Abstract
Myocardial ischemia/reperfusion (I/R) injury is a major cause of morbidity and mortality worldwide. Caveolae, caveolin-1 (Cav-1), and caveolin-3 (Cav-3) are essential for the protective effects of conditioning against myocardial I/R injury. Caveolins are membrane-bound scaffolding proteins that compartmentalize and modulate signal transduction. In this review, we introduce caveolae and caveolins and briefly describe the interactions of caveolins in the cardiovascular diseases. We also review the roles of Cav-1/-3 in protection against myocardial ischemia and I/R injury, and in conditioning. Finally, we suggest several potential research avenues that may be of interest to clinicians and basic scientists. The information included, herein, is potentially useful for the design of future studies and should advance the investigation of caveolins as therapeutic targets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronary artery reperfusion is currently the most effective therapy for acute myocardial infarction; however, the process of restoring blood flow to the ischemic myocardium can also induce myocardial injury, termed myocardial ischemia/reperfusion (I/R) injury [93]. Numerous studies have reported that caveolae, caveolin-1 (Cav-1), and caveolin-3 (Cav-3) are essential for protection against myocardial I/R injury [51, 80, 94]. Caveolae are 50–100 nm-wide flask-shaped plasma membrane invaginations that contain oligomeric caveolins [3, 67]. Caveolins are crucial drivers of caveola formation and have served as the major defining markers of caveolae since their discovery. There are three mammalian caveolins: Cav-1, Cav-2, and Cav-3 [28, 66, 85, 100, 114]. Both Cav-1 and Cav-3 play significant roles in myocardial protection against I/R injury; however, the role of Cav-2 remains unclear [14, 89]. Cav-1 and Cav-3 knockout (KO) mice have been shown to exhibit reduced survival after ischemic injury compared with wild-type mice [43], while Cav-3 overexpressing (OE) mice have been demonstrated to exhibit increased tolerance to myocardial I/R injury [102] (Fig. 1). Furthermore, Cav-1 and Cav-3 KO mice are resistant to the cardioprotective effects of ischemic conditioning [47, 98] and pharmacological conditioning (e.g., anesthetic preconditioning, opioid preconditioning) against myocardial I/R injury [89, 111, 123]. Cav-1 and Cav-3 have been shown to compartmentalize and regulate a number of cardioprotection-related signaling molecules, including phosphoinositide-3 kinase (PI3K)/protein kinase B (Akt) [39], extracellular signal-regulated kinases 1 and 2 (ERK1/2) [16], endothelial nitric oxide (NO) synthase (eNOS) [19], G proteins [69], tyrosine kinases, and protein kinase C (PKC) [61], etc. These findings suggest that caveolins play a role in cardioprotection.
The focus of this review is to summarize the latest progress regarding the protective effects of both Cav-1 and Cav-3 in myocardial ischemia and I/R injury. First, we introduce background information on caveolae and caveolins. Then, we summarize the roles of Cav-1/-3 in myocardial ischemia, I/R injury. Next, we provide in-depth descriptions of the involvement of Cav-1 and Cav-3 in conditioning against myocardial I/R injury. Finally, we discuss several novel potential directions for future research on caveolins and caveolae. The information compiled, herein, may serve as a comprehensive reference for the activities of both Cav-1 and Cav-3 in the cardiovascular system and may be helpful for the design of future studies and for the future development of caveolins as therapeutic targets.
General background on caveolae and caveolins
Caveolae and the caveolin family
Caveolae are considered a specialized subset of detergent-insoluble plasma membrane microdomains named lipid rafts, and they are characterized by high cholesterol, sphingolipid, and sphingomyelin concentrations [3, 67] (Fig. 2a). Traditionally, caveolae have been implicated in vesicular uptake associated with transcytosis, potocytosis, and pinocytosis [26]. However, with the advent of advanced molecular biological techniques, other functions of caveolae have been discovered, including roles in maintaining cholesterol homeostasis and the regulation of cell signaling [29]. Caveolae selectively sequester membrane-targeted proteins and create a unique signaling microdomain, thereby compartmentalizing and regulating numerous signaling molecules, including eNOS, G protein-coupled receptors (GPCRs), and PKC [95], etc. Complex details regarding caveolae have been discussed in previous reviews [67, 78].
