Abstract
It is worth looking forward to manufacturing a shape memory hydrogel (SMH) with good mechanical properties for practical applications in a simple and rapid manner. In this study, the one-pot method has been proposed to prepare a quadruple shape memory hydrogel (PAA-CS, poly(acrylic acid)-chitosan). The PAA-CS hydrogel obtained by mixing acrylic acid (AA), chitosan (CS), initiator, and cross-linker at 60 °C shows excellent function of shape memory and recovery less than 20 s. Moreover, its maximum deformation can reach to 100%. Scanning electron microscopy (SEM) showed that PAA-CS hydrogels would form new cross-linked networks in NaOH, NaCl, and FeCl3, and these new networks can trigger quadruple shape memory. In addition, the tensile and compressive strength tests showed the PAA-CS hydrogel possesses outstanding mechanical properties. Its excellent property has enormous potential in applications where multi-shape changes responding to different stimuli are required.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As one of the most important stimulus-responsive materials, shape memory polymers (SMPs) have attracted increasing interest over the past few decades due to wide range of potential applications from biomedicine to textiles. Initial SMPs can only remember one temporary shape in each shape memory cycle, resulting in dual shape memory effect [1,2,3,4,5,6]. Since the potential application of SMPs is determined normally by the number of temporary shapes that can be fixed, increasing attention has thus been paid to constructing multi-SMPs which could stabilize two or more temporary shapes [7,8,9,10,11]. However, most of the multi-SMPs are thermoresponsive polymers, in which temporary shapes are fixed by vitrification or crystallization of switching domains, and the shape recovery is induced by heat [12,13,14,15]. Heat is not a convenient stimulus in practical biomedical and textile applications; other external stimuli such as light, electricity, magnetic field, and chemicals have become increasingly attractive because of owning to corresponding shape memory ability at ambient temperature [16,17,18,19].
Nevertheless, it is still challenging to design and fabricate SMPs with two or more non-interfering reversible interactions. In order to achieve simple multi-shape memory, more non-interfering and reversible interactions should be explored by new ways. Hu et al. [20] reported a new SMP which maintains the thermal-induced shape switchable effect originally existing in the polymer matrix and simultaneously possesses water-induced shape memory effect due to the percolation network of the cellulose whiskers whose hydrogen bonding can be regulated by water reversibly.
Another effective strategy to establish two or more non-interfering reversible interactions is via chemical or physical interactions. Ladet et al. [21] reported that chitosan can form a kind of chain-entangled structure in NaCl and a kind of microcrystalline structure in NaOH; furthermore, changes above are noninterference and reversible interaction. Liu et al. have reported the shape memory hydrogel based on imidazole–zinc ion coordination for the first time, which was based on the property that imidazole groups can bind strongly to zinc ions [22, 23]. Their work points out a new direction to design SM hydrogels. It is well-documented that a variety of metal ions are able to strongly chelate amino and carboxyl groups at low concentrations. These pioneering works are tremendously useful to inspire the design of novel SMPs on the basis of supramolecular interactions.
Herein, a novel hydrogel (PAA-CS, poly(acrylic acid)-chitosan) with multi-responsive and quadruple shape memory properties was synthesized by free radical polymerization of acrylic acid (AA) in the presence of biopolymer (Scheme 1). PAA-CS hydrogel involves three reversible interactions including the crystallites and chain entanglement structure of chitosan and metal coordination interactions. The quadruple shape memory is affected by three different types of athermal stimuli. This research may provide a new strategy for designing multi-responsive SMPs and broaden the application of SMPs further.
Materials and methods
Materials
Chitosan (deacetylation, 80~95%), acetic acid, acrylic acid, hydrochloric acid (HCl), calcium chloride (CaCl2), potassium chloride (KCl), ferric chloride (FeCl3), potassium nitrate (KNO3), sodium chloride (NaCl), ammonium persulfate (APS), N,N′-methylenebisacrylamide (MBA), and sodium hydroxide were purchased from Chengdu Kelong Chemical Reagent Factory (Sichuan, China).
