Abstract
Biodegradable micelles based on disulfide-linked monomethoxy poly(ethylene glycol)-b-poly(methyl methacrylate)2 (mPEG-SS-PMMA2) miktoarm star block copolymers were synthesized through atom transfer radical polymerization procedure and applied to solubilization and delivery of methotrexate (MTX). The synthesized miktoarm star block copolymers were characterized by 1H nuclear magnetic resonance, Fourier transform infrared spectra, and gel permeation chromatography. The block copolymers were able to self-assemble into spherical micelles in water with an average diameter up to 130 nm by transmission electron microscopy observation and dynamic light scattering measurements and have a critical micelle concentration of 0.91 mg/L. When MTX was incorporated into obtained micelles, high drug loading capacity was found attributed to the designed miktoarm star-shaped nanostructure. Interestingly, in presence of dithiothreitol, the micelles could be degraded into polymer chains by cleavage of the disulfide linkages. The in vitro release studies revealed that these micelles released over 95 % of MTX within 48 h under a reductive environment analogous to that of the cell comparing to minimal drug release (<22 %) under nonreductive conditions. Cell cytoxicity experiments showed that the obtained micelles exhibited nontoxic, and the drug-loaded micelles exhibited high anticancer efficacy and better biocompatibility as compared to free MTX. All of these results showed that mPEG-SS-PMMA2 micelles are promising carriers for delivery of hydrophobic antitumor drugs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite the vigorous exploration of various antitumor drugs in recent years, clinical outcomes have been disappointing because of the severe side effects. As one of the most promising drug-carrier systems, during the past 2 decades, micelles prepared from amphiphilic macromolecules have been widely developed for application as antitumor drug carriers to overcome this problem [1–5]. Amphiphilic macromolecules can self-assemble into micelles in aqueous solution through the hydrophobic interactions among the core-forming blocks. Subsequently, the hydrophobic core serves as a container for hydrophobic drugs, and the outer shell composed of hydrophilic polymers forms hydration barrier to provide colloidal stability [6, 7]. These nanosized micelles possess several unique features, such as enhancing the water solubility of hydrophobic drugs, prolonging the circulation time, improving the preferential accumulation at tumor sites by the enhanced permeability and retention (EPR) effect, and reducing side effects [8–11]. The most studied was the micelles self-assembled from linear block polymers, which have been extensively investigated for use as drug delivery carriers [12, 13]. However, since the micelles can be formed in solution only above the critical micelle concentration (CMC), the micelles from linear block polymers suffer from instability in vivo once the concentration of the copolymer falls below the CMC in the bloodstream, which may cause serious toxicity problems and extremely limit their in vivo application [14]. In addition, another major point that influences the performance of polymeric micelles for drug delivery is low loading capacity due to low lipophilic interior space in hydrophobic core of linear block copolymers [15].
Compared to amphiphilic linear block copolymers, amphiphilic star block copolymers exhibit unique phase-separation behavior in selected solution [16, 17]. This feature makes them able to form micelles which are stable in selective media at relatively high concentrations without association and at infinite dilution without dissociation [18]. In the case of star block copolymers, as the number of arms increases, the CMC will be decreased until a certain threshold [19]; therefore, they have lower CMC compared to micelles from amphiphilic linear block copolymers. In addition, star block copolymer micelles possess larger interior space which can accommodate more drugs [20]. Benefiting from these advantages, star block copolymer micelles will make it possible to achieve an enhanced therapy effect for polymeric drug delivery system. Miktoarm star block copolymer is a classification of star copolymer which has more than two building blocks linked to a single junction point [21]. This feature has provoked considerable interest in the preparation of a variety of miktoarm star block copolymers with varying arm numbers, chemical composition, and chain topology [22–24]. The most studies of miktoarm star polymers were concerned with the synthesis of tailored architecture and structure-morphology relationships; however, in contrast to the case of conventional star block copolymers, miktoarm star block copolymer is much less explored in application as drug delivery carrier [25]. On the other hand, while entering into the body, the micelles based on conventional nondegradable polymers would accumulate in the host cells and tissues, and interact with them, causing a long-term toxicity. Accordingly, biodegradable drug carriers are optimal to remove side effect such as redox stimulus responsive polymeric micelles for intracellular drug delivery [26–30]. Although biodegradable polymeric micelles sensing pH decreases in the acidic endocytic compartments has been reported to achieve tumor-targeted drug delivery for years [31], recently, to extend the stimuli-sensitive drug delivery system, polymer micelles with redox stimulus sensitivity demonstrated effective drug release behavior by large difference in reducing potential between the tumor tissues and normal tissues, with at least fourfold higher concentrations of GSH in the tumor tissues over normal tissues. Therefore, redox stimulus responsive polymeric micelles formed from miktoarm star copolymers are of particular interest for hydrophobic drug delivery.
