delpech MC, Garcia MEF (2002) Polym Test 21:719
Mequanint K, Sanderson R (2006) Eur Polym J 42:1145
Coutinho FMB, Delpech MC (2000) Polym Degrad Stab 70:49
Mohaghegh SMS, Barikani M, Entezami AA (2006) Colloids Surf A 276:95
Lee H, Wu S, Jeng R (2006) Colloids Surf A 276:176
Jang JY, Jhon YK, Cheong IW, Kim JH (2002) Colloids Surf A 196:135
Delpech MC, Coutinho FMB (2000) Polym Test 19:939
Król P (2007) Prog Mater Sci 52:915
Bao L, Lan Y, Zhang S (2006) J Polym Res 13:507
Chuang FS, Tsen WC, Shu YC (2004) Polym Degrad Stab 84:69
Coutinho FMB, Delpech MC, Alves TL, Ferreira AA (2003) Polym Degrad Stab 81:19
Madorsky SL, Straus S (1959) J Polym Sci 36:183
Wang T, Hsieh T (1997) Polym Degrad Stab 55:95
Coutinho FMB, Delpech MC (1996) Polym Test 15:103
Chattopadhyay DK, Raju KVSN (2007) Prog Polym Sci 32:352
Wang CB, Cooper SL (1983) Macromolecules 16:775
Coutinho FMB, Delpech MC, Garcia MEF (2004) Polim Ciên Tecnol 14:230
Yoo S, Lee HS, Seo SW (1997) Pollimo 21:459
Coutinho FMB, Alves LS, Delpech MC (1998) Anais Assoc Bras Quím 47:255
Kim BK, Lee JC, Lee KH (1994) J Macromol Sci Pure Appl Chem A31:1241
Kim BK, Kim TK (1991) J Appl Polym Sci 43:393
Acknowledgements
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) for financial support and Centro Técnico Aeroespacial/Instituto de Aeronáutica e Espaço (CTA/IAE) and Dow Química for the donation of IPDI and polyethers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santos, C.C., Delpech, M.C. & Coutinho, F.M.B. Thermal and mechanical profile of cast films from waterborne polyurethanes based on polyether block copolymers. J Mater Sci 44, 1317–1323 (2009). https://doi.org/10.1007/s10853-009-3272-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-009-3272-7