Characterization
Scanning electron microscopy (SEM) measurement was conducted with a Hitachi S4800 microscope. The tensile and compression tests were conducted on a tensile-compressive tester (Instron 5567, Instron, Norwood, MA, USA). The samples for the tensile tests were prepared in a dumbbell shape 40 mm in length and 4 mm in width and were then measured at an extension rate of 100 mm/min until snapped. The samples for the compression tests were prepared in a cylinder shape with a length of 20 mm and a diameter of 14 mm and were measured at a compression rate of 10% original height/min and the final compressive strain is 90%.
Preparation of the PAA-CS
The chitosan solution (2%,w/v) is prepared by dissolving a certain amount of chitosan in acetic acid (2%,w/v) about 12 h. PAA-CS was prepared by mixing chitosan solution, AA (5 g), N′N-methylenebisacrylamide (0.01~0.02 g), ammonium persulfate (0.6 g), and deionized water. The mixture was polymerized at 65 °C for 1 h.
The shape fixity ratio
The quantitative shape memory property was determined according to the report method [24,25,26]. The shape fixity ratio (Rf) and shape recovery ratio (Rr) were defined by the following equation:
where, θd is the deformed angle, θt is the temporarily fixed angle, and θf is the final angle.
Results and discussion
The shape memory property of PAA-CS
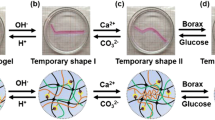
As show in Fig. 1a, the amine groups of chitosan would deprotonate under alkaline conditions and it could be induced to generation of the reversible CS microcrystalline cross-links. And the physical microcrystalline cross-linking points of PAA-CS hydrogel will be destroyed in HCl. In addition, CS chains can self-assemble to form chain entanglement structures under the treatment of NaCl solution. The second cross-linked network will be unlocked in pace with dipping PAA-CS hydrogel in deionized water because NaCl diffuses into the water [21, 26].
a The mechanisms of the shape memory hydrogel based on two physical interactions. The reversible CS microcrystalline cross-links and CS chain entanglement endowed the hydrogel with a programmable multiple shape memory ability. b The mechanisms of the shape memory hydrogel based on a chemical interactions. The reversible chemical interaction of metal ions with carboxyl groups
Fe3+ easily complexed with the carboxyl group to create a new temporary network which could be destroyed because iron ions would react with OH− to form Fe(OH)3 after the sample was transferred into NaOH solution (Fig. 1b) [6, 27, 28].
The microcrystal structure of chitosan
As shown in Fig. 2a, the shape of sample changed from straight line to “U” type under an external force. Moreover, in saturated NaOH solution, the “U” type can be fixed within 15 s due to the rapid formation of physical microcrystalline cross-link structure, and the shape fixity ratio can reach 100%. The gel will quickly return to the original shape (straight line) in HCl as a result of the destroyed microcrystalline physical cross-linking points.
The shape memory ability of PAA-CS hydrogel in NaOH solution is investigated. Figure 3 shows the shape fixity ratio of PAA-CS hydrogel changes with the following factors. First, as the immersing time increases, the rate of deformation increases. Second, the shape fixity ratio decreases with the content of cross-linkers (MBA) more than 0.01 g. Superfluous MBAs will lead to excessive cross-linking and over-concentration of the three-dimensional network structure, so CS cannot fully react with NaOH, which reduces the deformation rate of the sample [29]. Third, the deformation rate is maximum when initiator (APS) content is about 0.6 g. The APS content less than 0.6 g would lead to a shortage of free radicals which caused PAA network to loosen, and then PAA-CS hydrogel is low in strength, therefore it cannot fix shape effectively. On the other hand, large amounts of free radicals could be produced in the polymerization process when content of initiators is higher than 0.6 g; it will lead to excessive cross-linking of AA. Therefore, NaOH cannot react with CS, which has a negative effect on the shape fixity ratio [30]. Last, higher concentration of NaOH is beneficial to the greater rate of deformation. With increasing concentration of NaOH solution, concentration difference increased and NaOH could easily get into PAA-CS hydrogel, leading to the improvement of shape fixity ratio.
The chain entanglement structure of chitosan
Reversible arranging and disassociating process of the physical chain entanglement networks endow the hydrogel shape memory property. As shown in Fig. 2b, the straight samples were respectively deformed into a “U” shape under external stress and fixed by the CS chain physical entanglement cross-link junctions in NaCl, and this process only takes 3 min and the shape fix rate can reach about 70%. When they were immersed into deionized water, CS chain entanglement dissociates due to NaCl diffusion into the water, and temporary shapes would be recovered to the original straight shapes.
