Abstract
In this work, the swelling of pH- and temperature-sensitive [poly(2-(diethylamino) ethyl) methacrylate]-based nanogels has been analyzed with the help of coarse-grained simulations employed in the last decade for polyelectrolyte gels. This computational approach, which is based on particle-particle interactions between polymer units, constitutes an alternative to thermodynamic formalisms. Polymer-polymer hydrophobic forces are accounted for in the model through a solvent-mediated interaction potential whose depth increases with temperature. This model qualitatively captures the swelling behavior of such nanogels when their degree of protonation varies from 0 to 1 without requiring changes in the potential parameters. In addition, our study quantitatively reveals that [poly(2-(diethylamino) ethyl) methacrylate]-based particles are more hydrophobic than those based on poly(N-isopropylacrylamide).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanogels are nanometer-sized particles that consist of a cross-linked polymer network with the ability to swell in a thermodynamically good solvent [1]. More specifically, these soft nanoparticles can swell or shrink depending on many external stimuli such as temperature, pH, salt concentration, or solvent nature, which makes them very useful for many biotechnological applications [2]. In fact, there is a growing biomedical interest in multi-responsive nanogels that focuses to a great extent on their usage as controlled drug delivery systems, which are expected to overcome therapeutic issues such as poor intracellular delivery, lack of control over the release behaviors, or side effects. For instance, nanogels can be employed for siRNA Delivery [3], cancer therapy [4], and theranostic applications [5].
From a theoretical perspective, the classical Flory-Rhener (FR) formalism for gels and its refinements have been also applied to nanogels to achieve a better understanding of their swelling behavior (implicitly assuming that surface effects are negligible if nanogels are large enough) [6]. In the last decade, computer simulations within coarse-grained models have also been considerably useful for the analysis of certain nonspecific aspects on the swelling of gels and nanogels, such as size and charge effects, since excluded-volume and electrostatic interactions can be explicitly considered [7–20]. Coarse-grained models have also been extensively employed to shed light on issues of biotechnological interest, such as the adsorption and collapse of biopolymers [21–29], viral detection [30], or genome encapsidation [31]. It should be mentioned, however, that direct comparisons with experiments on specific microgels or nanogels have been scarcely done. In this sense, a solvent-mediated hydrophobic interaction was recently included in a coarse-grained model to capture the swelling of thermo-responsive microgels whose main comonomer was poly(N-isopropylacrylamide) (PNIPAM) [13]. This model succeeded in justifying why the transition from swollen to shrunken states was so gradual for these microgels, in contrast to abrupt volume changes previously reported for other PNIPAM-based gels and microgels. In particular, this work revealed that the abruptness of such transition is strongly conditioned by the number of monomer units per polymer chain. When this number is small, volume changes are very gradual and smaller in relative terms.
It should be pointed out, however, that the comparison performed by Quesada-Pérez et al. was restricted to slightly charged PNIPAM-based microgels with a high degree of cross-linking (small number of monomer units per chain) [13]. It would be desirable to find out if that coarse-grained model can justify (qualitatively at least) the swelling behavior of multi-responsive nanogels made of another stimuli-responsive polymers and/or under different conditions. In particular, it would be interesting to look into the case of nanogels with a high number of ionized monomer units per chain, since electrostatic interactions are not explicitly accounted for in most of the FR-inspired formalisms [6].
Recently, Pikabea et al. have reported an in-depth study of the swelling of multi-responsive nanogel particles based on poly(2-(diethylamino) ethyl) methacrylate (PDEAEMA) [32], which present thermo and pH sensitivity (tunable with the ionic strength). As a result of the protonation of amine groups in the monomer units, the degree of ionization of PDEAEMA chains can considerably increase when the pH decreases. In other words, slightly and highly charged nanoparticles can be achieved just changing the pH of the solution.
According to the preceding paragraphs, our main goal is to examine to what extent the abovementioned coarse-grained models for gels can capture the swelling of PDEAEMA-based nanogels with very different charges. We also want to test the hydrophobic potential (successfully applied to PNIPAM) with a different polymer.
