Abstract
Protein phosphatase 1 (PP1) is a key regulator of important cardiac signaling pathways. Dysregulation of PP1 has been heavily implicated in cardiac dysfunctions. Accordingly, pharmacological targeting of PP1 activity is considered for therapeutic intervention in human cardiomyopathies. Recent evidence from animal models implicated previously unrecognized, isoform-specific activities of PP1 in the healthy and diseased heart. Therefore, this study examined the expression of the distinct PP1 isoforms PP1α, β, and γ in human heart failure (HF) and atrial fibrillation (AF) and addressed the consequences of β-adrenoceptor blocker (beta-blocker) therapy for HF patients with reduced ejection fraction on PP1 isoform expression. Using western blot analysis, we found greater abundance of PP1 isoforms α and γ but unaltered PP1β levels in left ventricular myocardial tissues from HF patients as compared to non-failing controls. However, expression of all three PP1 isoforms was higher in atrial appendages from patients with AF compared to patients with sinus rhythm. Moreover, we found that in human failing ventricles, beta-blocker therapy was associated with lower PP1α abundance and activity, as indicated by higher phosphorylation of the PP1α-specific substrate eIF2α. Greater eIF2α phosphorylation is a known repressor of protein translation, and accordingly, we found lower levels of the endoplasmic reticulum (ER) stress marker Grp78 in the very same samples. We propose that isoform-specific targeting of PP1α activity may be a novel and innovative therapeutic strategy for the treatment of human cardiac diseases by reducing ER stress conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein phosphatase 1 (PP1) is the predominant dephosphorylating enzyme in the heart and accounts for up to 70% of the entire serine/threonine phosphatase activity [20]. Numerous studies have shown that PP1 dephosphorylates a huge subset of substrates in all compartments of the cardiomyocyte, including membrane, sarcoplasmic reticulum, and myofilament proteins, which work together in a finely tuned manner to enable sufficient excitation–contraction (EC) coupling [30]. Enhanced PP1 expression and activity along with decreased phosphorylation of several key calcium handling proteins [e.g., phospholamban (PLN), cardiac myosin binding protein-C (cMyBP-C), and L-type Ca2+-channel (CaV1.2)] has been shown in many cardiac pathologies including heart failure (HF) and atrial fibrillation (AF) [4, 10, 11, 23]. In mice, threefold heart-specific overexpression of PP1α was sufficient to induce severe cardiomyopathy accompanied by a significant decrease (69%) in the phosphorylation of PLN [3].
It is, therefore, not surprising that targeting of PP1 has been postulated as a potential strategy to improve HF phenotypes. However, the usually measured PP1 expression and activity is actually the sum of three different isoforms (α, β, and γ), which are encoded by distinct genes on different chromosomes [10]. Albeit the three isoforms share more than 90% sequence homology, they contain also divergent N- and C-termini with putatively unique interaction sites of substrate or phosphatase regulatory subunit [13]. A number of cell-based and extra-cardiac in vivo studies have demonstrated the isoform-specific dephosphorylation function of PP1. For example, PP1α or γ1, but not PP1β, interacts with neurabins and plays a key role in regulating synaptic transmission in mammalian neurons [27]. However, in skeletal muscle cells, PP1β interacts with the myosin phosphatase targeting subunit 2 (MYPT2) and enhances the dephosphorylation of skeletal muscle myosin [22].
In this study, we examined PP1 isoform expression in different human HF and AF samples using novel and validated PP1 isoform-specific antibodies. To our best knowledge, we are the first, who report selective abundance changes of different PP1 isoforms in human left ventricular (LV) tissues from patients with failing hearts and in right atrial appendage tissues from patients with AF. Interestingly, HF patients with beta-blocker pre-treatment showed lower PP1α abundance accompanied by greater phosphorylation of the PP1α-specific substrate eIF2α, which indicates an augmentation in the endoplasmic reticulum (ER) stress response in cardiomyocytes of HF patients [16].