It can be seen from Fig. 4, when PAA-CS hydrogels are soaked in NaCl solution, the factors affecting the shape fix rate are similar to those of NaOH solutions. In addition, the deformation rate of PAA-CS hydrogel can reach the maximum value of 73% when the content of MBA is 0.02 g and APS is 0.6 g.
The complexation of metal ions with carboxyl groups
Metal ion easily complexed with the carboxyl group to create a new temporary network which can provide shape memory properties for the PAA-CS hydrogel. Figure 2c shows that a straight hydrogel sample was bent to the “U” shape and immersed into the saturated FeCl3 solution, and the temporary shape was fixed within 15 s and the shape fixity ratio can reach 100%. The temporary cross-linked network could be destroyed and PAA-CS hydrogel quickly reverts to permanent shape because iron ions would complex with EDTA after the sample was transferred into EDTA solution.
Similar to the above two physical changes, the shape memory ability of the PAA-CS gel obtained from the complexation of Fe3+ with a carboxyl groups is also affected by the concentration of FeCl3 solution, immersion time, and content of MBA and APS. As shown in Fig. 5, PAA-CS hydrogel has the strongest shape memory ability when the content of MBA is 0.01 g and APS is 0.6 g respectively.
The multi-responsive properties of PAA-CS hydrogel
According to the formation mechanism of the CS physical entanglement cross-link structure, the physical microcrystalline cross-linked structure, and the network of carboxyl groups complexed with metal ions, it is envisioned that PAA-CS hydrogel not only responds to NaOH, NaCl, and FeCl3, but also forms a shape memory effect in other strong alkali solution, salt solution, and metal ion solution. As shown in Fig. 6, PAA-CS hydrogel can also be fixed shape in KOH, KCl, KNO3, NaNO3, ZnCl2, AgNO3, and CaCl2 solutions. The shape memory ability varies dramatically in different solutions. The degree of deformation is about 75% in KOH, 52% in KCl, 27% in KNO3, and 70% in CaCl2. Obviously, the shape fixity ratio is the highest when PAA-CS hydrogel was immersed in NaOH, NaCl, and FeCl3 solutions.
The quadruple shape memory property of PAA-CS hydrogel
Combining the shape memory capabilities of the above PAA-CS hydrogels can provide it with quadrilateral shape memory ability. As shown in Fig. 7, we take a long strip of sample and apply a continuous external force to bent its shape to α, then soaked it in NaOH solution to fixed shape. After that, changed shape α to β and keep the shape in β by immersing in NaCl solution. Next, the shape of the sample changed from type β to γ under an external force, and the shape would be fixed under the treatment of FeCl3 solution. Finally, the gel was sequentially placed in EDTA solution, deionized water, and HCl; it was found that the gel was sequentially restored to temporary shape β, temporary shape α, and permanent shape in turn.
Reusability of the PAA-CS hydrogel
Repeat the experimental steps in Fig. 7 and we can find the PAA-CS hydrogel still has not lost its original performance. So the PAA-CS hydrogel has recyclability.
The perimeter structure of PAA-CS hydrogel
In order to determine the forming of crystallites, chain entanglements, and complex network structures, the materials were subjected to electron microscopy (SEM) and tensile and compression tests.
As shown in Fig. 8, the original PAA-CS hydrogel (Fig. 8a) had smaller densities and larger pores than the hydrogel soaked in NaCl, NaOH, and FeCl3 (Fig. 8b–d). This indicates that the PAA-CS hydrogel forms a second cross-linked network after being soaked with NaCl, NaOH, and FeCl3. Conclusions are consistent with previous research [11].
The mechanical properties of PAA-CS hydrogel
The dumbbell type with a sample length of 20 mm and a width of 4 mm was selected to perform tensile tests (Fig. 9). It shows the relationship between tensile length and tensile stress of different samples. Especially, the elongation is 7.2 and the tensile stress is 0.33 MPa at break of the original PAA-CS hydrogel. The PAA-CS hydrogel reacts with NaCl, its elongation at break decreases to 4.4, and the tensile stress is 0.39 MPa. Similarly, immersed in NaOH, elongation at break of the PAA-CS hydrogel is 1.7, and the tensile stress increases to 0.42 MPa. Besides, in FeCl3 solution, the elongation at break becomes 0.7, and the tensile stress is 0.45 MPa.
It can be seen from Fig. 10 that the maximum compression ratio of the original PAA-CS hydrogel and the PAA-CS hydrogel soaked in NaCl, NaOH, and FeCl3 all can reach about 90%. Moreover, the maximum compression stress of the original PAA-CS hydrogel is about 4 MPa. Except that, the maximum compression stress of PAA-CS hydrogel soaked in NaCl solution would increase to about 5.2 MPa. Similarly, immersed in NaOH, the compression stress of PAA-CS hydrogel could be raised to 7 MPa approximately. In FeCl3, the maximum compressive stress of the PAA-CS hydrogel is close to 20 MPa.
The result of tensile and compression tests shows that PAA-CS hydrogel forms a second cross-linked network after being soaked with NaCl, NaOH, and FeCl3. The second cross-linked network not only endows PAA-CS hydrogel multiple shape memory capabilities but also improves its mechanical properties greatly.
Particularly, network formed by the complexation of iron ions with carboxyl groups gives PAA-CS the best mechanical properties and deformation effect. It shows that the cross-linked network is more close than others, which can also be seen from the cross-section SEM images. Besides, the second cross-linked network formed by PAA-CS hydrogel soaked in NaOH is tighter than that soaked in NaCl.
Conclusions
In summary, we prepared a quadruple shape memory hydrogel that can respond to environmental conditions via a simple one-pot process. The PAA-CS hydrogel which can be recycled owned excellent shape memory ability and mechanical properties. Simultaneously, there are several advantages, such as low cost, mild synthetic conditions, and environment friendly. It is potential for application in the areas of soft tissue of robots and arm bearing.
References
Li X, Serpe MJ (2016) Understanding the shape memory behavior of self-bending materials and their use as sensors. Adv Funct Mater 26:3282–3290
Li Z, Zhang XY, Wang SQ, Yang Y, Qin BY, Wang K, Xie T, Wei Y, Ji Y (2016) Polydopamine coated shape memory polymer: enabling light triggered shape recovery, light controlled shape reprogramming and surface functionalization. Chem Sci 7:4741–4747
Dong W (2013) Multiple-stimulus-responsive hydrogels of cationic surfactants and azoic salt mixtures. Colloid Polym Sci 291(12):2935–2946
Li Y, Chen HM, Liu D, Wang WX, Liu Y, Zhou SB (2015) pH-responsive shape memory poly(ethylene glycol)–poly(ε-caprolactone)-based polyurethane/cellulose nanocrystals nanocomposite. ACS Appl Mater Interfaces 7:12988–12999
Zhao Q, Qi HJ, Xie T (2015). Prog Polym Sci 49–50:79–120
Lu W, Le X, Zhang J et al (2017) Supramolecular shape memory hydrogels: a new bridge between stimuli-responsive polymers and supramolecular chemistry. Chem Soc Rev 46(5):1284–1294
Razzaq MY, Behl M, Kratz K, Lendlein A (2013) Triple-shape effect in polymer-based composites by cleverly matching geometry of active component with heating method. Adv Mater 25:5514–5518
Shao Y, Lavigueur C, Zhu XX (2012) Multishape memory effect of norbornene-based copolymers with cholic acid pendant groups. Macromolecules 45:1924–1930
Xiao YY, Gong XL, Kang Y, Jiang ZC, Zhang S, Li BJ (2016) Light-, pH-and thermal-responsive hydrogels with the triple-shape memory effect. Chem Commun 52(70):10609–10612
Le X, Lu W, Zheng J et al (2016) Stretchable supramolecular hydrogels with triple shape memory effect. Chem Sci 7(11):6715–6720
Xiao H, Lu W, Le X et al (2016) A multi-responsive hydrogel with a triple shape memory effect based on reversible switches. Chem Commun 52(90):13292–13295
Hao X, Liu H et al (2013) Thermal-responsive self-healing hydrogel based on hydrophobically;modified chitosan and vesicle. Colloid Polym Sci 291(7):1749–1758
Peng K, Yu H, Yang H, Hao X, Yasin A, Zhang X (2017) A mechanically robust hydrogel with thermally induced plasticity and a shape memory effect. Soft Matter 13(11):2135–2140
Lu X, Chan CY, Lee KI, Ng PF, Fei B, Xin JH, Fu J (2014) Super-tough and thermo-healable hydrogel–promising for shape-memory absorbent fiber. J Mater Chem B 2(43):7631–7638
Fan Y, Zhou W, Yasin A, Li H, Yang H (2015) Dual-responsive shape memory hydrogels with novel thermoplasticity based on a hydrophobically modified polyampholyte. Soft Matter 11(21):4218–4225
Yang X, Zhou L, Lv L et al (2016) Multi-stimuli-responsive poly (NIPA-co-HEMA-co-NVP) with spironaphthoxazine hydrogel for optical data storage application. Colloid Polym Sci 294(10):1–10
Xu B, Zhang YY, Liu WG (2015) Hydrogen-bonding toughened hydrogels and emerging CO2-responsive shape memory effect. Macromol Rapid Commun 36:1585–1591
Kalia S, Kango S, Kumar A, Haldorai Y, Kumari B, Kumar R (2014) Magnetic polymer nanocomposites for environmental and biomedical applications. Colloid Polym Sci 292(9):2025–2052
Jiang F, Hsieh YL (2014) Super water absorbing and shape memory nanocellulose aerogels from TEMPO-oxidized cellulose nanofibrils via cyclic freezing–thawing. J Mater Chem A 2(2):350–359
Luo H, Hu J, Zhu Y (2011) Polymeric shape memory nanocomposites with heterogeneous twin switches. Macromol Chem Phys 212:1981–1986
Ladet S, David L, Domard A (2008) Multi-membrane hydrogels. Nature 452(7183):76–79
Han Y, Bai T, Liu Y, Zhai X, Liu W (2012) Zinc ion uniquely induced triple shape memory effect of dipole-dipole reinforced ultra-high strength hydrogels. Macromol Rapid Commun 33:225–231
Nan W, Wang W, Gao H, Liu W (2013) Fabrication of a shape memory hydrogel based on imidazole–zinc ion coordination for potential cell-encapsulating tubular scaffold application. Soft Matter 9:132–137
Nan WJ, Wang W, Gao H, Liu WG (2013) Fabrication of a shape memory hydrogel based on imidazole-zinc ion coordination for potential cell-encapsulating tubular 2016 scaffold application. Soft Matter 9:132–137.[CrossRef]
Yasin A, Lu HZ, Rehman SU, Siddiqb M, Yang HY (2014) Compartmentalized multilayer hydrogel formation using a stimulus-responsive self-assembling polysaccharide. Soft Matter 10:972–977 [CrossRef]
Xiao H, Ma C, Le X et al (2017) A multiple shape memory hydrogel induced by reversible physical interactions at ambient condition. Polymers 9(4):138
Yasin A, Li H, Lu Z, Rehman S, Siddiq M, Yang H (2014) A shape memory hydrogel induced by the interactions between metal ions and phosphate. Soft Matter 10(7):972–977
Zhao L, Huang J, Zhang Y, Wang T, Sun W, Tong Z (2017) Programmable and bidirectional bending of soft actuators based on janus structure with sticky tough PAA-clay hydrogel. ACS Appl Mater Interfaces 9(13):11866–11873
Kabiri K, Omidian H, Hashemi SA, Zohuriaan-Mehr MJ (2003) Synthesis of fast-swelling superabsorbent hydrogels: effect of crosslinker type and concentration on porosity and absorption rate. Eur Polym J 39(7):1341–1348
Niki E (1990) Free radical initiators as source of water-or lipid-soluble peroxyl radicals. Methods Enzymol. Academic Press 186:100–108
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, X., Xu, S., Wang, Y. et al. A high strength hydrogel with quadruple-shape memory under the ambient condition. Colloid Polym Sci 297, 503–512 (2019). https://doi.org/10.1007/s00396-019-04475-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-019-04475-9