Lipid rafts, caveolae, and structural characteristics of Cav-1/-3. a This diagram summarizes the detailed organization of lipid rafts and caveola membranes. Lipid rafts are small, heterogeneous, highly dynamic, sterol- and sphingolipid-enriched domains formed by lipid–lipid interactions that compartmentalize cellular processes [67]. Moreover, small lipid rafts can be stabilized to form larger platforms though protein–protein and protein–lipid interactions [67]. The liquid-ordered phase in the outer leaflet of the membrane is enriched in cholesterol and exoplasmic-oriented sphingolipids. The liquid-disordered phase in the cytoplasmic leaflet of the membrane is composed essentially of phospholipids. Compared with lipid rafts, the liquid-ordered phase of caveolae forms a small flask-shaped invagination due to the composition of caveolins. Caveolins oligomerize to form large multimeric complexes, and these interactions serve as the driving force for caveola formation. Due to the role of caveolins, caveolae possess the ability to selectively sequester membrane-targeted proteins and to create a unique signaling microdomain, thereby compartmentalizing and regulating signal transduction. b Schematic representation showing the domain organizations of Cav-1 and Cav-3. CSD caveolin scaffolding domain
Caveolae are characterized by the presence of caveolins, which distinguishes caveolae from other lipid raft domains [67]. Caveolins are essential for caveola formation, because they oligomerize to form large multimeric complexes, and these interactions serve as the driving force for caveola formation [79, 97]. Currently, the caveolin family consists of three members: Cav-1, Cav-2, and Cav-3. Cav-1 is expressed in a variety of tissues, particularly in terminally differentiated cells, such as adipocytes, endothelial cells, muscle cells, macrophages, and type I pneumocytes. Cav-2 forms hetero-oligomers with Cav-1 and requires Cav-1 for stabilization and plasma membrane localization [85]. Cav-2 is expressed in the same cell types as Cav-1 [38]. Cav-1 KO mice exhibit significantly decreased Cav-2 expression, most likely because Cav-1 determines the localization of Cav-2 despite the presence of an adequate Cav-2 expression level [59]. In the absence of Cav-1, Cav-2 remains within the Golgi complex and is subsequently degraded by the proteasomal pathway [59, 66]. In Cav-2 KO mice, Cav-1 properly localizes to the plasma membrane and contributes to caveola formation [84]. In contrast, Cav-3 is muscle-specific [26, 66, 114], shares 65 % sequence identity and 85 % sequence similarity with Cav-1, and forms homo-oligomeric complexes [100]. Moreover, Cav-3 can act independently of Cav-1 to drive caveola formation [29]. Caveola formation and Cav-1 and Cav-2 expression are not affected by the loss of Cav-3 in nonmuscle tissues, whereas the density of sarcolemmal caveolae is decreased in the absence of Cav-3 [84]. Interestingly, Cav-1/-3 double-KO mice show a loss of caveola formation in all cells [65]. More detailed reviews of caveolins can be found in the literature [38, 84, 115].
Structural characteristics of caveolins
Cav-1 is a 22–24 kDa integral membrane protein consisting of 178 amino acid residues, and both its N- and C-termini face the cytoplasm [28]. Several domains have been identified in Cav-1, including an oligomerization domain (residues 61–101), a caveolin scaffolding domain (CSD) (residues 82–101), and a transmembrane domain (residues 102–134) [116]. Cav-2 consists of 162 amino acids and shares 38 % sequence identity and 58 % sequence similarity with Cav-1 [85]. Cav-3 consists of 151 amino acids and contains several separate domains: an N-terminal domain (residues 1–53), a CSD (residues 54–73), a transmembrane domain (residues 74–106), and a C-terminal domain (residues 107–151) [26] (Fig. 2b). Caveolins are more than just caveolae-associated proteins. Importantly, the CSD plays a role in mediating protein–protein interactions and is responsible for the interactions between caveolins and various signaling proteins [64, 97].
Caveolins and signal transduction
Caveolins not only anchor signaling molecules but also inhibit or promote the signaling capacities of these molecules in a CSD-dependent manner [71, 95]. Cav-1 co-localizes with and regulates various signaling molecules. For example, it directly negatively regulates the activities of epithelial growth factor receptor, eNOS, PKC, G proteins, and Src family proteins [54], whereas it activates the insulin receptor [64]. The evidence supporting the role for Cav-2 as a signaling modulator is less clear, perhaps partly because its CSD sequence is divergent from that of Cav-1 [115]. Moreover, the muscle-specific isoform Cav-3 performs regulatory functions similar to those of Cav-1 in skeletal muscle, smooth muscle, and cardiac muscle cells [26] (Fig. 3a).
Signaling network by which Cav-1/-3 are involved in myocardial protection and conditioning. a This diagram summarizes how caveolins integrate into cholesterol-rich caveola membranes and assist with caveola formation. Caveolins are integral membrane proteins, and both their N- and C-terminal ends face the cytoplasm. The caveolin scaffolding domain binds to and regulates the activities of various signaling molecules. Cav-1-, Cav-3- or both Cav-1/-3-binding signaling molecules in cardiomyocytes are selectively listed in the diagram. b Schematic diagram of typical protocols for ischemic preconditioning (IPC), ischemic perconditioning, and ischemic postconditioning (IPTC). In IPTC, transient myocardial ischemia is induced at the onset of reperfusion. c IPC impairs Cav-1–eNOS complex formation and activates eNOS; IPC, ceramide preconditioning, and tocotrienol preconditioning enhance the inhibitory effect of Cav-1 on the pro-apoptotic factor MAPKα, but suppress the inhibitory effect of Cav-3 on the anti-apoptotic factor MAPKβ; Cav-3 binds to and promotes the activity of