Methods
Patients
The study conforms with the principles outlined in the Declaration of Helsinki and was reviewed and approved by the ethics committee of the University Hospital Carl Gustav Carus Dresden (Az.: EK 446122011 and EK 1140 82202 and 28/3/07) and the University Medical Center Goettingen (Az.: 31/9/00, this approval is also applicable for the acquisition of samples at the University Hospital Regensburg). Written informed consent was obtained from all participants. A detailed list of patients with brief clinical characteristics can be found in Tables 1, 2, and 3. Frozen non-failing myocardial samples originated from 16 healthy donor hearts that could not be transplanted for technical reasons.
Antibodies, recombinant proteins, and PP1 overexpression lysates
Antibodies Anti-calsequestrin (1:1000, Thermo Fisher Scientific, #PA1-913), anti-PP1α (1:200, Santa Cruz, C-19, Lot #: H0114), anti-PP1β (1:1000, Abcam, ab16369, Lot #: GR279250-3), anti-PP1γ1 (1:1000, Abcam, ab16387, Lot #: GR54077-10), anti-BIP (1:1000, Cell Signaling, #3183, Lot #: 3), anti-eIF2α (1:1000, Cell Signaling, #9722, Lot #: 15), anti-phospho-eIF2α (Ser51) (1:1000, Cell Signaling, #9721, Lot #: 15), and anti-IRE1α (1:1000, Cell Signaling, #3294, Lot #: 9). Recombinant proteins PP1α with N-terminal HIS tag (Origene, Cat.-Nr.: TP760285), PP1β with C-terminal Myc-DDK tag (Origene, Cat.-Nr.: TP301142), and PP1γ with N-terminal HIS tag (Origene, Cat.-Nr.: TP720571). PP1 overexpression lysates lysate of HEK293 cells transfected with Myc-DDK-tagged human PP1α (Origene, Cat.-Nr.: LY400954), PP1β (Origene, Cat.-Nr.: LY404146), and PP1γ (Origene, Cat.-Nr.: LY419153).
Cardiac tissue preparation, SDS-Gel electrophoresis and immunoblotting
Human cardiac tissues were rapidly frozen by nitrogen, pulverized and lysed in lysis buffer (30 mmol/L Tris/HCl pH 8.8, 5 mmol/L EDTA, 30 mmol/L NaF, and 3% SDS) supplemented with protease inhibitor cocktail and phosphatase inhibitor cocktail (Roche Diagnostics). The protein samples were separated by weight using SDS-polyacrylamide gel electrophoreses and subsequently transferred to nitrocellulose membranes. Membranes were blocked with 5% non-fat milk (AppliChem) for 1 h and afterwards incubated with the respective primary antibodies overnight at 4 °C. After repeated washing steps and incubation with appropriate secondary antibodies at room temperature for 1 h, chemiluminescence was detected using a Fusion FX imaging system (Vilber Lourmat) and quantified with the Fusion-Capt Advance (Vilber Lourmat) software.
Statistics
Results are presented as mean ± standard error of the mean (SEM). Data sets were compared by unpaired two-tailed Student’s t test to assess differences between two groups. P values of <0.05 were considered as statistically significant.
Results and discussion
Due to the strong sequence homology of PP1 isoforms and multiple reports showing unspecific and isoform-independent binding of several PP1 isoform-specific antibodies, we first confirmed the specificity of the PP1 isoform-specific antibodies used in this study. We either applied recombinant proteins of the (human) PP1 isoforms α, β, or γ or protein lysates from HEK293 cells transfected with human PP1 isoforms (Fig. 1a, b). The molecular weight of the different PP1 isoforms detected was slightly higher than expected due to HIS or Myc-DDK tags, which are fused to the respective isoforms. To perform quantitative analysis of western blots, we then made a serial dilution of lysate of a left ventricular sample from an HF patient and carried out western blots using antibodies against PP1α, β, or γ, or calsequestrin. We were able to show that the expression of all three PP1 isoforms and calsequestrin in human samples was within the linear detection range of the chemiluminescence detection method used in these studies when 20 µg of protein was loaded (Figs. 1c–e, 2e–g) [12]. Based on these data, 20 µg of protein per lane was loaded for western blot analyses throughout the study.