Model and simulations
Model
The coarse-grained picture employed here is the so-called bead-spring model for polyelectrolyte, in which monomer units and ions are represented as spheres, whereas the solvent is considered as a dielectric continuum [17, 33]. It should be mentioned that, even within this level of coarse graining, simulating charged nanogel particles with diameters greater than some tens of nanometers could require long times, particularly in the presence of salt, since a significant part of the electric double layer of such particles should also be included in the simulation cell. Just as an example, simulating nanogels of about 30 nm in the presence of an electrolyte at 100 mM [15] took nearly 2 months (within a conventional work station of 2.2 GHz).
For some purposes, however, simulating an inner piece of the polymer network might provide valuable information. This method, adopted here, supplies the value of intensive quantities such as the polymer volume fraction, which can be used to estimate the swelling ratio. In any case, the reader should keep in mind that this idealized representation of reality implicitly assumes that the inner structure of the network is replicated periodically and surface effects are negligible. A unit cell is schematically represented in Fig. 1.
If we consider the sequence of monomer units comprised between two cross-linker molecules as a single chain, molecules such as ethylene glycol dimethacrylate can be considered tetrafunctional cross-linkers (since four chains are linked to this molecule). Thus, a diamond-like topology was assumed (as usual in previous simulations of gels). N bead stands for the number of monomer units (beads) per chain (between two cross-linker molecules or nodes), whereas N charged represents the number of charged monomers per chain. In a model of ideal polymer network, N bead and N charged are identical for all the chains of the network. In our simulations, N bead = 48 was employed. This value is of the order of the mean number of monomer units per chain that can be estimated from the synthesis recipe assuming an ideal network. The reader can find an example with PNIPAM-based microgels in a previous work [13]. In fact, it should be emphasized that the N bead value used in these simulations is considerably greater than the value previously used comparing with PNIPAM-based microgels (N bead = 8). In other words, the degree of cross-linking of the nanogels studied here is significantly smaller. As we are interested in PDEAEMA chains with different degrees of ionization, simulations with several N charged values were performed here.
Together with the beads of the polymer network, the simulation cell also contains a number of monovalent counterions that neutralize the charge of the protonated groups of the network. As the experiments with PDEAEMA-based nanogels were carried out in the presence of additional electrolyte, a monovalent electrolyte solution (at 10 mM) was also considered in simulations. For the sake of simplicity, the anion of the additional electrolyte was assumed to be identical to the counterion of the charged network, and both are collectively referred to as counterions. The solvent is only taken into account through its relative permittivity, ε r .
Monomer units of polymer chains, cross-linker molecules, and ions are explicitly modeled as spheres of diameter σ M , σ CL , and σ I , respectively. For PDEAEMA, σ M was estimated from the molecular weight of the corresponding monomer and its density in the liquid state assuming that the volume fraction of such state is similar to that of close packing (0.74) [13, 34]. According to this prescription, previously used also for PNIPAM, σ M = 0.7 nm. This value was also assumed for cross-linker molecules. σ I = 0.7 nm was employed for all the ionic species. This ionic size can be considered representative for many hydrated monatomic cations and anions (if the hydration shell is included) and was estimated averaging results obtained from different experimental techniques [34].
At this point, let us comment the interactions between the different constituents of the model. The short-range repulsion between any pair of particles (monomer units, cross-linker molecules, and solute molecules) due to excluded volume effects is taken into account by means of the purely repulsive Weeks–Chandler–Andersen potential [8–10, 35]:
where r is the center-to-center distance between a given pair of particles, ε LJ = 4.11 × 10−21 J, σ = (σ i + σ j )/2 (where i and j stand for interacting species). The beads of a given chain are connected by harmonic bonds, whose interaction potential is
where k bond is the elastic constant and r 0 is the equilibrium bond length. In this work, we have assumed that r 0 = σ M and k bond = 0.4 N/m. All the charged species (charged monomers and ions) interact electrostatically through the Coulomb potential:
where e is the elementary charge, Z i is the valence of species i, and ε 0 is the vacuum permittivity. Being interested in the thermo-responsive nanogels, the temperature dependence of the dielectric permittivity was also considered [36]:
Finally, we should keep in mind that, when nonpolar molecules (or macromolecules) are inserted into an aqueous medium, they tend to reduce the surface exposed to the solvent aggregating. The solvent-mediated attraction between nonpolar monomers required for this process is the so-called hydrophobic force, which is intrinsically temperature-sensitive and justifies why some polymer networks collapse upon heating. Admitting that the precise knowledge of hydrophobic forces is far from complete, other authors have employed phenomenological potentials for hydrophobic forces with different functional forms (square well, Lennard-Jones, and others) in previous simulations of flexible polymers [37–43]. In this work, the hydrophobic interaction was modeled through the same potential previously used for PNIPAM-based microgels [13], although some parameters were changed. In this way, the interaction potentials obtained for different polymers can be compared. The functional form u hyd (r) consists in a sigmoid approximation to the square well potential (also employed by other authors [39, 41, 44]):
where r h is the range of the potential and k h is related to the slope of the sigmoid. As mentioned in previous works, the depth of this potential should increase with temperature. The function for the T dependence of ε h previously proposed for PNIPAM was also adopted here:
where ε max is the maximum depth of the hydrophobic potential (reached at high temperatures), T ε/2 is the temperature for which ε = ε max / 2 (inflection point) and k ε/2 is proportional to the slope of the function at that point. Regarding the hydrophobic interaction, it should also be mentioned that charged chemical groups are usually hydrophilic rather than hydrophobic. Thus, the hydrophobic forces involving charged monomer units are expected to be considerably weakened. In addition, we also considered that hydrophobic forces are less intense for bonded monomeric units since their surface exposed to water molecules is smaller in this case. As a limiting case, we therefore assumed that the hydrophobic interaction only works between uncharged nonbonded monomeric beads.
Simulations
The simulation method of thermo-responsive gels in the presence of salt (in equilibrium with an external salt reservoir) has been described in detail elsewhere [35]. Here, its main features are outlined. First, the osmotic pressure and the activity coefficient of such reservoir were previously computed. The simulation cell consisted in a cubic box containing a piece of the polymer network (with a fixed number monomeric units), monovalent counterions neutralizing the charge of this network, and an electroneutral group of monovalent cations and anions in equilibrium with the salt reservoir. The equilibrium polymer volume fraction (φ) at a given temperature was calculated as follows. First, a set of systems at the same temperature but different polymer volume fractions were simulated adjusting the dimensions of the simulation cell. The osmotic pressure for each one of them was computed (see reference [35] for further details about the computation of this property). Then, φ was estimated as the polymer volume fraction for which the osmotic pressure of the simulation cell equaled the osmotic pressure of the electrolyte reservoir (previously determined, as mentioned before).
The Metropolis Monte Carlo (MC) protocol was used to generate Boltzmann-weighted gel configurations. Periodic boundary conditions were employed in the three directions of space. Translational MC moves were applied to all the particles in the simulation box adjusting their maximum displacements so that their acceptance ratios were of about 50 %. At least 1 × 107 and 2 × 107 trial moves were used for thermalization and statistics, respectively. It should be mentioned, however, that the electrolyte in equilibrium with the reservoir was simulated in the grand canonical ensemble implementing the method of Valleau and Cohen [45]. Accordingly, insertions and removals of ions were also attempted. As the condition of electrical neutrality of a system had to be preserved, electroneutral cation-anion pairs were inserted at random positions or were randomly selected from the ions present in the box and removed when such insertions and removals were attempted. The expressions for the probability of acceptance of these MC moves are again provided in reference [35].
Results
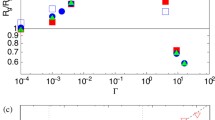
Given that the model employed here cannot provide extensive properties, comparisons between experiment and simulation will be done in relative terms defining the swelling ratio (S) as the quotient between the hydrodynamic diameters at a given state and at a reference state, which usually corresponds to the collapsed state. Figure 2 shows this parameter as a function of temperature for three pH values: 7.4, 6.3, and 5.6. The diameter of the nanogel at 353 K and pH = 6.3 was chosen as reference to compute the swelling ratio. According to the titration curve reported by Pikabea et al. [32], the polyelectrolyte chains are slightly and highly protonated at pH = 7.4 and 5.6, respectively. At pH = 6.3, the degree of protonation is close to 50 %. Thus, these systems exhibit very different charges and three clearly differentiated swelling behaviors. At pH = 7.4, nanogels are shrunken in the temperature window explored here, whereas at pH = 5.6, they are swollen. At pH = 6.3, a gradual transition from swollen to collapsed states is observed. The original results reported by Pikabea et al. [32] (which include data for other pH-values) also reveal that this transition shifts toward higher temperatures when the pH decreases and the nanogel charge increases. Finally, it should be mentioned that the swelling ratios observed in experimental data at pH = 5.6 and 7.4 are similar to those observed at pH = 6.3 when nanogels are swollen (low temperatures) or shrunken (high temperatures), respectively.
Swelling ratio observed in experiments with PDEAEMA-based nanogels (solid symbols) and predicted by simulations (open symbols) as a function of temperature at pH = 7.4 (solid and open squares, respectively), 6.3 (solid and open circles, respectively), and 5.6 (solid and open triangles, respectively)
Can the simple model described above justify the behavior observed for PDEAEMA-based nanogels with very different charges? To give an answer to this question, N charged = 2, 24, and 48 were chosen as representative number of ionized groups per chain for the slightly, moderately, and highly charged nanogels. Then, we tried to find a set of values for PDEAEMA-based nanogels that semiquantitatively captures their swelling behavior when their degree of ionization is 50 %. Such values were chosen taking as starting point the previously reported values for PNIPAM and/or from the experimental behavior observed for PNIPAM- and PDEAEMA-based nanogels. For instance, the lowest volume phase transition temperature (VPTT) observed for PDEAEMA-based nanogels (at 10 mM) by Pikabea et al. [32] is 292 K. As T ε/2 is expected to be related to the VPTT (if T ε/2 increases, the VPTT will shifts towards larger values), two T ε/2 values of this order were explored in our simulations (286 and 294 K). In relation to the depth and the range of the hydrophobic potential, r h and ε max , we explored a few values ranging from 0.9 to 1.4 nm and from 9.0 × 10−21 to 15.0 × 10−21 J, respectively. The swelling curves turned out to be quite sensitive to these parameters. As can be seen, such values are a bit larger than those reported for PNIPAM-based nanogels (see Table 1), in agreement with a recent work by Pelton [46] that concluded that PNIPAM chains have both hydrophobic and hydrophilic domains even above the lower critical solution temperature (LCST). In other words, this polymer is not as hydrophobic as many authors claim. k ε/2 is somehow related to the slope of volume phase transition observed in the collapse of the nanogels upon heating. The comparison of the experimental data shown in Fig. 2 for PDEAEMA-based nanogels with similar curves published for PNIPAM-based nanogels suggests that the slope of the transition is smaller for the former. Accordingly, we explored k ε/2 values smaller than the k ε/2 value reported for PNIPAM-based nanogels (0.03125 and 0.0385 K−1) looking for the agreement between our results and simulation data. Finally, k h is a technical parameter employed in constructing a differentiable approximation to the well square potential (which facilitates the computation of osmotic pressure). Swelling results are not expected to be very sensitive to this technical parameter. Thus, the same value reported for PNIPAM-based nanogels was used here. Indeed, this was not a strict fitting procedure, which would require a vast (and highly time-consuming) collection of simulation data at different conditions. However, it can provide some information about the hydrophobic forces inducing the collapse of PDEAEMA-based nanogels. Table 1 summarizes the values employed for the parameters of this potential for PNIPAM-based microgels (in a previous work) [13] and PDEAEMA-based nanogels.
In addition, Fig. 2 displays the swelling ratio obtained from simulations using the parameters included in Table 1. As mentioned before, this model cannot provide extensive quantities, such as the nanogel diameter. For an ideal network, however, the diameter is proportional to φ − 1/3, where φ is the polymer volume fraction. Thus, the swelling ratio can be computed as S = φ − 1/3/φ − 1/3 ref , where φ ref is the φ value at the reference state.
Figure 3 shows the hydrophobic interaction potential, u hyd (r), computed from these parameters as a function of r for these two polymer units at the lower and upper temperatures explored here (283 and 353 K, respectively). As can be seen, both the depth and the range of the potential are greater for PDEAEMA. In fact, Fig. 3 also reveals that at 283 K, the depth for PDEAEMA is of the order of the thermal energy (k B T) and very similar to the depth for PNIPAM at 353 K. This would justify why slightly charged PDEAEMA-based nanogels are shrunken at 283 K (since PNIPAM-based nanogels are collapsed under similar hydrophobic forces at 353 K).
Hydrophobic potential between polymer units employed for PNIPAM (in a previous work [13]) and PDEAEMA at 283 and 353 K
Concerning the predictions of the model using the parameters included in Table 1, Fig. 2 reveals that the model qualitatively and even semiquantitatively reproduces the gradual transition from swollen to shrunken states exhibited by the moderately charged nanogel (pH = 6.3). The model also predicts that the highly charged nanogel is swollen in the temperature window explored, whereas the slightly charged system is collapsed. Both predictions are in qualitative agreement with the experimental swelling ratio observed. In relation to the qualitative agreement between simulation and experiment, it should be stressed that the predictions of the model displayed in Fig. 2 for PDEAEMA-based nanogels with different charges and those previously reported for PNIPAM-based microgels were obtained with hydrophobic potential’s parameters that do not change when the nanogel charge varies. This is a strong point of the coarse-grained model as compared to FR-inspired formalisms, whose parameters can be extremely sensitive to the charge of the gel (even in the case of slightly charged systems) [13], which clearly limits the capability of prediction of these theoretical approaches.
It should be also mentioned, however, that there are quantitative discrepancies between simulation and experiment at pH = 5.6 and 7.4, since the S value predicted for N charged = 48 is considerably greater than that measured for pH = 5.6, whereas the swelling ratio obtained for N charged = 2 (pH = 5.6) is significantly smaller than the one corresponding to the slightly charged nanogel.
Different aspects of real nanogels not included in this model could contribute to justify this quantitative disagreement. For instance, the reader should bear in mind that coarse-grained models are usually developed on polymer networks made of chains with the same number of monomeric units. However, high-resolution NMR measurements reveal a bimodal distribution of the PDEAEMA chain mobility inside the nanogel particles from which the core-shell model can be inferred [32]. Obviously, this also means that there is considerable heterogeneity in the PDEAEMA chain distribution, which is not considered in our coarse-grained model. Edgecombe et al. have observed that highly charged polymer networks with chain length polydispersity swell less than the ideal ones [47]. This could partly justify why simulation data overestimate the swelling ratio at pH = 5.6. On the other hand, two factors might contribute to the numerical discrepancies reported for slightly charged systems (pH = 7.4). First, it should be mentioned that our model did not consider chain stiffness. Thus, the compactness of shrunken networks might be overestimated by the model. In addition, the parameters employed in Eqs. 6 and 7 might overestimate the intensity of the hydrophobic interaction at high polymer volume fractions (collapsed nanogels), because the surface of polymer units exposed to water is smaller under such circumstances. At pH = 7.4, simulations predict φ values of the order of 0.2. Thus, the hydrophobic interaction might have been overestimated to some extent in this case (predicting too shrunken nanogels). Finally, it should be kept in mind that we are simulating just an inner piece of the nanogel particle, which is identically replicated in the three directions of space. In other words, the core-shell structure inferred from NMR measurements is not included in the coarse-grained model either. Regarding this assumption (infinite and homogenous network), we should also point out that such hypothesis has been commonly employed in the study of microgels through classic RF formalisms as well as current sophisticated thermodynamic approaches [48–50]. However, a thermodynamic theory for core-shell particles has been recently proposed [51].
Conclusions
In this work, we have shown that coarse-grained simulations of polyelectrolyte gels qualitatively capture the swelling behavior of PDEAEMA-based nanogels with very different charges using a hydrophobic potential whose parameters do not depend on the degree of protonation, improving on the capability of prediction of those formalisms whose parameters are extremely sensitive to charge. Our simulations also suggest that the depth and the range of the hydrophobic interaction (responsible for the nanogel collapse) are greater for PDEAEMA polymer chains than for those of PNIPAM. In any case, the quantitative differences between simulation and experiment observed for highly and slightly charged nanogels could be reduced including some refinements in the model (for instance, chain length polydispersity, chain stiffness).
References
Pelton R (2000) Temperature-sensitive aqueous microgels. Adv Colloid Interface Sci 85:1–33. doi:10.1016/s0001-8686(99)00023-8
Ramos J, Imaz A, Callejas-Fernandez J et al (2011) Soft nanoparticles (thermo-responsive nanogels and bicelles) with biotechnological applications: from synthesis to simulation through colloidal characterization. Soft Matter 7:5067–5082. doi:10.1039/c0sm01409e
Smith MH, Lyon LA (2012) Multifunctional nanogels for siRNA delivery. Acc Chem Res 45:985–993. doi:10.1021/ar200216f
Liechty WB, Peppas NA (2012) Expert opinion: responsive polymer nanoparticles in cancer therapy. Eur J Pharm Biopharm 80:241–246, http://dx.doi.org/10.1016/j.ejpb.2011.08.004
Sierra-Martin B, Fernandez-Barbero A (2015) Multifunctional hybrid nanogels for theranostic applications. Soft Matter 11:8205–8216. doi:10.1039/C5SM01789K
Quesada-Perez M, Alberto Maroto-Centeno J, Forcada J, Hidalgo-Alvarez R (2011) Gel swelling theories: the classical formalism and recent approaches. Soft Matter 7:10536–10547. doi:10.1039/c1sm06031g
Mann BA, Holm C, Kremer K (2005) Swelling of polyelectrolyte networks. J Chem Phys. doi: 15490310.1063/1.1882275
Yin DW, Yan QL, de Pablo JJ (2005) Molecular dynamics simulation of discontinuous volume phase transitions in highly-charged crosslinked polyelectrolyte networks with explicit counterions in good solvent. J Chem Phys. doi: 17490910.1063/1.2102827
Yin D-W, Horkay F, Douglas JF, de Pablo JJ (2008) Molecular simulation of the swelling of polyelectrolyte gels by monovalent and divalent counterions. J Chem Phys. doi: 15490210.1063/1.2991179
Yin D-W, de la Cruz MO, de Pablo JJ (2009) Swelling and collapse of polyelectrolyte gels in equilibrium with monovalent and divalent electrolyte solutions. J Chem Phys. doi: 19490710.1063/1.3264950
Jha PK, Zwanikken JW, Detcheverry FA et al (2011) Study of volume phase transitions in polymeric nanogels by theoretically informed coarse-grained simulations. Soft Matter 7:5965–5975. doi:10.1039/c1sm05264k
Quesada-Pérez M, Ibarra-Armenta JG, Martín-Molina A (2011) Computer simulations of thermo-shrinking polyelectrolyte gels. J Chem Phys 135:94109
Quesada-Perez M, Ramos J, Forcada J, Martin-Molina A (2012) Computer simulations of thermo-sensitive microgels: quantitative comparison with experimental swelling data. J Chem Phys 136:244903–244909
Quesada-Perez M, Martin-Molina A (2013) Monte Carlo simulation of thermo-responsive charged nanogels in salt-free solutions. Soft Matter 9:7086–7094. doi:10.1039/c3sm00093a
Quesada-Pérez M, Ahualli S, Martín-Molina A (2014) Temperature-sensitive nanogels in the presence of salt: explicit coarse-grained simulations. J Chem Phys 141:124903. doi:10.1063/1.4895960
Höpfner J, Richter T, Košovan P, et al (2013) Seawater desalination via hydrogels: practical realisation and first coarse grained simulations. In: Sadowski G, Richtering W (eds) Intell. Hydrogels. Springer International Publishing, Switzerland, pp 247–263
Košovan P, Richter T, Holm C (2013) Molecular simulations of hydrogels. In: Sadowski G, Richtering W (eds) Intell. Hydrogels. Springer International Publishing, Switzerland, pp 205–221
Mann BAF, Kremer K, Lenz O, Holm C (2011) Hydrogels in poor solvents: a molecular dynamics study. Macromol Theory Simul 20:721–734. doi:10.1002/mats.201100050
Claudio GC, Kremer K, Holm C (2009) Comparison of a hydrogel model to the Poisson-Boltzmann cell model. J Chem Phys. doi: 09490310.1063/1.3207275
Mann BA, Everaers R, Holm C, Kremer K (2004) Scaling in polyelectrolyte networks. Europhys Lett 67:786–792. doi:10.1209/epl/i2004-10121-x
Jorge AF, Sarraguca JMG, Dias RS, Pais A (2009) Polyelectrolyte compaction by pH-responsive agents. Phys Chem Chem Phys 11:10890–10898. doi:10.1039/b914159f
Dias RS, Pais A (2010) Polyelectrolyte condensation in bulk, at surfaces, and under confinement. Adv Colloid Interface Sci 158:48–62. doi:10.1016/j.cis.2010.02.007
Nunes SCC, Cova T, Pais A (2013) A new perspective on correlated polyelectrolyte adsorption: positioning, conformation, and patterns. J Chem Phys. doi: 05490610.1063/1.4817338
Seijo M, Pohl M, Ulrich S, Stoll S (2009) Dielectric discontinuity effects on the adsorption of a linear polyelectrolyte at the surface of a neutral nanoparticle. J Chem Phys. doi: 17470410.1063/1.3251767
Ulrich S, Seijo M, Stoll S (2006) The many facets of polyelectrolytes and oppositely charged macroions complex formation. Curr Opin Colloid Interface Sci 11:268–272. doi:10.1016/j.cocis.2006.08.002
Luque-Caballero G, Martín-Molina A, Quesada-Pérez M (2014) Polyelectrolyte adsorption onto like-charged surfaces mediated by trivalent counterions: a Monte Carlo simulation study. J Chem Phys 140:174701. doi:10.1063/1.4872263
Jin J, Wu J (2008) A theoretical study for nanoparticle partitioning in the lamellae of diblock copolymers. J Chem Phys 128:074901. doi:10.1063/1.2827470
Wang L, Liang H, Wu J (2010) Electrostatic origins of polyelectrolyte adsorption: theory and Monte Carlo simulations. J Chem Phys 133:044906. doi:10.1063/1.3463426
de Carvalho SJ, Metzler R, Cherstvy AG (2014) Critical adsorption of polyelectrolytes onto charged Janus nanospheres. Phys Chem Chem Phys 16:15539–15550. doi:10.1039/C4CP02207F
Shin J, Cherstvy AG, Metzler R (2014) Sensing viruses by mechanical tension of DNA in responsive hydrogels. Phys Rev X 4:21002
Kim J, Wu J (2014) A molecular thermodynamic model for the stability of hepatitis B capsids. J Chem Phys 140:235101. doi:10.1063/1.4882068
Pikabea A, Ramos J, Forcada J (2014) Production of cationic nanogels with potential use in controlled drug delivery. Part Part Syst Charact 31:101–109. doi:10.1002/ppsc.201300265
Stoll S (2014) Chapter 10 Computer simulations of soft nanoparticles and their interactions with DNA-like polyelectrolytes. In: Soft Nanoparticles Biomed. Appl. The Royal Society of Chemistry, Cambridge, pp 342–371
Israelachvili JN (1991) Intermolecular and surface forces / Jacob N. Israelachvili. Academic, London ; San Diego
Quesada-Pérez M, Ahualli S, Martín-Molina A (2014) Thermo-responsive gels in the presence of monovalent salt at physiological concentrations: a Monte Carlo simulation study. J Polym Sci Part B Polym Phys 52:1403–1411. doi:10.1002/polb.23576
Catenaccio A, Daruich Y, Magallanes C (2003) Temperature dependence of the permittivity of water. Chem Phys Lett 367:669–671. doi:10.1016/S0009-2614(02)01735-9
Anderson JA, Travesset A (2006) Coarse-grained simulations of gels of nonionic multiblock copolymers with hydrophobic groups. Macromolecules 39:5143–5151. doi:10.1021/ma061120f
Chodanowski P, Stoll S (1999) Monte Carlo simulations of hydrophobic polyelectrolytes: evidence of complex configurational transitions. J Chem Phys 111:6069–6081
Escobedo FA, de Pablo JJ (1996) Monte Carlo simulation of branched and crosslinked polymers. J Chem Phys 104:4788–4801
Khalatur PG, Khokhlov AR, Mologin DA, Reineker P (2003) Aggregation and counterion condensation in solution of charged proteinlike copolymers: a molecular-dynamics study. J Chem Phys 119:1232–1247. doi:10.1063/1.1579683
Khan MO, Mel’nikov SM, Jonsson B (1999) Anomalous salt effects on DNA conformation: experiment and theory. Macromolecules 32:8836–8840. doi:10.1021/ma9905627
Lee N, Thirumalai D (2001) Dynamics of collapse of flexible polyelectrolytes in poor solvents. Macromolecules 34:3446–3457. doi:10.1021/ma001604q
Micka U, Holm C, Kremer K (1999) Strongly charged, flexible polyelectrolytes in poor solvents: molecular dynamics simulations. Langmuir 15:4033–4044. doi:10.1021/la981191a
Schneider S, Linse P (2004) Discontinuous volume transitions in cross-linked polyelectrolyte gels induced by short-range attractions and strong electrostatic coupling. Macromolecules 37:3850–3856. doi:10.1021/ma035512n
Valleau JP, Cohen LK (1980) Primitive model electrolytes. I. Grand canonical Monte Carlo computations. J Chem Phys 72:5935–5941. doi:10.1063/1.439092
Pelton R (2010) Poly(N-isopropylacrylamide) (PNIPAM) is never hydrophobic. J Colloid Interface Sci 348:673–674. doi:10.1016/j.jcis.2010.05.034
Edgecombe S, Linse P (2007) Monte Carlo simulation of polyelectrolyte gels: effects of polydispersity and topological defects. Macromolecules 40:3868–3875. doi:10.1021/ma0700633
Kim SM, Choi JS, Bae YC (2014) Swelling behaviors of poly(N-isopropylacrylamide) nanosized hydrogel particles/poly(vinyl alcohol)/water systems: effect of the degree of hydrolysis of PVA. Macromol Chem Phys 215:210–216. doi:10.1002/macp.201300662
Lee SM, Lee JH, Bae YC (2014) Swelling behaviors of poly(methyl methacrylate) nano-sized gels in PEG/alcohol solutions. Fluid Phase Equilib 382:107–115. doi:10.1016/j.fluid.2014.09.003
Lee SM, Bae YC (2014) Enhanced solvation effect of re-collapsing behavior for cross-linked PMMA particle gel in aqueous alcohol solutions. Polymer (Guildf) 55:4684–4692. doi:10.1016/j.polymer.2014.07.033
Lee SM, Bae YC (2014) Swelling behaviors of doubly thermosensitive core-shell nanoparticle gels. Macromolecules 47:8394–8403. doi:10.1021/ma5020897
Acknowledgments
The authors thank the financial support from the following institutions: (i) ‘Ministerio de Economía y Competitividad, Plan Nacional de Investigación, Desarrollo e InnovaciónTecnológica (I + D + i)’, Projects MAT2012-36270-C04-01 and -04; and (ii) European Regional Development Fund (ERDF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ahualli, S., Maroto-Centeno, J.A., Pikabea, A. et al. Coarse-grained simulation study of dual-stimuli-responsive nanogels. Colloid Polym Sci 294, 735–741 (2016). https://doi.org/10.1007/s00396-016-3832-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-016-3832-8