AdipoR1; and IPC and IPTC promote formation of the Cav-3–MG53–PI3K complex, activating the PI3K/Akt cascades. Thus, all of these treatments contribute to cardioprotection against myocardial I/R injury. d This diagram depicts the trafficking of caveolins to mitochondria, which enhances myocardial cellular stress adaptation by improving mitochondrial energetics, reducing ROS generation and enhancing calcium tolerance. First, caveolins are synthesized in the rough endoplasmic reticulum as integral membrane proteins. Then, they traffic through the Golgi complex to the cell surface. They also traffic to mitochondria. Following IPTC, Cav-3 forms structures that may drive the translocation of ERK1/2, GSK3β, and Akt to mitochondria, thereby regulating mPTP opening. CSD caveolin scaffolding domain, GPCRs G protein-coupled receptors, PI3K phosphoinositide-3 kinase, Akt protein kinase B, ERK1/2 extracellular signal-regulated kinases 1 and 2, PKC protein kinase C, MAPK mitogen-activated protein kinase, eNOS endothelial nitric oxide synthase, ERα estrogen receptor alpha, HO-1 heme oxygenase-1, GLUT-4 translocation of glucose transporter-4, δ-OR delta opioid receptor, AdipoR1 adiponectin receptor 1, Popdc1 Popeye domain-containing 1, AMPK AMP-activated protein kinase, AC adenylate cyclase, PKA protein kinase A, NO nitric oxide, GSK3β glycogen synthase kinase-3β, mPTP mitochondrial permeability transition pore
Caveolins and the cardiovascular system
Caveolins have essential functions in the cardiovascular system. Studies using gene KO mice have shown that the loss of caveolins results in various pathological cardiovascular conditions [20]. Cav-1 or Cav-3 KO results in cardiac hypertrophy, contractile dysfunction, and heart failure [64]. Moreover, Cav-1/3 double-KO mice develop severe cardiomyopathy with a dramatic increase in left ventricular wall thickness compared with Cav-1 KO, Cav-3 KO, and wild-type mice [65]. However, Cav-2 KO mice do not exhibit signs of either cardiovascular dysfunction or lipid disorders, although they have severe pulmonary dysfunction despite the lack of disruption of caveola formation [77]. Furthermore, human CAV3 gene mutations are associated with long QT syndrome and limb-girdle muscular dystrophy [58, 109]; and a recent study conducted by Schilling et al. has reported that cardiac myocyte-specific Cav-3 OE mice have decreased heart rates and increased cardiac ion channels expression (Kv1.4 and Kv4.3 channels, Nav1.5 channels, and connexin 43), and have changes in electrocardiogram intervals (prolonged PR intervals and shortened QTc intervals) [86].
Roles of Cav-1/-3 in protection against myocardial ischemia and I/R injury
Analyses of the phenotypes of mice with genetic deletion or overexpression of specific caveolin isoforms have provided key evidence of the protective roles of caveolins against myocardial ischemia [102]. Cav-1 KO mice subjected to permanent left anterior descending coronary artery ligation exhibit reduced survival compared with the corresponding wild-type mice. Mechanistically, Cav-1 KO mice subjected to myocardial ischemia display reduced β-adrenergic receptor density at the plasma membrane and decreased cyclic adenosine monophosphate and protein kinase A (PKA) phosphorylation, and these effects exacerbate cardiac dysfunction and result in reduced survival after ischemia [43]. Moreover, cardiac myocyte-specific Cav-3 OE mice show enhanced ischemic tolerance through increased basal Akt and glycogen synthase kinase-3β (GSK3β) phosphorylation in cardiomyocytes, which inhibits mitochondrial permeability transition pore (mPTP) opening, thereby contributing to cardiac protection [102].
Cav-1/-3 play cardioprotective roles during myocardial I/R injury, as demonstrated by numerous studies showing that Cav-1 and Cav-3 KO mice exhibit increased myocardial I/R injury [70, 113] and are resistant to induction of myocardial protection by conditioning treatments [47, 70, 103, 123], whereas cardiac-specific OE of Cav-3 mimics the preconditioning phenotype against I/R injury [102]. Peart et al. have recently suggested that myocardial I/R tolerance declines with age and that age-dependent reductions in both Cav-3 expression and caveola abundance may contribute to this phenomenon [73]. Caveolins function as scaffolds for signaling molecules, providing temporal and spatial regulation of signal transduction [87]. An emerging concept may help to understand the cardioprotective roles of caveolins, suggesting that caveolins and signaling molecules exist as multiprotein complexes, “signalosomes”, which form and dissociate under basal and stimulated conditions [21, 87]. (i) Under basal conditions, Cav-1/-3 may allosterically inhibit a wide range of signaling molecules involved in cardioprotection, including GPCRs [34, 69], Gα subunits of heterotrimeric G proteins [52], Src kinases [16, 69], PI3K [48, 87], Akt [46, 69], ERK1/2 [4], eNOS [21, 25, 47], PKC isoforms [61, 120], mitogen-activated protein kinases (MAPKs) [14, 16], and heme oxygenase-1 (HO-1) [16], etc. (ii) However, Cav-1/-3 promote increased signaling following stress (e.g., I/R or metabolic inhibition) [4, 13, 21] or conditioning (e.g., ischemic conditioning or pharmacological conditioning) stimulation [14, 16, 47, 70]. (iii) Mechanistically, Cav-1/-3 phosphorylation via Src activation alters the properties of Cav-1/-3 during stress or conditioning treatments [13, 48, 70, 82, 83, 99]; moreover, the levels of caveolae and caveolins are significantly reduced after myocardial infarction and I/R injury, which may also facilitate the disassociation of caveolins from myocardial-protective signaling molecules [4, 12, 76, 92]. However, Cav-1/-3 appear to confer myocardial protection against I/R injury in a variety of ways, for example, via epigenetic regulation [15]. Thus, further studies will facilitate an increased understanding of the complex cardioprotective mechanisms of Cav-1/-3.