Validation of protein phosphatase 1 (PP1) isoform-specific antibodies. a Western blot analysis of human recombinant PP1 α (with HIS tag), β (with Myc-DDK tag), and γ (with HIS tag) (10 ng each lane) or b lysate of HEK293 cells transfected with human PP1α, β, or γ fused with Myc-DDK-tag (10 µg each lane) using isoform-specific antibodies. c–e Western blot analysis of PP1α, β, γ, and calsequestrin (CSQ) in one experiment with dilution series of human left ventricular heart failure samples. Unspecific bands are marked by a hash (#)
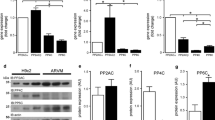
Expression of protein phosphatase 1 isoform α (PP1α) and γ (PP1γ) is enhanced in left ventricular tissues of patients with heart failure compared to non-failing patients. a Western blot and b–d densitometric analysis of PP1 isoforms in left ventricular human non-failing (NF; n = 16) versus heart failure (HF; n = 21) samples. Unspecific bands are marked by a hash (#). Results are presented as mean ± SEM. Data sets were compared by unpaired two-tailed Student’s t test to assess differences between groups. **p < 0.01 HF versus NF. All samples were normalized to calsequestrin (CSQ). The patient data are shown in Table 1. e–g Serial dilution of the human recombinant PP1α, β, and γ protein to calibrate endogenous abundance of PP1α, β, and γ in 20 µg of total protein of a left ventricular human HF sample. The PP1α, β, and γ band intensity was plotted as a function of protein input with a nonlinear regression fit model
Previous reports showed consistent increase of overall PP1 expression and activity within the most common cardiac pathologies of HF and AF. However, we are not aware of previous studies, which dissected the PP1 isoform expression in the heart. Here, we studied PP1 isoform expression in LV heart tissue samples of patients with (terminal) HF (Table 1) and in right atrial appendages of patients with AF (Table 2) versus non-failing (NF) heart tissues or SR patient samples, respectively. The pattern of PP1 isoform expression was strikingly different within these two human cardiac pathologies compared to NF heart tissues or SR patient samples. In detail, HF patient samples revealed greater abundance of PP1α and γ of 1.6- and 1.5-fold, respectively, when compared to NF samples, whereas the abundance of PP1β was not changed (Fig. 2a–d). In contrast, AF samples showed higher expression of all three PP1 isoforms (2.4-, 3.0-, and 1.8-fold increase of PP1α, β, and γ, respectively), with the most pronounced expression change for PP1β (Fig. 3a–d). These results fit well with the previously reported increase of overall PP1 protein abundance in the human HF and AF settings [5, 6, 21, 24], but highlight a special role of PP1β in different cardiac pathologies. Furthermore, we could show that PP1β is the predominant PP1 isoform in human heart tissue (approximately tenfold higher abundance than PP1 α or γ) (Fig. 2e–g). We propose that the distinct expression pattern of PP1 isoforms is very likely to reflect the different pathogenesis and progression of the underlying cardiac diseases, including significant differences in cardiac protein phosphorylation patterns. It would be of great importance to decipher the specific downstream targets of the different PP1 isoforms, especially PP1β. While cardiomyocyte-restricted overexpression of PP1α in mice induced dilated cardiomyopathy and pump failure strikingly similar to human HF [3], unfortunately, there are no published reports about the outcome of cardiomyocyte-specific overexpression of the other isoforms. It would be interesting to see whether mice with cardiomyocyte-specific PP1β or PP1γ overexpression without concomitant PP1α upregulation would resemble functional phenotypes of AF patients. Furthermore, cardiomyocyte-specific knock-out of the three PP1 isoforms in embryonic and adult mouse shed light into the previously unknown in vivo function and regulation of the different PP1 isoforms in the heart [17]. While embryonic and adult deletion of PP1α or PP1γ had negligible effects overall, the deletion of PP1β led to concentric remodelling of the heart, interstitial fibrosis, and contractile dysregulation. At the cardiomyocyte level, the deletion of any of the three PP1 isoforms had no effect on phosphorylation of phospholamban; however, PP1β knock-out showed enhanced contractility with concomitant elevated phosphorylation of myosin light chain 2 and cardiac myosin binding protein-C but unchanged Ca2+ handling dynamics. Furthermore, the potentially important role of PP1β for heart function was confirmed by a study in adult rat cardiomyocytes using adenovirally delivered shRNAs against the different PP1 isoforms. Here, PP1β knockdown not only led to a comparable increase in cardiac force, but also had influence on Ca2+ transients and phosphorylation of phospholamban at position serine 16 [1]. Finally, there were very recent findings showing that PP1β de novo missense variants were associated with intellectual disability and congenital heart disease [19].
Comparison of protein phosphatase 1 isoform abundance in right atrial appendages of AF and sinus rhythm (SR) patients. a Western blot and b–d densitometric analysis of PP1 isoforms in human right atrial appendages of patients with sinus rhythm (SR; n = 6) versus atrial fibrillation (AF; n = 6). Unspecific bands are marked by a hash (#). All samples were normalized to calsequestrin (CSQ). The patient data are shown in Table 2. Results are presented as mean ± SEM. Data sets were compared by unpaired two-tailed Student’s t test to assess differences between groups. **p < 0.01 AF versus SR
Beta-blockers such as metoprolol, bisoprolol, and carvedilol are first-line recommended drugs for the treatment of HF patients with reduced ejection fraction, which have been shown to improve survival in HF patients with reduced ejection fraction [25]. Numerous studies have shown beneficial beta-blocker effects by targeting the chronically hyper-activated β-adrenergic signaling pathway. Nevertheless, there are no data about PP1 expression and virtually no data regarding regulation of PP1 isoform expression after chronic beta-blocker treatment available. Here, we observed significantly lower PP1α isoform abundance in the LV tissues from HF patients with beta-blocker treatment (see Table 3), while the expression of the other two PP1 isoforms remained unchanged (Fig. 4a–d). As previous studies showed, PP1α is uniquely interacting with the central ER stress complex Gadd34/eIF2α [2]. We, therefore, postulated that specific PP1α downregulation after chronic beta-blocker treatment may lead to increased or prolonged phosphorylation of eIF2α at position Ser51. Indeed, we observed a higher ratio of phosphorylated (p-Ser51) to unphosphorylated eIF2α in LV tissues from HF patients treated with beta-blocker (HF + BB) versus HF patients without beta-blocker treatment (Fig. 4a, fourth and fifth panels, e, f). Phosphorylation of eIF2α at serine 51 inhibits the translation and subsequent opportunity for the cell to get rid of misfolded proteins or to improve chaperone-mediated protein folding [28]. This process was accompanied by greater expression of ER stress markers, e.g., BIP (also known as Grp78) and IRE1α. Conversely, we observed lower expression of the latter ER stress markers in HF patients, who were on beta-blocker treatment (Fig. 4a, sixth and seventh panels, g, h). These findings fit well with previous studies showing beta-blocker mediated protection against HF-induced ER stress in the heart and cardiomyocytes [9]. Notably, a very recent publication convincingly showed Nox4-dependent inactivation of a similar PP1/p-eIF2α/Grp78 signaling axis in a murine HF model of ischemia–reperfusion [26]. However, it should be noted that counteracting p-Ser51-eIF2α kinases, e.g., PERK, PKR, and GCN2, were supposed to be regulated under HF conditions as well. It would be interesting to study the expression dynamics of these kinases in response to beta-blocker treatment as well as to receive a more comprehensive picture of this signaling pathway in the future [15, 18, 29]. This further underlines our results, which propose a previously unknown role of the PP1 isoform α in the idea that PP1α-specific downregulation in HF augments beneficial outcome in patients by decreasing ER stress [26].
Expression of protein phosphatase 1 isoform α (PP1α) is decreased in human left ventricular tissues of heart failure patients after beta-blocker treatment. a Western blot analysis of PP1 isoforms, eIF2α, phospho-eIF2α (Ser51), and ER stress marker BIP and IRE1α in human left ventricular samples of patients with heart failure (HF) with (HF + BB; n = 5) and without (HF; n = 6) beta-blocker treatment. b–d Densitometric analysis of western blots against PP1 isoforms in human left ventricular samples of HF patients treated with (HF + BB) and without (HF) beta-blocker. e–f Densitometric analysis of western blots against PP1α specific substrate eIF2α and the resulting in phospho-eIF2α (Ser51) in human left ventricular samples of HF patients with (HF + BB) and without (HF) beta-blocker treatment. g–h Densitometric analysis of western blots against ER stress marker BIP and IRE1α in human left ventricular samples of HF patients with (HF + BB) and without (HF) beta-blocker treatment. Results are presented as mean ± SEM. Data sets were compared by unpaired two-tailed Student’s t test to assess differences between groups. *p < 0.05 HF + BB versus HF. **p < 0.01 HF + BB versus HF. All samples were normalized to calsequestrin (CSQ). The patient data are shown in Table 3
Potential limitations of the study
While we tried to avoid sex- and age bias as best as possible, the limited number of available human samples did not allow for perfect matching of all potentially confounding factors. This holds especially true when interpreting the results, which we obtained after evaluating PP1α isoform expression and activity in HF patients with or without beta-blocker treatment. It would be of great interest to study, e.g., the expression of PP1α isoform in LV tissues of HF patients before and after beta-blocker treatment to decide if differences in the abundance of PP1α are really a direct response towards beta-blocker treatment. Finally, we did not study the impact of beta-blocker treatment on the abundance of other cardiac phosphatases under heart failure conditions, e.g., protein phosphatase 2A (PP2A), calcineurin, or dual-specific phosphatase 14 (Dusp14), although studies have shown that the abundance and activity of these phosphatases might be changed as well [7, 8, 14].
In conclusion, we believe that differential expression of PP1 isoforms is an obvious issue of human cardiomyopathies and that our novel findings may pave the way to innovative strategies to tackle cardiovascular diseases in the future.
Change history
07 July 2017
An erratum to this article has been published.
References
Aoyama H, Ikeda Y, Miyazaki Y, Yoshimura K, Nishino S, Yamamoto T, Yano M, Inui M, Aoki H, Matsuzaki M (2011) Isoform-specific roles of protein phosphatase 1 catalytic subunits in sarcoplasmic reticulum-mediated Ca(2+) cycling. Cardiovasc Res 89:79–88. doi:10.1093/cvr/cvq252
Brush MH, Weiser DC, Shenolikar S (2003) Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol 23:1292–1303. doi:10.1128/MCB.23.4.1292-1303.2003
Carr AN, Schmidt AG, Suzuki Y, del Monte F, Sato Y, Lanner C, Breeden K, Jing SL, Allen PB, Greengard P, Yatani A, Hoit BD, Grupp IL, Hajjar RJ, DePaoli-Roach AA, Kranias EG (2002) Type 1 phosphatase, a negative regulator of cardiac function. Mol Cell Biol 22:4124–4135. doi:10.1128/MCB.22.12.4124-4135.2002
Chiang DY, Li N, Wang Q, Alsina KM, Quick AP, Reynolds JO, Wang G, Skapura D, Voigt N, Dobrev D, Wehrens XH (2014) Impaired local regulation of ryanodine receptor type 2 by protein phosphatase 1 promotes atrial fibrillation. Cardiovasc Res 103:178–187. doi:10.1093/cvr/cvu123
Christ T, Boknik P, Wohrl S, Wettwer E, Graf EM, Bosch RF, Knaut M, Schmitz W, Ravens U, Dobrev D (2004) L-type Ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation 110:2651–2657. doi:10.1161/01.CIR.0000145659.80212.6A
El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, Dobrev D (2006) Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation 114:670–680. doi:10.1161/CIRCULATIONAHA.106.636845
Eleftheriadou O, Boguslavskyi A, Longman MR, Cowan J, Francois A, Heads RJ, Wadzinski BE, Ryan A, Shattock MJ, Snabaitis AK (2017) Expression and regulation of type 2A protein phosphatases and alpha4 signalling in cardiac health and hypertrophy. Basic Res Cardiol 112:37. doi:10.1007/s00395-017-0625-2
Fan J, Zou L, Cui K, Woo K, Du H, Chen S, Ling Z, Zhang Q, Zhang B, Lan X, Su L, Zrenner B, Yin Y (2015) Atrial overexpression of angiotensin-converting enzyme 2 improves the canine rapid atrial pacing-induced structural and electrical remodeling. Fan, ACE2 improves atrial substrate remodeling. Basic Res Cardiol 110:45. doi:10.1007/s00395-015-0499-0
George I, Sabbah HN, Xu K, Wang N, Wang J (2011) Beta-adrenergic receptor blockade reduces endoplasmic reticulum stress and normalizes calcium handling in a coronary embolization model of heart failure in canines. Cardiovasc Res 91:447–455. doi:10.1093/cvr/cvr106
Heijman J, Dewenter M, El-Armouche A, Dobrev D (2013) Function and regulation of serine/threonine phosphatases in the healthy and diseased heart. J Mol Cell Cardiol 64:90–98. doi:10.1016/j.yjmcc.2013.09.006
Heijman J, Ghezelbash S, Wehrens XH, Dobrev D (2017) Serine/threonine phosphatases in atrial fibrillation. J Mol Cell Cardiol 103:110–120. doi:10.1016/j.yjmcc.2016.12.009
Janes KA (2015) An analysis of critical factors for quantitative immunoblotting. Sci Signal 8:rs2. doi:10.1126/scisignal.2005966
Korrodi-Gregorio L, Esteves SL, Fardilha M (2014) Protein phosphatase 1 catalytic isoforms: specificity toward interacting proteins. Transl Res 164:366–391. doi:10.1016/j.trsl.2014.07.001
Li CY, Zhou Q, Yang LC, Chen YH, Hou JW, Guo K, Wang YP, Li YG (2016) Dual-specificity phosphatase 14 protects the heart from aortic banding-induced cardiac hypertrophy and dysfunction through inactivation of TAK1-P38MAPK/-JNK1/2 signaling pathway. Basic Res Cardiol 111:19. doi:10.1007/s00395-016-0536-7
Liu M, Dudley SC Jr (2015) Role for the unfolded protein response in heart disease and cardiac arrhythmias. Int J Mol Sci 17:52. doi:10.3390/ijms17010052
Liu MQ, Chen Z, Chen LX (2016) Endoplasmic reticulum stress: a novel mechanism and therapeutic target for cardiovascular diseases. Acta Pharmacol Sin 37:425–443. doi:10.1038/aps.2015.145
Liu R, Correll RN, Davis J, Vagnozzi RJ, York AJ, Sargent MA, Nairn AC, Molkentin JD (2015) Cardiac-specific deletion of protein phosphatase 1beta promotes increased myofilament protein phosphorylation and contractile alterations. J Mol Cell Cardiol 87:204–213. doi:10.1016/j.yjmcc.2015.08.018
Lu Z, Xu X, Fassett J, Kwak D, Liu X, Hu X, Wang H, Guo H, Xu D, Yan S, McFalls EO, Lu F, Bache RJ, Chen Y (2014) Loss of the eukaryotic initiation factor 2alpha kinase general control nonderepressible 2 protects mice from pressure overload-induced congestive heart failure without affecting ventricular hypertrophy. Hypertension 63:128–135. doi:10.1161/HYPERTENSIONAHA.113.02313
Ma L, Bayram Y, McLaughlin HM, Cho MT, Krokosky A, Turner CE, Lindstrom K, Bupp CP, Mayberry K, Mu W, Bodurtha J, Weinstein V, Zadeh N, Alcaraz W, Powis Z, Shao Y, Scott DA, Lewis AM, White JJ, Jhangiani SN, Gulec EY, Lalani SR, Lupski JR, Retterer K, Schnur RE, Wentzensen IM, Bale S, Chung WK (2016) De novo missense variants in PPP1CB are associated with intellectual disability and congenital heart disease. Hum Genet 135:1399–1409. doi:10.1007/s00439-016-1731-1
MacDougall LK, Jones LR, Cohen P (1991) Identification of the major protein phosphatases in mammalian cardiac muscle which dephosphorylate phospholamban. Eur J Biochem 196:725–734. doi:10.1111/j.1432-1033.1991.tb15871.x
Mishra S, Gupta RC, Tiwari N, Sharov VG, Sabbah HN (2002) Molecular mechanisms of reduced sarcoplasmic reticulum Ca(2+) uptake in human failing left ventricular myocardium. J Heart Lung Transplant 21:366–373. doi:10.1016/S1053-2498(01)00390-4
Moorhead G, Johnson D, Morrice N, Cohen P (1998) The major myosin phosphatase in skeletal muscle is a complex between the beta-isoform of protein phosphatase 1 and the MYPT2 gene product. FEBS Lett 438:141–144. doi:10.1016/S0014-5793(98)01276-9
Neumann J (2002) Altered phosphatase activity in heart failure, influence on Ca2+ movement. Basic Res Cardiol 97(Suppl 1):I91–I95. doi:10.1007/s003950200036
Neumann J, Eschenhagen T, Jones LR, Linck B, Schmitz W, Scholz H, Zimmermann N (1997) Increased expression of cardiac phosphatases in patients with end-stage heart failure. J Mol Cell Cardiol 29:265–272. doi:10.1006/jmcc.1996.0271
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37:2129–2200. doi:10.1093/eurheartj/ehw128
Santos CX, Hafstad AD, Beretta M, Zhang M, Molenaar C, Kopec J, Fotinou D, Murray TV, Cobb AM, Martin D, Zeh Silva M, Anilkumar N, Schroder K, Shanahan CM, Brewer AC, Brandes RP, Blanc E, Parsons M, Belousov V, Cammack R, Hider RC, Steiner RA, Shah AM (2016) Targeted redox inhibition of protein phosphatase 1 by Nox4 regulates eIF2alpha-mediated stress signaling. EMBO J 35:319–334. doi:10.15252/embj.201592394
Terry-Lorenzo RT, Carmody LC, Voltz JW, Connor JH, Li S, Smith FD, Milgram SL, Colbran RJ, Shenolikar S (2002) The neuronal actin-binding proteins, neurabin I and neurabin II, recruit specific isoforms of protein phosphatase-1 catalytic subunits. J Biol Chem 277:27716–27724. doi:10.1074/jbc.M203365200
Tsaytler P, Harding HP, Ron D, Bertolotti A (2011) Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science 332:91–94. doi:10.1126/science.1201396
Wang H, Xu X, Fassett J, Kwak D, Liu X, Hu X, Falls TJ, Bell JC, Li H, Bitterman P, Bache RJ, Chen Y (2014) Double-stranded RNA-dependent protein kinase deficiency protects the heart from systolic overload-induced congestive heart failure. Circulation 129:1397–1406. doi:10.1161/CIRCULATIONAHA.113.002209
Weber S, Meyer-Roxlau S, Wagner M, Dobrev D, El-Armouche A (2015) Counteracting protein kinase activity in the heart: the multiple roles of protein phosphatases. Front Pharmacol 6:270. doi:10.3389/fphar.2015.00270
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was supported by DFG Grant EL270/7-1, DZHK (Grant-Nr.: 81X2800134) (to A. E.-A.), the National Institutes of Health (HL131517 to D.D.) and research stipend of the German Society of Cardiology (to S.W.).
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Additional information
The original version of this article was revised: The spelling of the author name Samuel Sossalla was incorrect.
An erratum to this article is available at https://doi.org/10.1007/s00395-017-0638-x.
Rights and permissions
About this article
Cite this article
Meyer-Roxlau, S., Lämmle, S., Opitz, A. et al. Differential regulation of protein phosphatase 1 (PP1) isoforms in human heart failure and atrial fibrillation. Basic Res Cardiol 112, 43 (2017). https://doi.org/10.1007/s00395-017-0635-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-017-0635-0