Poly(methyl methacrylate) (PMMA) had been widely used for the hydrophobic segment in biomedical field as a nontoxic polymer. PMMA had a significant history and track record of safety in implantable medical device applications [32] and was reported to release a variety of drugs for many different applications [33–35]. Therefore, because of its excellent biocompatibility, biodegradability, and hydrophobicity, PMMA could be employed for the fabrication of drug delivery systems both in vitro and in vivo.
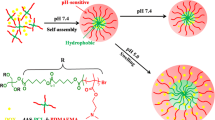
In the present work, we reported the preparation of biodegradable micelles from miktoarm star block copolymers, mPEG-SS-PMMA2, via atom transfer radical polymerization (ATRP) for controlled release of methotrexate (MTX) (Scheme 1). The characterization data were reported from analyses using 1H nuclear magnetic resonance (NMR), gel permeation chromatography (GPC), and Fourier transform infrared (FTIR) spectra. On the basis of its amphiphilic structure, mPEG-SS-PMMA2 self-assembled into nanosized micelles by utilizing PMMA blocks as the hydrophobic core and PEG the hydrophilic shell in aqueous solution, which was investigated by transmission electron microscopy (TEM) observation and dynamic light scattering (DLS) measurements. The CMC and drug loading capacity of the micelles for the anticancer drug MTX were measured. In addition, in vitro release behavior and cell cytotoxicity of MTX-loaded micelles were examined respectively. According to the experimental results, a high drug loading content, redox stimulus sensitivity, as well as good cell-biocompatibility for mPEG-SS-PMMA2-based anticancer drug delivery system, was observed. To the best of our knowledge, this is the first reported example for MTX delivery based on miktoarm star block copolymer.
Experimental section
Materials
Monomethoxy poly(ethylene glycol) cystamine (mPEG-cystamine) (Mn = 2000, BK Biochem Tech Co., Chengdu), 3,5-dihydroxy benzoic acid (98 %, Adamas-beta), 2-bromo-2-methylpropanoyl bromide (98 %, Adamas-beta), N,N-dicyclohexylcarbodiimide (DCC) (99 %, Sigma), N-hydroxysuccinimide (NHS) (98 %, Aladdin), N,N,N′,N″,N″-pentamethyl diethylenetriamine (PMDETA) (98 %, TCI), and triethylamine (98 %, Changzheng Chemical Reagents Co., Chengdu) were used as received. Methyl methacrylate (MMA), N,N-dimethylformamide (DMF), and tetrahydrofuran (THF) (Changzheng Chemical Reagents Co., Chengdu) were used after purified and distilled. Chlorobenzene was purified by washing with concentrated sulfuric acid to remove residual thiophenes, followed by washing with a 5 % sodium carbonate solution, and again with water, then dried with anhydrous calcium chloride and finally distilled.
Synthesis of 3,5-bis(2-bromo-2-methylpropanoyloxy)benzoic acid (BPA) (1)
3,5-Dihydroxy benzoic acid (4.0 g, 25.9 mmol) and K2CO3 (19.4 g, 130.0 mmol) were dissolved in the mixer of deionized water (64.0 mL) and isopropanol (26.0 mL), and 2-bromo-2-methylpropionyl bromide (6.0 mL, 57.1 mmol) was added dropwise slowly. The reaction mixture was cooled in ice water and was stirred at 0 °C for 2 h and then at room temperature for 7 h. The filtered solution was washed with water for three times and distilled under vacuum. A purified product was easily obtained by passing through a silica gel column (yield 7.9 g (68 %), 1H NMR (CDCl3), δ 7.7 (d, 2H, C6H3), 7.2 (t, 1H, C6H3), 2.1 (s, 12H, (CH3)2CBr).
Synthesis of bromo-functionized macroinitiator (2)
To a round flask was added mPEG-cystamine (1.2 g 0.5 mmol), the obtained 3,5-bis(2-bromo-2-methylpropanoyloxy)benzoic acid (0.4 g, 0.8 mmol), DCC (0.1 g, 0.5 mmol), NHS (59 mg, 0.5 mmol), and 20 mL of dichloromethane under nitrogen. The mixture was stirred at room temperature for 30 h. After filtration, the eluent was concentrated and precipitated in cooled, anhydrous diethyl ether. The sediments were collected and dried to constant weight in a vacuum oven at room temperature to yield macroinitiator, mPEG-Br2, as a white powder.
Synthesis of mPEG-SS-PMMA2 star block copolymers (3) and characterization
In a typical experiment for polymerization, MMA (0.6 g, 6 mmol), bromo-functionalized macroinitiator (72.9 mg, 30 μmol), CuBr (4.2 mg, 30 μmol), PMDETA (5.1 mg, 30 μmol), and chlorobenzene (2 mL) were charged into a polymerization tube under N2 atmosphere. After stirring and degassing by three freeze-thaw cycles, the tube was sealed under vacuum. Subsequently, the tube was immerged into a thermostated oil bath at 90 °C for 12 h and then quenched in cold water and diluted in THF to room temperature. The solution was passed through a neutral alumina column in order to remove copper salt. The polymer was precipitated in a large volume of cold methanol and dried in a vacuum overnight. The different feed of reactant weight ratios are shown in Table 1.
1H NMR spectra were obtained using a Bruker 400-MHz spectrometer with deuterated chloroform (CDCl3) as solvent and tetramethylsilane (TMS) as an internal standard. GPC experiments were conducted on a Waters 1515 solvent pump instrument equipped with a Waters 2410 refractive index detector. DMF was used as eluent and delivered at a flow rate of 1.0 mL/min. The calibration curve was obtained with linear polystyrenes as standards. FTIR data were gathered in solid state, utilizing FTIR at 4-cm−1 resolution (Spectrum One, PE).
For 1H NMR analysis of macroinitiator, the ratio of the integrated peak areas of the methylene group in the glycol unit to the methyl proton of end group of mPEG was determined and utilized to calculate the molecular weight. For miktoarm star copolymers, the ratio of the integrated peak areas of the methylene group in backbone of PMMA blocks to the methyl proton of end group of mPEG was determined, and the molecular weight was calculated with the following equation:
where n is the number of arms (2), M m is the molecular weight of MMA monomer, and M I is the molecular weight of the macroinitiator. IMethylene and IMethyl are the corresponding NMR proton integration areas in PMMA backbone and mPEG end group, respectively.
Micelles preparation and characterization
The MTX-loaded micelles and empty micelles were prepared by a dialysis method by dissolving mPEG-SS-PMMA2 (10 mg) in 2 mL of DMF with and without MTX (2 mg). Under gentle stirring, the solution was added dropwise to 5 mL of double-distilled water in 1 h. The solution was transferred to a dialysis bag (MWCO 5000) and dialyzed for 24 h to remove the organic solvents and free MTX. The micellar solution was further filtered to remove the residual MTX aggregates. To obtain micelle powders, the micellar solution was frozen and lyophilized.
The CMC of the micelles was measured using fluorescent pyrene as a probe on a fluorescence spectrophotometer (Hitach F7000 FL). The final concentration of pyrene was kept at 6.0 × 10−7 g/L. The excitation spectra were scanned from 215 to 370 nm at a fixed emission wavelength of 390 nm with bandwidth 2.5 nm. The ratios of pyrene probe fluorescence intensity at 339 and 334 nm (I 339/I 334) were calculated and plotted against the concentration logarithm of micelles. CMC was obtained from the intersection of two tangent plots of intensity ratio I 339/I 334 versus the logarithm of polymer concentrations.
DLS measurements were performed in aqueous solution using a Malvern Zetasizer Nano-ZS90 apparatus equipped with a 4.0-mW laser operating at λ = 633 nm. All samples of 1 mg/mL were measured at 20 °C, and intensity for each sample was collected in three replicates and yielded the size fraction distribution plots. TEM observation was performed with a Hitachi H-600 instrument. The samples were prepared by directly dropping the solution of micelles onto carbon-coated copper grids and dried at room temperature overnight without staining before measurement.
DL content and EE
To determine MTX loading level and encapsulation efficiency, a known amount of freeze-dried MTX-loaded micelles was dissolved in DMF, and the absorbance at 302 nm was measured on a UV-Vis spectrophotometer. The weight of encapsulated MTX in the micelles was calculated with a standard curve obtained from MTX/DMF solutions at a series of MTX concentrations. Drug loading (DL) and encapsulation efficiency (EE) were calculated as Eqs. 1 and 2
Reduction-triggered disassembly of mPEG-SS-PMMA2 micelles
The size change of micelles in response to reductive condition in PBS buffer was followed by DLS measurement [27]. Ten millimolar DTT was added in the solution of mPEG-SS-PMMA2 micelles in PBS buffer (pH 7.4, 10 mM). At different time points, the size of micelles was measured by DLS.
In vitro release of MTX
MTX-loaded micelles at a concentration of 1 mg/mL (2 mL) in a dialysis membrane tube (MWCO 5000) were incubated in three different media: PBS (10 mM, pH 7.4) with 1 mM DTT, PBS (10 mM, pH 7.4) with 10 mM DTT, and PBS (10 mM, pH 7.4) without DTT at 37 °C under gentle stirring. At specified time intervals, the MTX content in the samples was analyzed with a UV absorbance at 302 nm. Three replicate measurements were carried out for each time point.
In vitro cell experiments
Human cervical carcinoma HeLa cells were provided by school of biomedical science at Chengdu medical college. HeLa cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10 % heat-inactivated fetal bovine serum (FBS) and penicillin at 50 U/mL at 37 °C in a humidified atmosphere of 5 % CO2. For experiments, HeLa cells were seeded into 96-well plate at a density of 5000 cells/well. The medium was replaced by 90 μL of fresh DMEM medium containing 10 % FBS, and then 10 μL samples with final concentrations (10−3∼1.0 mg/mL) of the micelle suspensions in phosphate buffer (10 mM, pH 7.4) were added.
After a 48-h incubation period, the relative cytotoxicity of micelles with and without MTX was estimated by an MTT viability assay. Twenty microliters of MTT solution in PBS (5 mg/mL) was added to each well and incubated for 4 h. Afterward, the MTT-containing medium was aspirated, and 200 μL of dimethyl sulfoxide (DMSO) was added to each well to extract the formazan products with gentle agitation for 10 min. The absorbance of the extracts was measured at 570 nm on a Bio-Rad 550 microplate reader. The cell viability was calculated as follows:
where OD was the absorbance at the presence of sample solutions and ODControl was the absorbance without treatment. All tests were performed in three replicate measurements.
Results and discussion
Synthesis of biodegradable miktoarm star block copolymers
In this study, different miktoarm star block copolymers, mPEG-SS-PMMA2, were synthesized via a copper-mediated ATRP procedure by varying the macroinitiator/MMA feed weight ratio (shown in Table 1). The synthetic route is shown in Scheme 2, which was started with the preparation of star-shaped bifunctional agent, 3,5-bis(2-bromo-2-methylpropanoyloxy)benzoic acid, followed by an amidation reaction with mPEG-cystamine in the presence of DCC and NHS to synthesize the bromo-functionized macroinitiator. The macroinitiator was used to trigger ATRP of MMA monomers in chlorobenzene using CuBr complexed by PMDETA according to published procedure [36].
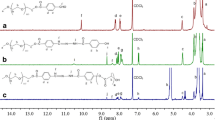
The proton of bromo-functionized macroinitiator and miktoarm star copolymer was clearly identified by 1H NMR. Figure 1a shows the 1H NMR spectrum of synthesized mPEG-Br2 in CDCl3 as described in experimental section. The resonance signals of protons of phenyl group (e and f), glycol unit (b), methyl end group (a) in PEG block, methylene protons (c and d) neighboring to the disulfide bond, and methyl group (g) closed to bromine atom appeared at δ = 7.6, 7.2, 3.6–3.7, 3.4, 3.5, 3.1, and 2.0 ppm, respectively. The 1H NMR spectrum of miktoarm star copolymer, mPEG-SS-(PMMA39)2, is shown in Fig. 1b. The proton signals of methoxy group (f), vinyl backbone (g), and methyl group (h) in PMMA block appeared at δ = 3.6–3.7, 1.6, and 0.8–1.0 ppm, respectively. FTIR was also used to investigate the group structure of macroinitiator and miktoarm star copolymers for the completion of the ATRP polymerization procedure. The FTIR spectrum of macroinitiator (shown in Fig. 2a), mPEG-Br2, clearly revealed the presence of the vibrational band of methylene group at 2886 cm−1, and 1116 cm−1 assigned to the C-O group, which were characteristics of glycol unit in PEG blocks. In Fig. 2b, the strong absorbance at 1735 cm−1 was assigned to the vibrations of C = O in PMMA blocks, which was indicated the completion of ATRP polymerization.
The structure of the copolymer was further verified by GPC to determine the difference of polymer molecular weight and PDI, despite the fact that GPC often underestimates the molecular weight of star polymers because these star polymers are more globular than linear coil polymers [37]. GPC analyses showed the total disappearance of macroinitiator signal from miktoarm star copolymers indicating an effective ATRP polymerization (shown in Fig. 3), and narrow molecular weight distributions denoted high control of the polymerization process. The results of GPC analysis and 1H NMR estimation of the molecular weight of copolymers are summarized in Table 1.
Micellization of mPEG-SS-PMMA2 and redox-responsive destabilization
For their distinctive core-shell structure, most amphiphilic macromolecules can form micellar structures in water. Accordingly, the dialysis method was used to induce self-assembly of the micelles of mPEG-SS-PMMA2 copolymers in water by hydrophobic interactions between PMMA arms, whereas the outer shell of hydrophilic PEG maintains a hydration barrier to provide colloidal stability to the micelles. The CMC of micelles was evaluated by the fluorescence technique using pyrene as a probe. With increasing concentration, a red shift from 334 to 339 nm of pyrene in the excitation spectrum occurs, reflecting the change in environmental polarity. The ratio of pyrene fluorescence intensities excited at 339 and 334 nm (I 339/I 334) was plotted as a function of the logarithm of micellar concentrations. As illustrated in Fig. 4, the intensity ratio remained almost unchanged at low copolymer concentrations. As the copolymer concentration increased, once the polymer concentration reached the CMC, an abrupt increase occurred indicating the formation of micelles, that was, pyrene transferred into the hydrophobic core of the micelles. Based on the experimental results, mPEG-SS-(PMMA39)2 showed a low CMC of 0.91 mg/L, which provided the possibility of keeping stabilization of micelles and allowing their use in very dilute aqueous solution such as bloodstream and body fluids. However, the CMC of mPEG-SS-(PMMA76)2 could not be obtained by the same method, which can be explained by that the long hydrophobic segments of PMMA would lead to fast aggregation into precipitations by hydrophobic interaction [38, 39].
Due to the size of the micelle which is an important parameter for drug delivery, micelles less than 200 nm are beneficial to maintaining a lower level of reticuloendothelial system (RES) uptake, minimal renal excretion, and the ability to take advantage of the EPR effect for passive targeting delivery for tumor sites. The micellar structure was further confirmed by DLS and TEM. DLS measurements showed that mPEG-SS-(PMMA39)2 formed micelles with sizes of about 130 nm in average (shown in Fig. 5a). In Fig. 5b, TEM micrograph revealed that these micelles assembled into a spherical morphology with an average size of about 100 nm. The different diameter obtained from DLS measurement and TEM observation may be due to the shrinkage of the PEG shell induced by water evaporation under the high vacuum conditions before TEM observation [40].
The size change of micelles in response to DTT in PBS buffer (pH 7.4, 10 mM) was followed by DLS measurement (Fig. 6). Following addition of 10 mM DTT, fast aggregation was observed for mPEG-SS-(PMMA39)2 micelles, in which micelle size increased from 125 to 300 nm in 2 h, reaching over 1000 nm after 24 h (shown in Fig. 5). The aggregates were formed because of probably reductive cleavage of the intermediate disulfide bonds, which results in shedding of the PEG shells. In contrast, no remarkable change in micelle sizes was discerned after 24 h in the absence of DTT under the same conditions. Although the DLS data collected from 6 to 24 h were similar, the micelle sizes were quite different. The average micelle size was about 300 nm in 6 h after addition of DTT, but parts of the aggregates reached over 1000 nm in 24 h. It was indicated that parts of the micelles formed large aggregates due to reductive cleavage of the disulfide linkers by DTT, despite the fact that the other parts were not so sensitive to the reductive reagent because of the chemical efficiency. This phenomenon revealed that the copolymer mPEG-SS-(PMMA39)2 possessed the redox stimulus sensitivity and might be a potential intracellular stimulus sensitive micelles for drug delivery.
Loading and reduction-triggered release of MTX
MTX, an antineoplastic agent with a very low solubility in water, is one of the most routinely used and potent drug in anticancer treatment [41]. In order to improve the solubility and enhance the therapeutic efficiency, MTX was chosen as a model drug to be entrapped into the hydrophobic cores of the polymeric micelles. Recently, several examples of linear polymeric micelle have been explored for MTX delivery [42, 43]. However, no example concerned with redox-responsive, miktoarm star block polymeric micelles was reported so far. Therefore, it will be very interesting to develop star-shaped polymer-based redox stimulus responsive delivery systems for MTX delivery, which may lead to the enhanced cancer chemotherapy.
The MTX-loaded micelles were prepared by the dialysis technique, which was detailed in the experimental section. The drug loading content and encapsulation efficiency were determined by fluorescence measurement. The experimental results showed a high drug loading and encapsulation efficiency up to 64 % and 16 % respectively for mPEG-SS-(PMMA39)2 micelles. The release behavior of free MTX and that from mPEG-SS-(PMMA39)2 micelles was investigated using a dialysis membrane tube in PBS (10 mM, pH 7.4) with 1 mM DTT, PBS (10 mM, pH 7.4) with 10 mM DTT, and PBS (10 mM, pH 7.4) without DTT at 37 °C. The drug release data was shown in Fig. 7. In contrast with the burst release of free MTX in first 7 h, sustained releases from the mPEG-SS-(PMMA39)2 micelles were observed. The sustained release of MTX from mPEG-SS-(PMMA39)2 micelles could be attributed to the hydrophobic interactions between the MTX molecules and the hydrophobic PMMA core of the nanosized micelles.
From Fig. 7, it was found that the reductive condition had a significant effect on the MTX release from polymeric micelles. The experimental results showed that in the presence of 10 mM DTT, a reductive environment analogous to that of the intracellular compartments such as cytosol and the cell nucleus, the drug-loaded micelles exhibited a much faster drug release rate and 95 % of the encapsulated MTX was released within 48 h, while it released only 22 % of loaded MTX in the absence of DTT. In this study, drug release rates from mPEG-SS-(PMMA39)2 micelles increased in parallel with increasing DTT concentrations: 10 mM DTT > 1 mM DTT > 0 mM DTT, indicating that the polymer degradation was the key factor affecting the drug release rate. It was also verified the feasibility to modulate the release kinetics of micelle-encapsulated drug by chemically cleaving the hydrophilic shell from the micelles. Therefore, regarding the reductive condition in tumor cell, disassembly of mPEG-SS-(PMMA39)2 micelles via reductive cleavage of disulfide linkage seems a promising approach to accelerate payload release under tumor-relevant conditions.
In vitro cytotoxicity
A cell cytotoxicity study was carried out to investigate whether the redox stimulus responsive micelles affect cell proliferation of the Human cervical carcinoma HeLa cell. Results of in vitro cytotoxicity are described in Fig. 8. It should be noted that the blank micelles of mPEG-SS-(PMMA39)2 show almost nontoxicity to HeLa cells up to concentration of 1 mg/mL following 48-h incubation (shown in Fig. 8a). However, encapsulation of MTX into these nanomicelles effectively reduced viability of HeLa cells (Fig. 8b). It showed that the cell viability was dose-dependent, and MTX-loaded mPEG-SS-(PMMA39)2 micelles had a lower cytotoxicity by the observed IC50 (half-maximal inhibitory concentration) of 4.19 μg/mL, comparing with that observed for free MTX (IC50 = 0.71 μg/mL), which was mostly due to the slower release of MTX from micelles and the delayed nuclear uptake of MTX in HeLa cells by PEG shell of micelles as barriers for drug release. Based on the above experimental results, these redox-sensitive biodegradable micelles with good biocompatibility and intracellular redox-responsive drug release are highly promising for cancer therapy.
Conclusion
In summary, biodegradable micelles based on miktoarm star block copolymer, mPEG-SS-PMMA2, have been prepared and characterized. The micelles were of low CMC, a high drug loading efficiency for MTX, and displayed low drug release under a nonreductive environment but a rapid release of MTX in response to the reductive conditions. The cell cytotoxicity study showed that the micelles exhibited nontoxic, and the drug-loaded micelles displayed pronounced antitumor activity toward HeLa cells. We are convinced that this kind of polymeric micelle holds great promise for efficient cytoplasm delivery and the potential usage as a hydrophobic drug carrier to enhance the efficiency of cancer chemotherapy.
References
Scarano W, Duong HTT, Lu H, De Souza PL, Stenzel MH (2013) Folate conjugation to polymeric micelles via boronic acid ester to deliver platinum drugs to ovarian cancer cell lines. Biomacromolecules 14:962–975
Liu J, Pang Y, Huang W, Huang X, Meng L, Zhu X, Zhou Y, Yan D (2011) Bioreducible micelles self-assembled from amphiphilic hyperbranched multiarm copolymer for glutathione-mediated intracellular drug delivery. Biomacromolecules 12:1567–1577
Laouini A, Koutroumanis KP, Charcosset C, Georgiadou S, Fessi H, Holdich RG, Vladisavljević GT (2013) pH-Sensitive micelles for targeted drug delivery prepared using a novel membrane contactor method. ACS Appl Mater Inter 5:8939–8947
Zhou Q, Lin J, Wang J, Li F, Tang F, Zhao X (2009) A designed amphiphilic peptide containing the silk fibroin motif as a potential carrier of hydrophobic drugs. Prog Nat Sci 19:1529–1536
Jin X, Mo R, Ding Y, Zheng W, Zhang C (2014) Paclitaxel-loaded n-octyl-o-sulfate chitosan micelles for superior cancer therapeutic efficacy and overcoming drug resistance. Mol Pharm 11:145–157
Zhang L, Eisenberg A (1995) Multiple morphologies of “Crew-Cut” aggregates of polystyrene-b-poly (acrylic acid) block copolymers. Science 268:1728–1731
Zhang L, Yu K, Eisenberg A (1996) Ion-induced morphological changes in “Crew-Cut” aggregates of amphiphilic block copolymers. Science 272:1777–1779
Torchilin VP (2006) Multifunctional nanocarriers. Adv Drug Delivery Rev 58:1532–1555
Kwon GS, Kataoka K (1995) Block copolymer micelles as long-circulating drug vehicles. Drug Delivery Rev 16:295–309
Kataoka K, Harada A, Nagasaki Y (2001) Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Delivery Rev 47:113–131
Lin J, Luo J-B, Yang S-T, Zhou Q-H (2013) Template-directed self-assembly of a designed amphiphilic hexapeptide on mica surface. Colloid Polym Sci 291:2263–2270
Yang J, Yan J, Zhou Z, Amsden BG (2014) Dithiol-PEG-PDLLA micelles: preparation and evaluation as potential topical ocular delivery vehicle. Biomacromolecules 15:1346–1354
Ma R, Yang H, Li Z, Liu G, Sun X, Liu X, An Y, Shi L (2012) Phenylboronic acid-based complex micelles with enhanced glucose-responsiveness at physiological ph by complexation with glycopolymer. Biomacromolecules 13:3409–3417
Wang F, Bronich TK, Kabanov AV, Rauh RD, Roovers J (2005) Synthesis and evaluation of a star amphiphilic block copolymer from poly (ε-caprolactone) and poly (ethylene glycol) as a potential drug delivery carrier. Bioconjugate Chem 16:397–405
Wang F, Bronich TK, Kabanov AV, Rauh RD, Roovers J (2008) Synthesis and characterization of star poly (ε-caprolactone)-b-poly (ethylene glycol) and poly (l-lactide)-b-poly (ethylene glycol) copolymers: evaluation as drug delivery carriers. Bioconjugate Chem 19:1423–1429
Pavlov GM, Knop K, Okatova OV, Schubert US (2013) Star-brush-shaped macromolecules: peculiar properties in dilute solution. Macromolecules 46:8671–8679
Rao J, Zhang Y, Zhang J, Liu S (2008) Facile preparation of well-defined AB2 Y-shaped miktoarm star polypeptide copolymer via the combination of ring-opening polymerization and click chemistry. Biomacromolecules 9:2586–2593
Cao W, Zhou J, Mann A, Wang Y, Zhu L (2011) Folate-functionalized unimolecular micelles based on a degradable amphiphilic dendrimer-like star polymer for cancer cell-targeted drug delivery. Biomacromolecules 12:2697–2707
Poree DE, Giles MD, Lawson LB, He J, Grayson SM (2011) Synthesis of amphiphilic star block copolymers and their evaluation as transdermal carriers. Biomacromolecules 12:898–906
Ni C, Wu G, Zhu C, Yao B (2010) The Preparation and characterization of amphiphilic star block copolymer nano micelles using silsesquioxane as the core. J Phys Chem C 114:13471–13476
Lin J, Zhou Q-H, L-d L, Li Z-N (2014) Synthesis and self-assembly in bulk of star-shaped block copolymers based on helical polypeptides. Colloid Polym Sci 292:3177–3185
Junnila S, Houbenov N, Karatzas A, Hadjichristidis N, Hirao A, Iatrou H, Ikkala O (2012) Side-chain-controlled self-assembly of polystyrene–polypeptide miktoarm star copolymers. Macromolecules 45:2850–2856
Ostermann J, Merkl J-P, Flessau S, Wolter C, Kornowksi A, Schmidtke C, Pietsch A, Kloust H, Feld A, Weller H (2013) Controlling the physical and biological properties of highly fluorescent aqueous quantum dots using block copolymers of different size and shape. ACS Nano 7:9156–9167
Ye C, Zhao G, Zhang M, Du J, Zhao Y (2012) Precise synthesis of ABCDE star quintopolymers by combination of controlled polymerization and azide–alkyne cycloaddition reaction. Macromolecules 45:7429–7439
Liu H, Li S, Zhang M, Shao W, Zhao Y (2012) Facile synthesis of ABCDE-type H-shaped quintopolymers by combination of ATRP, ROP, and click chemistry and their potential applications as drug carriers. J Polym Sci A-Polym Chem 50:4705–4716
McRae Page S, Martorella M, Parelkar S, Kosif I, Emrick T (2013) Disulfide cross-linked phosphorylcholine micelles for triggered release of camptothecin. Mol Pharm 10:2684–2692
Sun H, Guo B, Cheng R, Meng F, Liu H, Zhong Z (2009) Biodegradable micelles with sheddable poly (ethylene glycol) shells for triggered intracellular release of doxorubicin. Biomaterials 30:6358–6366
Sun Y, Huang Y, Bian S, Liang J, Fan Y, Zhang X (2013) Reduction-degradable PEG-b–PAA-b–PEG triblock copolymer micelles incorporated with MTX for cancer chemotherapy. Colloids Surf B 112:197–203
Zhang Y, Jin T, Zhuo R-X (2005) Methotrexate-loaded biodegradable polymeric micelles: preparation, physicochemical properties and in vitro drug release. Colloids Surf B Biointerfaces 44:104–109
Chassepot A, Gao L, Nguyen I, Dochter A, Fioretti F, Menu P, Kerdjoudj H, Baehr C, Schaaf P, Voegel J-C (2012) Chemically detachable polyelectrolyte multilayer platform for cell sheet engineering. Chem Mater 24:930–937
Sui X, Zhang Z, Guan S, Xu Y, Li C, Lv Y, Chen A, Yang L, Gao L (2015) A facile strategy for the synthesis of block copolymers bearing an acid-cleavable junction. Polym Chem 6:2777–2782
Jäger M, Wilke A (2003) Comprehensive biocompatibility testing of a new PMMA-HA bone cement versus conventional PMMA cement in vitro. J Biomater Sci Polymer Edn 14:1283–1298
Ding X, Liu Y, Li J, Luo Z, Hu Y, Zhang B, Liu J, Zhou J, Cai K (2014) Hydrazone-bearing PMMA-functionalized magnetic nanocubes as pH-responsive drug carriers for remotely targeted cancer therapy in vitro and in vivo. ACS Appl Mater Interfaces 6:7395–7407
Streubel A, Siepmann J, Bodmeier R (2003) Multiple unit gastroretentive drug delivery systems: a new preparation method for low density microparticles. J Microencapsul 20:329–347
Sparnacci K, Laus M, Tondelli L, Bernardi C, Magnani L, Corticelli F, Marchisio M, Ensoli B, Castaldello A, Caputo A (2005) Core–shell microspheres by dispersion polymerization as promising delivery systems for proteins. J Biomater Sci Polym Ed 16:1557–1574
Zhou Q-H, Zheng J-K, Shen Z, Fan X-H, Chen X-F, Zhou Q-F (2010) Synthesis and hierarchical self-assembly of rod-rod block copolymers via click chemistry between mesogen-jacketed liquid crystalline polymers and helical polypeptides. Macromolecules 43:5637–5646
Matyjaszewski K, Miller PJ, Pyun J, Kickelbick G, Diamanti S (1999) Synthesis and characterization of star polymers with varying arm number, length, and composition from organic and hybrid inorganic/organic multifunctional initiators. Macromolecules 32:6526–6535
Hanson JA, Li Z, Deming TJ (2010) Nonionic block copolypeptide micelles containing a hydrophobic rac-leucine core. Macromolecules 43:6268–6269
Ren T-B, Xia W-J, Dong H-Q, Li Y-Y (2011) Sheddable micelles based on disulfide-linked hybrid PEG-polypeptide copolymer for intracellular drug delivery. Polymer 52:3580–3586
Hu Y, Zhang L, Cao Y, Ge H, Jiang X, Yang C (2004) Degradation behavior of poly (ε-caprolactone)-b-poly (ethylene glycol)-b-poly (ε-caprolactone) micelles in aqueous solution. Biomacromolecules 5:1756–1762
Kang H, Kim J-D, Han S-H, Chang I-S (2002) Self-aggregates of poly (2-hydroxyethyl aspartamide) copolymers loaded with methotrexate by physical and chemical entrapments. J Control Release 81:135–144
Ashwanikumar N, Kumar NA, Nair SA, Kumar GSV (2014) Dual drug delivery of 5-fluorouracil (5-FU) and methotrexate (MTX) through random copolymeric nanomicelles of PLGA and polyethylenimine demonstrating enhanced cell uptake and cytotoxicity. Colloids Surf B Biointerfaces 122:520–528
Baranello MP, Bauer L, Benoit DSW (2014) Poly (styrene-alt-maleic anhydride)-based diblock copolymer micelles exhibit versatile hydrophobic drug loading, drug-dependent release, and internalization by multidrug resistant ovarian cancer cells. Biomacromolecules 15:2629–2641
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 21404086) and the Project of Postgraduate Degree Construction, Southwest University for Nationalities (No. 2015XWD-S0703).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhou, QH., Lin, J., Li, LD. et al. Biodegradable micelles self-assembled from miktoarm star block copolymers for MTX delivery. Colloid Polym Sci 293, 2291–2300 (2015). https://doi.org/10.1007/s00396-015-3610-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3610-z