Roles of Cav-1/-3 in conditioning phenomena
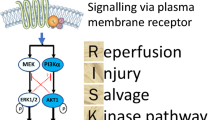
Conditioning (e.g., ischemic conditioning or pharmacological conditioning) phenomena exert powerful protective effects against myocardial I/R injury [32, 36]. Ischemic conditioning refers to an endogenous phenomenon in which brief episodes of nonlethal ischemia and reperfusion confer protection against I/R injury [32]. The conditioning stimulus can be applied before ischemia (ischemic preconditioning, IPC), after the onset of ischemia (ischemic perconditioning), within the first minute of myocardial reperfusion (ischemic postconditioning, IPTC) or even to a distant organ or tissue (remote ischemic conditioning) [32, 36, 37] (Fig. 3b). Also, pharmacological conditioning, which mimics ischemic conditioning, has emerged with elucidation of the mechanistic pathways underlying ischemic conditioning [8, 36]. The roles of Cav-1/-3 in IPC, IPTC, and pharmacological conditioning have been studied; however, their roles in ischemic perconditioning and remote ischemic conditioning remain unknown [14, 35, 70]. The mechanisms underlying ischemic conditioning are rather complex and result in the recruitment of a number of pro-survival protein kinase pathways, such as the reperfusion injury salvage kinase (RISK, involving the PI3K/Akt and ERK1/2 cascades) and survivor activating factor enhancement (SAFE, involving the TNFα and STAT3 cascades) pathways [31, 36, 49]. The emerging evidence regarding Cav-1/-3 may help to elucidate the cardioprotective signaling mechanisms and establish them as spatially and temporally concerted actions that occur during conditioning. Mechanistically, in addition to the involvement of caveolins, “signalosomes”, which associate with and dissociate from signaling molecules under basal and stimulated conditions [21, 87], and conditioning, which increases the formation of caveolae and the amount of caveolins, in contrast with I/R injury, have also been studied [102]. Interestingly, caveolins promote cardioprotective signaling by enhancing receptor–effector coupling or by enhancing receptor affinity when their expression is increased [74, 87, 113]. Next, we summarize recent findings related to the involvement of Cav-1/-3 in conditioning against myocardial I/R injury.
Cav-1 involvement in conditioning against myocardial I/R injury
IPC and ceramide preconditioning
Cav-1, as a scaffold for signaling molecules, interacts with and inhibits the activities of various signaling proteins (e.g., p38 MAPK, eNOS, and HO-1) [14, 17], while Cav-1 phosphorylation via Src activation alters the properties of Cav-1 and contributes to cardioprotection during IPC and anesthetic preconditioning [70]. Das and colleagues have determined that IPC, ceramide preconditioning, and tocotrienol preconditioning significantly increase the interaction of Cav-1 with the pro-apoptotic factor p38 MAPKα and decrease the association of Cav-3 with the anti-apoptotic factor p38 MAPKβ, thereby enhancing cardioprotection in preconditioned hearts [14, 16, 17] (Fig. 3c). Moreover, reduced interactions of Cav-1 with HO-1 and eNOS have been reported in rat hearts treated with tocotrienol compared with those subjected to I/R alone. Because HO-1 and eNOS are pro-survival signaling components, inhibiting their interactions with Cav-1 promotes survival signaling in tocotrienol-treated hearts [14, 16].
Ceramide, which is generated from sphingomyelin during I/R, induces cardiomyocyte death, whereas its metabolite sphingosine-1-phosphate (sphingosine-1-P) promotes both the survival and proliferation of cardiomyocytes [6, 14]. Der and colleagues have reported that ceramide extensively accumulates in caveolin-rich membranes during I/R, indicating that increased interactions between Cav-1 and eNOS contribute to myocardial dysfunction. Desipramine, an inhibitor of ceramide formation, suppresses the interaction between eNOS and Cav-1, thereby inducing cardioprotection [19]. Although ceramide plays a deleterious role in ischemic injury, it is essential for IPC-induced cardioprotection. Additionally, ceramide itself mimics the effects of IPC. In rat hearts subjected to either IPC or ceramide preconditioning, a decrease in the ceramide concentration has been associated with enhancement of the sphingosine-1-P concentration in caveolae, suggesting that both types of preconditioning trigger the degradation of ceramide and the accumulation of sphingosine-1-P, thereby inducing myocardial protection [14, 19]. Furthermore, both types of preconditioning enhance the association of Cav-1 with pro-apoptotic p38 MAPKα; this enhancement can be partially attenuated by desipramine treatment, demonstrating the cardioprotective role of ceramide preconditioning. In summary, ceramide accumulates in caveolin-rich membranes and induces cardiomyocyte death during I/R. However, conditioning results in the degradation of ceramide, and this effect is essential for promoting cardioprotection. Although the roles of ceramide in both I/R and conditioning remain controversial, Cav-1 mediates these activities; therefore, these potential roles warrant further investigation.
Other types of pharmacological preconditioning
Several studies have suggested that either the activation or preservation of Cav-1 plays a cardioprotective role in myocardial I/R injury and that the upstream mediators of Cav-1 may contribute to those effects. Epigallocatechin-3-gallate (EGCg), the most physiologically potent compound in green tea, displays anti-oxidative properties and ameliorates myocardial injury in the setting of myocardial ischemia [42, 53]. Using in vivo myocardial ischemia and in vitro oxidative stress models, Hsieh and colleagues have found that Cav-1 activation promotes the increased activity of the EGCg-induced cardioprotective Akt/GSK-3β signaling pathway [41]. Additionally, Chaudhary and colleagues have reported that epoxyeicosatrienoic acid (EET)-mediated myocardial protection from I/R injury involves the preservation of Cav-1 [12]. EETs are metabolized from arachidonic acid by cytochrome P450 epoxygenases [91]. Ischemia causes damage to mitochondrial cristae, resulting in the disappearance of both caveolae and Cav-1 from the wild-type mouse heart. However, treatment of the wild-type mouse heart with EETs results in enhanced post-ischemic functional recovery and increased Cav-1 expression [12]. Despite these findings, the mechanisms underlying these effects remain unclear, and further studies are needed to explore the role of Cav-1 in pharmacological conditioning.
Cav-3 involvement in conditioning against myocardial I/R injury
IPC and IPTC
Cav-3 plays a key role in the spatiotemporal regulation of signaling molecules during ischemic conditioning. Recent studies have reported that the MG53-mediated interaction between Cav-3 and PI3K is essential for the IPC-/IPTC-induced activation of RISK signaling [9, 122]. MG53, also known as TRIM72, is a novel TRIM family protein that plays a cardioprotective role in the setting of I/R injury [125]. The N-terminal TRIM domain of MG53 interacts with Cav-3, whereas its C-terminal SPRY domain binds to PI3K, forming a functional complex, Cav-3–MG53–PI3K. Moreover, either MG53 or Cav-3 KO blocks the IPC-induced activation of PI3K [9, 122] (Fig. 3c). Cardiac myocyte-specific Cav-3 OE mimics the cardioprotective effects of IPC by increasing both Akt and GSK3β phosphorylation [102]. Therefore, further investigation is warranted to determine whether Cav-3 OE induces cardioprotection by mimicking IPC in an MG53-dependent manner.
The fate of cardiomyocytes under pathological conditions is closely associated with mitochondrial function; the mPTP is not only associated with a major cause of reperfusion injury but is also an effective target for cardioprotection [30, 63]. The activation of both ERK1/2 and Akt prevents GSK3β-mediated mPTP opening [10]. Hernández and colleagues have reported that in a rat heart I/R model subjected to IPTC, Cav-3 forms structures that may drive the transport of ERK1/2, Akt, and GSK3β into the mitochondria, regulating mPTP opening and contributing to the cardioprotective effects of IPTC [35] (Fig. 3d). Additionally, Sun et al. have provided evidence that IPC results in further increases in subsarcolemmal mitochondrial eNOS and Cav-3 expression, thereby increasing eNOS/NO/S-nitrosylation signaling in subsarcolemmal mitochondria [98]. Mitochondrial proteins are major targets of S-nitrosylation, and NO-mediated S-nitrosylation signaling promotes IPC-induced cardioprotection [75]. In summary, mitochondria play central roles in cardiomyocyte death and survival, and potentially represent the end effectors of cardioprotective interventions.
Anesthetic preconditioning
Volatile anesthetics, such as isoflurane and sevoflurane, have long been used in the clinical management of anesthesia; however, evidence suggests that they also have cardioprotective functions [45]. Anesthetic preconditioning (APC) refers to the protective effects of volatile anesthetics on the body, including the heart, prior to a lethal ischemic insult. The myocardial protection conferred by APC can be classified into two types: acute APC and delayed APC. Acute APC is transient and subsides after a few hours, whereas delayed APC begins 12–24 h after the initial anesthetic treatment [11, 101]. Acute APC-induced cardioprotection cannot be elicited in either Cav-1- or Cav-3-deficient mice in vivo, indicating that both Cav-1 and Cav-3 are essential for acute APC-induced cardioprotection in the setting of I/R injury [40, 70]. However, Tsutsumi and colleagues have reported that Cav-3, but not Cav-1, is required for delayed APC-induced myocardial protection [103]. Furthermore, these Cav-3-dependent delayed cardioprotective effects are accompanied by the translocation of glucose transporter-4 (GLUT-4) to caveolae after 24 h of isoflurane exposure. The upregulation of Cav-3 and GLUT-4 and an increase in the interaction between Cav-3 and GLUT-4 are observed after isoflurane treatment [103]. Therefore, the interaction between Cav-3 and GLUT-4 contributes to delayed APC-induced cardioprotection. Additionally, Wang et al. have recently revealed that isoflurane preconditioning results in the accumulation of Cav-1 and Cav-3 in mitochondria and improves mitochondrial functioning in a GPCR/Gi signaling-dependent manner [111]. Furthermore, Zhao and colleagues have provided evidence that sevoflurane preconditioning mediates cardioprotection against I/R injury by inhibiting cyclooxygenase-2 (COX-2) in a Cav-3-dependent manner [123]. However, the molecular mechanisms underlying this phenomenon are currently unknown, and whether COX-2 inhibition plays a role in either acute or delayed APC warrants further investigation.
Opioid preconditioning
Opioids, which are a class of neurohormones, are released acutely from nerve endings and are also synthesized in cardiomyocytes. Exogenous administration of opioids and activation of opioid receptors (ORs) induce cardioprotection against I/R injury [36, 72]. Delta opioid receptor (δ-OR), the dominant OR isoform in the heart, is involved in opioid preconditioning and ischemic conditioning [68, 88]. Tsutsumi and colleagues have reported that Cav-3 organizes ORs in caveolae and that Cav-3 expression appears to be essential for δ-OR-induced cardioprotection against myocardial I/R injury. Moreover, the innate cardioprotection exerted by Cav-3 OE in mice is opioid-dependent [104]. However, See Hoe et al. have recently suggested that the mechanisms underlying the cardioprotection conferred by acute OR activation (acute ligand-activated preconditioning) differ from those underlying sustained OR activation (SOA) [89]. Methyl-β-cyclodextrin (MβCD) depletes membrane cholesterol, whereas Cav-3 KO selectively depletes caveolae, and both MβCD treatment and Cav-3 KO disrupt caveolae and attenuate acute δ-OR-mediated myocardial protection. However, the mechanisms underlying the protective effects of SOA are distinct from those associated with acute OR activation-mediated protection, as the effects of SOA are less sensitive to cholesterol depletion than those of the acute OR response, and they are completely independent of both Cav-3 expression and caveolae [89]. Accordingly, further investigation is warranted to clarify the potential mechanisms underlying the cardioprotective effects of SOA.
Adiponectin preconditioning
Adiponectin (APN) is an anti-diabetic and anti-atherogenic adipocytokine secreted by adipose tissue. The plasma APN level is decreased in obesity, insulin resistance, and type 2 diabetes [62]. Additionally, both experimental and clinical studies have demonstrated that APN acts as an endogenous cardiovascular protective molecule [10]. APN regulates cellular functions by binding to and activating adiponectin receptors (AdipoRs), including AdipoR1 and AdipoR2 [44]. APPL1 and adenylate cyclase (AC) are the most prominent downstream signaling molecules in APN-mediated AMP-activated protein kinase (AMPK) activation and anti-oxidative signaling (involving PKA activation), respectively. Wang and colleagues have reported that Cav-3 co-localizes with and interacts with AdipoR1, APPL1, and AC to form a signaling complex within caveolae [113]. Moreover, Cav-3 KO mice express normal levels of APN-induced signaling molecules, but the cardioprotective effects exerted by APN are apparently lost in these mice, suggesting that the Cav-3-AdipoR1 interaction is essential for APN-initiated AMPK-dependent and anti-oxidative intracellular cardioprotective signaling [113] (Fig. 3c).
Other types of pharmacological preconditioning
Cav-3 participates in other pharmacological conditioning phenomena against myocardial I/R injury. Melatonin (N-acetyl-5-methoxytryptamine), the major secretory product of the pineal gland, displays anti-atherogenic, anti-oxidant, anti-inflammatory, anti-apoptotic, and vasodilatory properties [56]. Melatonin-induced cardioprotection is associated with activation of the RISK pathway, including activation of both Akt and ERK1/2 [55]. Lamont et al. have reported that melatonin protects the heart from I/R injury via the activation of the powerful pro-survival SAFE pathway, which involves the activation of both TNFα and STAT3 [50]. Moreover, a recent study conducted by our group has revealed that melatonin attenuates myocardial I/R-induced mitochondrial oxidative damage via JAK2/STAT3 signaling [117]. Also, Şehirli and colleagues have observed that melatonin treatment significantly increases the Cav-3 level in rats with heart failure, suggesting that Cav-3 may play an essential role in melatonin-induced myocardial protection [90]. However, the downstream signaling pathway involving Cav-3 under melatonin treatment is unclear. These results indicate that a potential relationship exists between Cav-3 and either the RISK or SAFE pathway in the presence of melatonin.
Geranylgeranylacetone (GGA), an acyclic polyisoprenoid, is commonly used as an oral anti-ulcer medication in Asia [108]. It exerts delayed cardioprotective effects associated with an increased number of caveolae and increased expression of Cav-3 [124]. Moreover, isoflurane preconditioning-induced myocardial protection may be enhanced by the combination of GGA administration with sub-therapeutic isoflurane preconditioning. The protective effects of both of these treatments are abolished in Cav-3 KO mice, indicating that these effects are Cav-3-dependent [107]. Recently, Wang et al. reported that GGA-induced HSP70 OE promotes myocardial protection against humid heat stress [112]. HSP70 is a protein that also directly co-localizes with and interacts with Cav-3 [106]. These results suggest that a novel pathway exists by which Cav-3 interacts with and influences HSP70 in the presence of GGA, resulting in myocardial protection.
Glucagon-like peptide-1 (GLP-1) is an intestinal hormone that stimulates insulin secretion and inhibits glucagon secretion [24]. Both in vitro and in vivo models have demonstrated that GLP-1 contributes to myocardial protection against I/R injury and that exendin-4 (Ex-4), an exogenous GLP-1 receptor (GLP-1R) agonist, exerts similar effects [7, 96]. Tsutsumi et al. have reported that both caveolae and Cav-3 are essential for GLP-1- and Ex-4-induced myocardial protection against I/R injury and that these factors interact with and co-localize with GLP-1R [105]. Further, Alcalay and colleagues have reported that the evolutionarily conserved membrane protein Popeye domain-containing 1 (Popdc1), also referred to as Bves, co-localizes with Cav-3 in the sarcolemma and protects against I/R injury via preservation of the structural and functional integrity of caveolae [2]. Table 1 summarizes the involvement of Cav-1/-3 in conditioning against myocardial I/R injury and also presents information regarding the experimental models used and bibliographic references.
Collectively, the targeting of Cav-3 expression and its activation, as mediated by pharmacological conditioning, may represent a promising therapeutic strategy for myocardial protection against ischemia.
Physical therapy
In addition to the previously mentioned ischemic and pharmacological preconditioning, Cav-3 plays a role in physical therapy against myocardial ischemia. Giusti and colleagues have suggested that long-term mild exercise increases Cav-3 expression in the mouse heart, thereby promoting cardioprotection against I/R injury [27].
Chung and colleagues have reported that estrogen receptor alpha (ERα) co-localizes with Cav-3 on the plasma membrane of rat cardiomyocytes. Metabolic inhibition in an in vitro model mimicking myocardial infarction induces the tyrosine phosphorylation of Cav-3 via an Src activation-mediated mechanism, and this phosphorylation decreases its association with ERα. Accordingly, this dissociation attenuates the suppressive effects of Cav-3 on ERα, resulting in the increased stimulation of ERα by estradiol, thereby triggering downstream signaling pathways by inhibiting the metabolic inhibition-induced phosphorylation of connexin-43 (Cx43) [13]. Shi and colleagues have demonstrated that chronic hypoxia increases eNOS expression but that it decreases the levels of both Cav-3 expression and Cav-3–eNOS complexes in the heart. Therefore, enhanced cardiomyocyte eNOS activity results in both increased NO production and enhanced resistance to ischemia [92].
Potential directions
Cav-1 and Cav-2 form hetero-oligomers [85]; moreover, Cav-2 is degraded in the absence of Cav-1 in lung and adipose tissues [20]. In the hearts of rats with experimental autoimmune myocarditis, Cav-1 and Cav-2 expression is increased [1]. This finding at least demonstrates that Cav-2 participates in pathological processes involving the heart. However, few studies have examined the role of Cav-2 in myocardial I/R injury. Whether Cav-2 has Cav-1-independent functions in myocardial tissue is unknown. The influence of caveolins on I/R injury has been demonstrated in animals with Cav-1 KO and Cav-3 KO or OE but not in those with Cav-1/-3 double-KO. Therefore, the use of other animals with multiple genetic alterations related to caveolins may reveal as-yet unknown functions of caveolins in I/R injury.
The current evidence regarding the association between caveolins and I/R injury remains limited to animal experiments. Undoubtedly, the next step in the process of elucidating the roles of the various caveolin family members in human myocardial ischemia is to apply what we have learned from murine models to the human population. This strategy has already been applied to some extent in research of other cardiovascular diseases, such as familial hypertrophic cardiomyopathy [33, 60] and long QT syndrome [109], but research progress is limited to Cav-3 [26]. A noninhibitory mutant of the Cav-1 scaffolding domain has also been demonstrated to enhance eNOS-derived NO synthesis and vasodilation [5]. Unfortunately, whether caveolin mutations are associated with myocardial ischemia and I/R remain unclear. Animal studies have revealed that caveolin expression and caveolae abundance decrease with aging and that these decreases may contribute to a decline in aging-related myocardial I/R tolerance [73]; moreover, the concentrations of caveolins and caveolae are correlated with the protective effects of conditioning against myocardial ischemia and I/R injury [47, 70, 102, 123]. Therefore, clinical importance may lie in (i) identifying conditions in which either the concentrations of caveolins and caveolae in myocytes are reduced or their functions are impaired through mutations or molecular interactions that block the protective effects of conditioning; and (ii) determining how to maintain caveolins at appropriate levels to prevent myocardial ischemia and I/R injury.
Through multiple experiments performed both in vitro and in vivo, the interactions between caveolins and various signaling molecules have been widely investigated. Most of the signaling molecules targeted by caveolins, including GPCRs, G proteins, PI3K, Akt, MAPK, PKC, HO-1, eNOS, and Src, are involved in myocardial protection against I/R injury; however, whether other complicated crosstalk occurs between these signaling molecules and caveolins requires further study. Moreover, determination of the mechanisms underlying the regulation of caveolins by opioids and APN is still in the initial stages, and the exact mechanisms involved remain to be elucidated. Collectively, caveolin-targeted molecules, especially those in the heart, require further investigation. This information should be useful for the treatment and prevention of myocardial ischemia and I/R injury.
The raft hypothesis involves the lipid-dependent segregation of specific membrane components in the plasma membrane. Lipid rafts are classified by their structures and compositions and are grouped into various subclasses, and caveolae are just one example [57]. The distinctive feature of caveolae is the presence of caveolins, whereas other lipid rafts are characterized by the presence of other proteins. These proteins drastically change the morphology and/or the functioning of lipid rafts. Modifiers of raft functions (MORFs) refer to a newly emerging class of structural proteins [78]. The first MORF identified was the Cav-1 protein. In addition to caveolins, several other proteins have been identified that may be enriched in lipid rafts, resulting in dramatic structural and functional changes, including the flotillins [81, 110, 118], stomatins [81], 36-kDa vesicular integral membrane protein (VIP36) [22], and MAL/BENE [18]. Interestingly, flotillin-1 expression is also affected by myocardial ischemic injury [121]. Whether community ecology exists between caveolins and these proteins during myocardial ischemia or in protection against myocardial ischemia and I/R is unknown. Undoubtedly, further research is necessary to address the associations and distinctions between these proteins and caveolins, as well as their contributions to ischemia and I/R.
Notably, several recent studies have focused on the role of caveolins in modulating mitochondrial function and thereby, contributing to myocardial protection. Wang and colleagues have reported that isoflurane preconditioning promotes the trafficking of both Cav-1 and Cav-3 to mitochondria. However, the functions and mechanisms of Cav-1 and Cav-3 in mitochondria are unclear [111]. It has been documented that caveolins localize to mitochondria and that the transport of caveolins to mitochondria enhances myocardial cellular stress adaptation by improving mitochondrial energetics, reducing reactive oxygen species (ROS) generation and enhancing calcium tolerance [23] (Fig. 3d). Sun et al. have provided evidence that IPC results in further increases in subsarcolemmal mitochondrial eNOS and Cav-3 expression, thereby enhancing cardioprotective eNOS/NO/S-nitrosylation signaling in subsarcolemmal mitochondria [98]. We speculate that eNOS/NO/S-nitrosylation signaling may be involved in the modulation of caveolins in mitochondria during APC, ultimately contributing to myocardial protection.
Concluding remarks
Increasing evidence suggests that both Cav-1 and Cav-3 protect the heart from I/R injury. The modulation of caveolin expression and function appears to be a promising strategy for attenuating I/R injury. Young et al. have reported that infusion of the CSD peptide of Cav-1 into I/R hearts results in the recovery of cardiac function [119]. This finding implies that the CSD peptide of caveolin has potentially therapeutic effects. The currently available data indicate that a complex signaling network is involved in Cav-1 and Cav-3 regulation. Their numerous regulators and signaling targets have provided researchers with many opportunities to explore their underlying mechanisms. However, many issues regarding the functions of caveolins in the heart must be addressed. Future work should clarify the roles of caveolins in cardiac cell biology before they can be considered as viable therapeutic targets in translational studies of myocardial ischemia.
Abbreviations
- Akt:
-
Protein kinase B
- APC:
-
Anesthetic preconditioning
- Cav:
-
Caveolin
- CSD:
-
Caveolin scaffolding domain
- eNOS:
-
Endothelial nitric oxide synthase
- ERK1/2:
-
Extracellular signal-regulated kinases 1 and 2
- GPCRs:
-
G protein-coupled receptors
- GSK3β:
-
Glycogen synthase kinase-3β
- HO-1:
-
Heme oxygenase-1
- IPC:
-
Ischemic preconditioning
- IPTC:
-
Ischemic postconditioning
- I/R:
-
Ischemia/reperfusion
- KO:
-
Knockout
- MAPKs:
-
Mitogen-activated protein kinases
- mPTP:
-
Mitochondrial permeability transition pore
- OE:
-
Overexpression
- PI3K:
-
Phosphoinositide-3 kinase
- PKC:
-
Protein kinase C
- RISK:
-
Reperfusion injury salvage kinase
- SAFE:
-
Survivor activating factor enhancement
References
Ahn M, Kim H, Matsumoto Y, Shin T (2006) Increased expression of caveolin-1 and -2 in the hearts of Lewis rats with experimental autoimmune myocarditis. Autoimmunity 39:489–495. doi:10.1080/08916930600929321
Alcalay Y, Hochhauser E, Kliminski V, Dick J, Zahalka MA, Parnes D, Schlesinger H, Abassi Z, Shainberg A, Schindler RF, Brand T, Kessler-Icekson G (2013) Popeye domain containing 1 (Popdc1/Bves) is a caveolae-associated protein involved in ischemia tolerance. PLoS One 8:e71100. doi:10.1371/journal.pone.0071100
Anderson RG, Jacobson K (2002) A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296:1821–1825. doi:10.1126/science.1068886
Ballard-Croft C, Locklar AC, Kristo G, Lasley RD (2006) Regional myocardial ischemia-induced activation of MAPKs is associated with subcellular redistribution of caveolin and cholesterol. Am J Physiol Heart Circ Physiol 291:H658–H667. doi:10.1152/ajpheart.01354.2005
Bernatchez P, Sharma A, Bauer PM, Marin E, Sessa WC (2011) A noninhibitory mutant of the caveolin-1 scaffolding domain enhances eNOS-derived NO synthesis and vasodilation in mice. J Clin Invest 121:3747–3755. doi:10.1172/JCI44778
Bielawska AE, Shapiro JP, Jiang L, Melkonyan HS, Piot C, Wolfe CL, Tomei LD, Hannun YA, Umansky SR (1997) Ceramide is involved in triggering of cardiomyocyte apoptosis induced by ischemia and reperfusion. Am J Pathol 151:1257–1263
Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM (2005) Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes 54:146–151
Bulluck H, Hausenloy DJ (2015) Ischaemic conditioning: are we there yet? Heart 101:1067–1077. doi:10.1136/heartjnl-2014-306531
Cao CM, Zhang Y, Weisleder N, Ferrante C, Wang X, Lv F, Zhang Y, Song R, Hwang M, Jin L, Guo J, Peng W, Li G, Nishi M, Takeshima H, Ma J, Xiao RP (2010) MG53 constitutes a primary determinant of cardiac ischemic preconditioning. Circulation 121:2565–2574. doi:10.1161/circulationaha.110.954628
Cao T, Gao Z, Gu L, Chen M, Yang B, Cao K, Huang H, Li M (2014) AdipoR1/APPL1 potentiates the protective effects of globular adiponectin on angiotensin II-induced cardiac hypertrophy and fibrosis in neonatal rat atrial myocytes and fibroblasts. PLoS One 9:e103793. doi:10.1371/journal.pone.0103793
Cason BA, Gamperl AK, Slocum RE, Hickey RF (1997) Anesthetic-induced preconditioning: previous administration of isoflurane decreases myocardial infarct size in rabbits. Anesthesiology 87:1182–1190. doi:10.1097/00000542-199711000-00023
Chaudhary KR, Cho WJ, