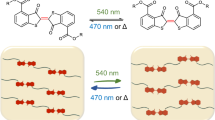
Abstract
A multi-stimuli-responsive poly(NIPA-co-HEMA-co-NVP) with spironaphthoxazine hydrogel (poly(NIPA-co-HEMA-co-NVP-co-SPO)) was prepared by radical polymerization. The structure of this hydrogel was characterized by FTIR, 13C NMR spectra, and SEM. Equilibrium swelling measurement and UV-vis spectra were applied into evaluating thermo-responsive, pH-responsive and light-responsive properties. These results exhibited poly(NIPA-co-HEMA-co-NVP-co-SPO) hydrogel with excellent stimuli-responsive characteristics including thermal, pH, and light stimulation. Meanwhile, the obvious change for internal microstructure of hydrogel was observed by SEM after UV irradiation and in acidic condition. In addition, the poly(NIPA-co-HEMA-co-NVP-co-SPO) hydrogel with good mechanical property can be utilized in erasable and rewritable photoimaging based on the photochromic effect, which makes it a potential application in rewritable optical memory media or imaging processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrogels are formed by three-dimensional hydrophilic polymers that can swell in water to an equilibrium state. They have received considerable attention because of their exceptional promise in a wide range of application [1, 2]. A special class of hydrogels, stimuli-responsive hydrogels can change their shape in a reversible and controllable way in response to stimulus like chemical, photo, thermal, or sound [3–6]. They have been extensively studied as potential candidates for applications in diverse engineering applications such as drug delivery [6, 7], actuators [8], sensors [9, 10], and smart devices [11, 12]. In recent years, significant researches have been focused on stimuli-responsive hydrogels that can respond simultaneously and independently to different stimuli [13, 14].
Among various types of smart hydrogel systems, poly(N-isopropylacrylamide) (PNIPA) gels are well-known thermo-responsive polymer systems and have been most intensively investigated recently [15–17]. Below the volume phase transition temperature (VPTT), these polymeric systems are hydrophilic, where the chains exist in coiled conformation and absorb a large amount of water. Whereas, above the VPTT, the polymer loses water and undergoes coiling to a globule transition. These unique characteristics make PNIPA-based hydrogels useful in biomedical applications.
Organic photochromic materials have attracted much attention because of their photochromic reactions which make them potentially suitable for optical storage [18, 19], chemical sensors [20], and so on. Among various types of photochromic compounds, spironaphthoxazine (SPO) and its derivates [21, 22] are well-known photochromic compounds due to their excellent photostability, compatibility with a variety of matrices, distinctive changes in structure, and absorption spectra upon irradiation. Photochromism in spironaphthoxazine compounds is attributable to the UV-induced dissociation of the heterolytic spiro C–O bond, from the oxazine ring to form a planar structure. Although, a number of attempts to incorporate spironaphthoxazine molecules into PNIPA copolymers with multi-stimuli-response have been reported [23–25]. However, the swelling properties of hydrogels with spironaphthoxazine moieties under light, heat, and proton stimuli have not been discussed deeply and need further improvement of mechanical performance to meet practical application.
Poly(hydroxyethyl methacrylate) (PHEMA) is a well-known hydrophilic polymer that exhibits good chemical stability and mechanical property due to pendant hydroxyl functionalities [26]. It has been reported as the backbone for synthesizing stimuli-responsive hydrogels [27, 28]. Meanwhile, poly(N-vinylpyrrolidone) (PNVP) is a water-soluble and biocompatible polymer, which has found numerous applications in modern material sciences and technologies [29, 30]. Therefore, the copolymer of HEMA and NVP with high swelling ratio and great mechanical behavior was widely used for the production of smart materials in industrial and medical fields.
In this paper, we have designed and synthesized a novel multi-stimuli-responsive hydrogel as shown in Scheme 1. We took the following points into consideration: at first, HEMA-NVP copolymer as backbone can increase swelling ratio and mechanical performance of hydrogel. Secondly, incorporation of N-isopropylacrylamide unit in the polymer system also could lead to a smart polymer that is responsive to temperature. Thirdly, incorporation of the photochromic spironaphthoxazine in the polymer system could lead to a response to light as well as pH. To our knowledge, this is the first report of preparation and characterization of poly(NIPA-co-HEMA-co-NVP-co-SPO). Swelling ratio of this hydrogel is measured at different temperature, pH, ionic strength, and light irradiation. The absorption spectra indicate poly(NIPA-co-HEMA-co-NVP-co-SPO) hydrogel could respond to temperature, proton, and light stimuli. Furthermore, this new hydrogel with better mechanical performance has successfully been applied into erasable and rewritable optical information.
Experimental
Materials
N-isopropylacrylamide (NIPA) was purchased from Sigma-Aldrich Chemicals and purified by recrystallization from a mixture of toluene/hexane (1/4). 2,2-Azobis-(isobutyronitrile) (AIBN) was purchased from aladdinm and recrystallized from methanol. Hydroxyethyl methacrylate (HEMA) and N-vinylpyrrolidone (NVP) were obtained from Shanghai Jingchun Industries Co. Ltd., China and distilled under vacuum before use. The other chemicals were of the highest grade available and used as received.
Synthesis of 1,3,3-Trimethyl-9′-methacryloyloxy-spiro[indoline-2,3′(3H)naphtho[2,1 -b][1, 4]oxazine](5)
The synthetic methods for the photochromic 1,3,3-Trimethyl-9′-methacryloyloxy -spiro[indoline-2,3′(3H)naphtho[2,1-b][1, 4]oxazine] (5) were adopted and modified from our previously published procedure [31]. Gray solid, yield: 98.6 %, m.p. 151–152 °C. FTIR (KBr, cm−1): 3049, 2970, 1732, 1628, 1481, 1438, 1360, 1258, 1082, 1172, 1121, 977, 902, 825, 745. 1H NMR(CDCl3, 500 MHz): δ 8.26 (1H, d, J = 2.3 Hz, ArH), 7.74 (1H, d, J = 8.9 Hz, ArH), 7.72 ( 1H, s , 2′-H), 7.63 (1H, d, J = 8.9 Hz, ArH), 7.22–7.17 (2H, m, ArH), 7.09 (1H, d, J = 7.1 Hz, ArH), 6.98 (1H, d, J = 8.9 Hz, ArH), 6.91 (1H, t, J = 7.4 Hz, ArH), 6.56 (1H, d, J = 7.7 Hz, ArH), 6.42 (1H, s, CH), 5.78 (1H, s, CH), 2.77 (3H, s, CH3), 2.12 (3H, s, CH3), 1.36 (6H, s, CH3). 13C NMR (DMSO, 300 MHz): δ18.0, 20.4, 25.1, 29.2, 51.5, 98.4, 107.1, 1123, 116.5, 120.5, 119.6, 121.5, 122.2, 126.8, 127.8, 127.8, 129.7, 130.2, 130.9, 135.3, 135.4, 144.4, 147.2, 149.6, 151.6, 165.4. Anal. calcd. for C26H24N2O3: C, 75.71; H, 5.86; N, 6.79. Found C, 75.57; H, 5.84; N, 6.81.
Synthesis of poly(NIPA-co-HEMA-co-NVP-SPO)
N-isopropylacrylamide (NIPA) monomer 8 (1.54 g, 13.67 mmol) was dissolved in distilled water (5 ml) under dry nitrogen, and then 1,3,3-Trimethyl-9′-methacryloyloxy-spiro[indoline-2,3′(3H)naphtha [2,1-b][1, 4]oxazine] monomer (0.09 g, 0.22 mmol), 2-hydroxyethyl methacrylate (HEMA) monomer (1.54 g, 11.85 mmol), N-vinylpyrrolidon (NVP) monomer (1.03 g, 9.28 mmol), and the initiator azodiisobutyronitrile (AIBN) (0.0164 g, 0.1 mmol) were added. After being heated for 2.5 h at 70 °C in the oven, the resultant mixture was brought to room temperature, filtered, and rinsed with ethanol five times to remove non-conjugated monomer. The resulting polymer was dried in vacuum (yield 90 %).
Characterization
Melting points were determined using an X-4 microscope electrothermal apparatus and remained corrected. Elemental analyses were carried out on a Vario EL III elemental analyzer. 1H NMR was recorded on a Bruker AV-500 spectrometer using TMS as internal standard. Solid-state 13C NMR spectra (CP/MAS) were obtained with a Bruker AV-400 spectrometer analyzer. The contact time was 1 ms, and the relaxation delay was 2 s. Optical absorption spectra were obtained on a CARY 1101 UV-Vis spectrophotometer. A 40-W UV lamp (365 nm) was used as the irradiation source (Beijing CBIO Bioscience & Technologies CO., Ltd., China). The interior morphologies of the hydrogel were observed by using a SU8010 scanning electron microscope. Fourier transformation infrared (FTIR) spectra were obtained at room temperature on a Nicolet IS10 using KBr pellets.
Swelling measurement
The swelling behavior of hydrogels was measured by immersing them in water at room temperature to swelling equilibrium. As-prepared cylindrical samples with 140-mm diameter and 0.4-mm height were used for swelling experiments. The swelled samples were taken out periodically and weighed after blotting free of surface water with wet filter paper, until a constant weight. The swelling ratio of the samples (SR) was determined according the following equation:
Where W t is the weight of the swollen sample at time t and W d is the weight of the dry state. All results are obtained by averaging three measurements. The equilibrium swelling ratio (ESR) was obtained from the SR value when the swollen time was 12 h.
Results and discussion
The design and preparation of hydrogel
The synthetic route of the poly(NIPA-co-HEMA-co-NVP-co-SPO) is depicted in Scheme 2. The poly(NIPA-co-HEMA-co-NVP-co-SPO) copolymer will exhibit a cooperatively responsive behavior to changes in temperature, pH, and light irradiation. The photochromic monomer 5 was synthesized by reacting 3 with methacryloyl chloride. The chemical structures were characterized by FTIR, 1H NMR, 13C NMR, and MS. Then this monomer 5 was copolymerized with thermal-responsive monomer (NIPA) and main monomers (HEMA, NVP) by conventional radical polymerization using AIBN as initiator. The poly(NIPA-co-HEMA-co-NVP-co-SPO) is identified by FTIR and 13C NMR spectra.
FTIR analysis
The FTIR spectra of poly(HEMA-co-NVP)-based hydrogels were shown in Fig. 1 . In the spectrum of HEMA-NVP copolymer, the peak at 1720 cm−1 shows carbonyl stretching band of the HEMA and the peak at 1660 cm−1 is attributed to the NVP carbonyl stretching band. Similarly, the peaks at 1160 cm−1 and 1450 cm−1 correspond to the C–O–C bending band and the C–N stretching band, respectively. In addition, the broad peak at 3350 cm−1 is assigned to hydrogen-bonded OH group, with reference to the earlier work [32]; the hydrogen-bonded OH group can subsidize to raise the hydrophilicity. In the spectrum of HEMA-NVP-NIPA copolymer, compared with (a) spectrum, new characteristic band of amide group appeared at 1550 cm−1, which demonstrates the grafting of NIPA on the HEMA-NVP copolymer [33]. In the (c) spectrum, characteristic bands at around 900 cm−1 and 3100 cm−1 corresponding to the vinyl groups of monomers disappeared completely, which indicates nonexistence of unreacted monomers. The absorption band at 1367 cm−1 is attributed to the C–N stretching vibration in SPO. The absorption bands at 847 cm−1 and 750 cm−1 are ascribed to the stretching vibration outside surface of =C–H. These well supported the successful entry of SPO moieties into the network formation of hydrogels [34].
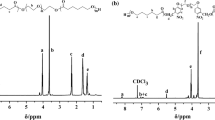
Solid 13C NMR spectra of HEMA-NVP and HEMA-NVP-NIPA-SPO copolymer
The solid-state13C NMR spectra of HEMA-NVP and HEMA-NVP-NIPA-SPO copolymer is shown in Fig. 2. In Fig. 2a, the results for the chemical shifts of carbon could be that signal at 17.8 ppm evidently corresponds to carbons a + j; signal at 32.04 ppm is attributed to carbons c + h; signal at 45.21 ppm is assigned to carbons d + m + l; signals at 54.79 and 177.32 ppm are ascribed to carbon b and carbon e + k, respectively; signals at 67.24 and 60.24 ppm correspond to carbon f and g of HEMA units, which is similar to early reports [35, 36]. Compared with Fig. 2a, the new chemical shifts around 120–130 ppm are assigned to the carbon of aromatic ring in spirooxazine in Fig. 2b, which is accorded with the 13C NMR spectrum of 5. Meanwhile, the vinyl groups of monomers disappeared completely in the FTIR spectrum, which further implied incorporation of spirooxazine into copolymer. In addition, the characteristic peak of 176.6 ppm could be corresponding to the carbon of carbonyl group, which has a little shift compared with that in HEMA-NVP copolymer due to different chemical environments. Most of chemical shifts located at 15–70 ppm could be the carbons of alkyl group in copolymer that originate from those of HEMA-NVP copolymer. It is worth noting that peak at 22.19 ppm is assigned to methyl carbon and 42.15 ppm corresponds to main chain CH and CH2 and isopropyl CH carbons of NIPA units [37].
The swelling behavior of the poly(NIPA-co-HEMA-co-NVP-co-SPO) hydrogel
Thermo-response of poly(NIPA-co-HEMA-co-NVP-co-SPO) at different temperature (15–60 °C) is shown in Fig. 3 a. At low temperature, the hydrogel had higher ESR due to the fact that the PNIPA component was swellable at temperature lower than VPTT. As temperature increased, the delicate hydrophobic/hydrophilic balance in the hydrogel network was broken. Dehydration took place in the PNIPA, which resulted in the subsequent aggregation of PNIPA chain and led to the deswelling of hydrogel. The relative higher ESR of poly(N-isopropylacrylamide) hydrogels having spironaphthoxazines [25] is due to addition of HEMA and NVP, which helps to improve ESR. Moreover, as shown in the inset of Fig. 3 a, poly(NIPA-co-HEMA-co-NVP-co-SPO) had VPTT of about 43 °C. The VPTT was higher than that of PNIPA-co-SPO hydrogel (31 °C) [23], which is also attributed to the introduction of HEMA and NVP monomer into the backbone of poly(NIPA-co-HEMA-co-NVP-co-SPO) obviously. Other authors have reported that the increase hydrophilicity in networks structure could shift the VPTT of the copolymer to a higher temperature [38].
a Influence of temperature on the hydrogel ESR. (pH = 7.0, ionic strength = 0), Inset: derivative of ESR vs temperature. b Influence of pH value on the hydrogel ESR (ionic strength = 0.1, at room temperature). c Influence of the ionic strength on the hydrogel ESR (pH = 7.0, at room temperature). d Influence of irradiation time on the hydrogel ESR (pH = 7.0, ionic strength = 0, at room temperature)
The influence of pH values on hydrogel’s ESR at room temperature (23 °C) was investigated. The experiments were performed in buffer solutions in a range of pH = 1–9 with an ionic strength of 0.1 mol/L, and the results are shown in Fig. 3b. ESRs for samples decrease with the increase of pH and the highest value of ESRs was about 6 at pH = 1. Furthermore, when pH was adjusted from 2 to 6, ESRs changes exhibited a near linear relationship. ESRs for samples reach a plateau at pH = 7–9. This change might be attributed to protonation behavior of tertiary amine groups in SPO segment. When the pH was relatively low, protonation behavior of tertiary amine groups in SPO segment leads to the ring opening of the pendant SPO, which further changes in the surface morphology of polymer. Therefore, hydrogel could absorb highest amount of water at pH = 1. With the improvement of pH, protonating tertiary amine groups became difficult. So, ESRs of hydrogel display a trend of gradual decrease and no obvious change were observed beyond pH = 7. In order to explain the response of this hydrogel for pH, the UV-vis spectra of SPO molecule at pH = 1–7 were obtained (Fig. S1). The result shows that SPO in acidic condition will open ring to form MEH, which can imply the behavior of response to pH well.
Figure 3c shows ESR values of the hydrogels in NaCl solutions (from 10−5 to 3.0 M) at room temperature. As can be seen, ESR values declined with increasing NaCl concentration and there are two ESR transitions with the changing of NaCl concentrations. In dilute NaCl solution, the shrinking process was continuous and rapid, and the ESR decreased quickly with the increase of the NaCl concentration. This conformational change can be attributed to the osmotic deswelling process inside the hydrogel, which might have been caused by some counterions and Donnan-equilibria effects [39]. In concentrated NaCl solutions, the hydrogel ESR decreased slower than that in dilute NaCl solution, with reference to earlier work, this result assigned to the higher ionic strength, which could suppress VPTT of the poly(NIPA-co-HEMA-co-NVP-co-SPO) hydrogels and trigger them to shrink at a temperature that could still be under the original VPTT [40, 41].
To examine the role of irradiation time on the swelling process for the hydrogels, dry poly(NIPA-co-HEMA-co-NVP-co-SPO) in water were exposed to UV or visible light at room temperature, then the water absorption measured at 10-min intervals. The results for SR are exhibited in Fig. 3b. When SRs of the hydrogel with UV and visible light irradiation tested in this study were compared, a lower swelling ratio is observed for hydrogel with visible light irradiation. The higher ESR of poly(NIPA-co-HEMA-co-NVP-co-SPO) under UV light irradiation may result in changing the surface morphology of poly(NIPA-co-HEMA-co-NVP-co-SPO) [25]. In Fig. S2, the picture displays that the size of samples after swelling at UV light is bigger than after swelling at visible light.
SEM analysis
In addition, to gain visual images of microstructures of poly(NIPA-co-HEMA-co-NVP-co-SPO) hydrogel, the interior morphology of the hydrogel was observed by using a SU8010 scanning electron microscope. The samples were freeze-dried at −60 °C with a pressure of 7–8 Pa for 48 h to completely remove the imbibed water. Many closed holes could be seen on the SEM image of the visible light-irradiated hydrogel (Fig. 4a), while some interconnected holes appeared on the UV light-irradiated hydrogel surface (Fig. 4b). UV light exposure disturbed the surface of the swollen poly(NIPA-co-HEMA-co-NVP-co-SPO) hydrogel. Moreover, it is noted that the holes of swollen poly(NIPA-co-HEMA-co-NVP-co-SPO) hydrogel in buffer solutions (pH = 1) (Fig. 4c) were bigger than those in buffer solutions (pH = 7) (Fig. 4a). The changes in surface morphology occurred were due to the ring opening of the pendant SPO during UV irradiation and in acidic condition. This photo and proton-stimulated isomerization has induced a change in polymer structure, by which more water molecules could penetrate through the surface, which is corresponding to Fig. 3b, d).
The multiple response behavior of poly(NIPA-co-HEMA-co-NVP-co-SPO)
The absorption spectra variations and schematic representation of poly(NIPA-co-HEMA-co-NVP-co-SPO) hydrogel by light, proton, and temperature stimuli were shown in Fig. 5 . Like other spirooxazine molecules, the poly(NIPA-co-HEMA-co-NVP-co-SPO) in water undergoes reversible photochromic reaction. As shown in Fig. 5a, line 1, upon UV irradiation at 365 nm, a broad absorption band at around 610 nm appears and the nearly colorless poly(NIPA-co-HEMA-co-NVP-co-SPO) turned blue (Fig. 5b: 1), which is attributed to the dissociation of the spiro C–O bond; generation of the open merocyanine form with a planar structure in Scheme 1 (poly(NIPA-co-HEMA-co-NVP-co-ME). The generated opened merocyanine form was thermally unstable; it underwent thermal bleaching to the closed spiro form after visible light irradiation (shown in Fig. 5a: line 2 and Fig. 5b: 2). The inset shows the decoloration at 610 nm of poly(NIPA-co-HEMA-co-NVP-co-SPO) hydrogel at room temperature. The original spectral band was recovered within 980 s. The poly(NIPA-co-HEMA-co-NVP-co-SPO) showed interesting acidchromic reaction like photochromic compound spiropyran [42]. The interaction of poly(NIPA-co-HEMA-co-NVP-co-SPO) with proton was investigated in water solution through spectrophotometric titration experiment. In Fig. 5a, line 3 shows the titration spectra of poly(NIPA-co-HEMA-co-NVP-co-SPO) with proton. Upon addition of proton, the band with a peak at around 540 nm occurred and produced the protonated poly(NIPA-co-HEMA-co-NVP-co-MEH) merocyanine, and the nearly colorless poly(NIPA-co-HEMA-co-NVP-co-SPO) became red in Fig. 5b: 3. After visible light irradiation, the original absorption spectrum was converted back to the initial state of poly(NIPA-co-HEMA-co-NVP-co-SPO). Furthermore, when the poly(NIPA-co-HEMA-co-NVP-co-SPO) in water was heated to 316 K (the VPTT value), the average absorbance of the system increased dramatically (shown in Fig. 5a, line 4), the color of hydrogel changed into milk white (shown in Fig. 5b:4), after cooling to room temperature, the hydrogel gradually returned to the initial transparent state (shown in Fig. 5, line 2). Furthermore, the multiple switching could be repeated more than 20 times. Thus, this system constitutes a multi-mode complete reversible switch by light, thermal, and proton stimuli.
a Absorption spectra of poly(NIPA-co-HEMA-co-NVP-co-SPO) in water solution at different conditions:(1) upon UV light, (2) upon visible light,(3) in acid solution (pH = 3), and (4) at 318 K. b Schematic representation for multiple response behavior of poly(NIPA-co-HEMA-co-NVP-co-SPO) copolymer in beaker with the trigger of light, thermal, and acid. (1) Gel (closed) (2) Gel (open) + H+ (3) Gel (closed) (4) Gel (open)
Erasable and rewritable (EARW) properties
According to significant photochromic switching feature of poly(NIPA-co-HEMA-co-NVP-co-SPO), a new technology for data recording could be designed. However, hydrogel needs great mechanical property to meet practical application for data recording. In order to demonstrate the role of HEMA-NVP in this hydrogel, the tensile strength of poly(NIPA-co-HEMA-co-NVP-co-SPO) and poly(NIPA+SPO) was characterized by an Instron 5943 testing material instrument and tensile strengths are 0.01080 and 0.00174 MPa, respectively. This result indicates that the addition of HEMA-NVP can improve tensile performance of our hydrogel, which realizes the purpose of our design. Therefore, a possible procedure for data recording and erasing is presented in Fig. 6a. Upon UV light irradiation on poly(NIPA-co-HEMA-co-NVP-co-SPO), the optical data can be recorded on irradiation region, while nothing changes on mask region. Furthermore, when visible light irradiates on irradiation region, the optical data were erased. To obtain visual photoswitching images of poly(NIPA-co-HEMA-co-NVP-co-SPO), the optical storage device consisted of two quartz glasses (75 mm × 25 mm × 1 mm) was separated by a 0.1-mm thickness spacer of TPU film. The cell filled with poly(NIPA-co-HEMA-co-NVP-co-SPO) was placed among two quartz glasses. Figure 6b shows the process of the photoswitching in poly(NIPA-co-HEMA-co-NVP-co-SPO) for optical data storage. The practical application of rewritable photoimaging on hydrogel was evaluated by patterned illumination through photomasks. The word “OK” was recorded at a first image by UV irradiation, which could be subsequently erased by visible light. Similarly, the word “SPO” can also be recorded at second image when the same method is adopted, whose cycles of writing and erasing were repeated more than 20 times. This procedure for data recording and erasing successfully demonstrates the potential application of poly(NIPA-co-HEMA-co-NVP-co-SPO) to rewritable optical memory media or imaging processes.
a Schematic diagram of the optical data recording on poly(NIPA-co-HEMA-co-NVP-co-SPO). b The switching and optical storage images of poly(NIPA-co-HEMA-co-NVP-co-SPO). (1) Gel, (2) writing, (3) erasing, and (4)rewriting. Photo-rewritable imaging on the poly(NIPA-co-HEMA-co-NVP-co-SPO) by using UV light (365 nm) and visible light (500 nm). The blue regions represent the writing optical data parts irradiated with UV light
Conclusion
In summary, we have successfully designed and synthesized a multi-stimuli-responsive poly(NIPA-co-HEMA-co-NVP-SPO) hydrogel, which was prepared by radical polymerization. This hydrogel exhibits variable ESR and absorption spectra at different thermal, pH, and light stimulation. Taking its superb reversible photochromic property, good mechanical property, and high fatigue resistance, which makes it a promising candidate for rewritable storage of optical information. Therefore, poly(NIPA-co-HEMA-co-NVP-SPO) hydrogel based on photochromic behavior was utilized in erasable and rewritable photoimaging.
References
Peppas NA, Hilt JZ, Khademhosseini A, Langer R (2006) Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater 18:1345–1360
Kuckling D (2009) Responsive hydrogel layers—from synthesis to applications. Colloid Polym Sci 287:881–891
Das D, Ghosh P, Ghosh A, Haldar C, Dhara S, Panda AB, Pal S (2015) Stimulus-responsive, biodegradable, biocompatible, covalently cross-linked hydrogel based on dextrin and poly(N-isopropylacrylamide) for in vitro/in vivo controlled drug release ACS. Appl Mater Interfaces 7:14338–14351
Garcia A, Marquez M, Cai T, Rosario R, Gust Z, Hayes M, Vail SA, Park CD (2007) Photo-, thermally, and pH-responsive microgels. Langmuir 23:224–229
Rutkevičius M, Mehla 1 G H, Paunov VN, Qin Q, Rubini PA, Stoyanov SD, Petkov J (2013) Sound absorption properties of porous composites fabricated by a hydrogel templating technique. J Mater Res 28:2409–2414
Hoare TR, Kohane DS (2008) Hydrogels in drug delivery: progress and challenges. Polymer 49:1993–2007
Eeckman F, Moes AJ, Amighi K (2004) Synthesis and characterization of thermosensitive copolymers for oral controlled drug delivery. Eur Polym J 40:873–881
Bassik N, Abebe BT, Laflin KE, Gracias DH (2010) Photolithographically patterned smart hydrogel based bilayer actuators. Polymer 51:6093–6098
Mueller M, Tebbe M, Andreeva DV, Karg M, Puebla RAA, Pazos-Perez N, Fery A (2012) Large-area organization of pNIPA-coated nanostars as SERS platforms for polycyclic aromatic hydrocarbons sensing in gas phase. Langmuir 28:9168–9173
Richter A, Paschew G, Klatt S (2008) Jens Lienig Karl-Friedrich Arndt and Hans-Jürgen P. Adler, review on hydrogel-based pH sensors and microsensors. Sensors 8:561–581
Schroeder V, Korten T, Linke H, Diez S, Maximov I (2013) Dynamic guiding of motor-driven microtubules on electrically heated smart polymer tracks. Nano Lett 13:3434–3438
Chatterjee P, Pan Y, Stevens EC, Ma T, Jiang H, Dai LL (2013) Controlled morphology of thin film silicon integrated with environmentally responsive hydrogels. Langmuir 29:6495–6501
Lee EM, Gwon SY, Ji BC, Wang S, Kim SH (2012) Multiple switching behaviors of poly(N-isopropylacrylamide) hydroel with spironaphthoxazine and D-π-a type dye. J Lumin 132:665–670
Feng Q, Li F, Yan QZ, Zhu YC, Ge CC (2010) Frontal polymerization synthesis and drug delivery behavior of thermo-responsive poly(N-isopropylacrylamide) hydrogel. Colloid Polym Sci 288:915–921
Dutta S, Dhara D (2015) Improved swelling–deswelling behavior of poly(N-isopropyl acrylamide) gels with poly(N,N′-dimethyl aminoethyl methacrylate) grafts. J Appl Polym Sci 132:42749–42758
Banerjee R, Dhara D (2014) Functional group-dependent self-assembled nanostructures from thermo-responsive triblock copolymers. Langmuir 30:4137–4146
Šťastná J, Hanyková L, Sedláková Z, Valentová H, Spěváček J (2013) Temperature-induced phase transition in hydrogel of interpenetrating networks poly(N-isopropylmethacrylamide)/poly(N-isopropylacrylamide). Colloid Polym Sci 291:2409–2417
Petriashvili G, Santo MPD, Devadze L, Zurabishvili T, Sepashvili 1 N, Gary R, Barberi R (2016) Rewritable optical storage with a spiropyran doped liquid crystal polymer film. Macromol Rapid Commun 37:500–505
Yuan W, Sun L, Tang H, Wen Y, Jiang G, Huang W, Jiang L, Song Y, Tian H, Zhu D (2005) A novel thermally stable spironaphthoxazine and its application in rewritable high density optical data storage. Adv Mater 17:156–160
Shiraishi Y, Tanaka K, Hirai T (2013) Colorimetric sensing of Cu(II) in aqueous media with a spiropyran derivative via a oxidative dehydrogenation mechanism. ACS Appl Mater Interfaces 5:3456–3463
Watkins DL, Fujiwara T (2012) Synthesis characterization, and solvent-independent photochromism of spironaphthooxazine dimers. J Photoch Photobio A 228:51–59
Feczkó T, Varga O, Kovács M, Vidóczy T, Voncina B (2011) Preparation and characterization of photochromic poly(methyl methacrylate) and ethyl cellulose nanocapsules containing a spirooxazine dye. J Photoch Photobio A 222:293–298
Wang S, Choi MS, Kim SH (2008) Bistable photoswitching in poly(N-isopropylacrylamide) with spironaphthoxazine hydrogel for optical data storage. J Photochem Photobiol A 198:150–155
Wang S, Choi MS, Kim SH (2008) Multiple switching photochromic poly(N-isopropylacrylamide) with spironaphthoxazine hydrogel. Dyes Pigments 78:8–14
Kulardana E, Mudiyanselage TK, Neckers DC (2009) Dual responsive poly(N-isopropylacrylamide) hydrogels having spironaphthoxazines as pendant groups. J Polym Sci A Polym Chem 47:3318–3325
Zırıh T, Orakdogen N (2016) Evaluation of pH/temperature double responsivity of copolymerized methacrylate-based networks: solvent diffusion analysis with adjustable swelling kinetics. Eur Polym J 75:75371–75387
Brahim S, Narinesingh D, Elie AG (2003) Synthesis and hydration properties of pH-sensitive p(HEMA)-based hydrogels containing 3-(trimethoxysilyl)propyl methacrylate. Biomacromolecules 4:497–503
Orakdogen N, Celik T (2016) Ion-stimuli responsive dimethylaminoethyl methacrylate/hydroxyethyl methacrylate copolymeric hydrogels: mutual influence of reaction parameters on the swelling and mechanical strength. J Polym Res 23:1–17
Fares MM, Assaf SM, Jaber AA (2011) Biodegradable amphiphiles of grafted poly(lactide) onto 2-hydroxyethyl methacrylate-co-N-vinylpyrrolidone copolymers as drug carriers. J Appl Polym Sci 122:840–848
Telford AM, James M, Meagher L (2010) Thermally cross-linked PNVP films as antifouling coatings for biomedical applications. ACS Appl Mater Interfaces 2:2399–2408
Yang XL, Yang BJ, Liu YY, Zhu HJ (2012) Microwave-assisted synthesis of novel spirooxazines and their photochromic behaviors in polymer matrices optoelectronics. Adv Mater 6:1146–1152
Yang J, Webb AR, Ameer GA (2004) Novel citric acid-based biodegradable elastomers for tissue engineering. Adv Mater 16:511–516
Kumar V, Chaudhari CV, Bhardwaj YK, Goel NK, Sabharwal S (2006) Radiation induced synthesis and swelling characterization of thermo-responsive N-isopropylacrylamide-co-ionic hydrogels. Eur Polym J 42:235–246
Al-Jallo HN, Jalhxoom MG (1975) Spectral correlations for α,β-unsaturated acid halides. Spectrochim Acta A 31:265–271
Horak D, Krystufek M, Spevacek J (2000) Effect of reaction parameters on the dispersion polymerization of 1-vinyl-2-pyrrolidone. J Polym Sci A Polym Chem 38:653–663
Toman L, Janata M, Spěváček J, Dvořánková B, Látalová P, Vlček P, Sikora A, Michálek J, Pekárek M (2006) One-pot synthesis of isocyanate and methacrylate multifunctionalized polyisobutylene and polyisobutylene-based amphiphilic networks. J Polym Sci A Polym Chem 44:2891–2900
Toman L, Janata M, Spěváček J, Brus J, Sikora A, Látalová P, Holler P, Vlček P, Dvořánková B (2006) Amphiphilic conetworks. II. Novel two-step synthesis of poly[2-(dimethylamino)ethyl methacrylate]–polyisobutylene, poly(N-isopropylacrylamide)-polyisobutylene, and poly(N,N-dimethylacrylamide)-polyisobutylene hydrogels. J Polym Sci A Polym Chem 44:6378–6384
Zhuang YF, Yang H, Wang GW, Zhu ZQ, Song WQ, Zhao HD (2003) Radiation polymerization and controlled drug release of polymer hydrogels with NIPA and NVP. J Appl Polym Sci 88:724–729
Ricka J, Tanaka T (1984) Swelling of ionic gels: quantitative performance of the Donnan theory. Macromolecules 17:2916–2921
Zhang XM, ZB H, Li Y (1997) The phase transition and modulus of ionic N-isopropylacrylamide gels in concentrated salt solutions. J Appl Polym Sci 63:1851–1856
Liu YY, Fan XD (2003) Preparation and characterization of a novel responsive hydrogel with a β-cyclodextrin-based macromonomer. J Appl Polym Sci 89:361–367
Doron A, Katz E, Tao G, Willner I (1997) Photochemically-, chemically-, and pH-controlled electrochemistry at functionalized spiropyran monolayer electrodes. Langmuir 13:1783–1790
Acknowledgments
This work was supported by the Research Fund for the Doctoral Program of Jinling Institute of Technology (jit-2012-27), the Natural Science Foundation of China (grant numbers 51103071).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 81 kb)
Rights and permissions
About this article
Cite this article
Yang, X., Zhou, L., Lv, L. et al. Multi-stimuli-responsive poly(NIPA-co-HEMA-co-NVP) with spironaphthoxazine hydrogel for optical data storage application. Colloid Polym Sci 294, 1623–1632 (2016). https://doi.org/10.1007/s00396-016-3915-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-016-3915-6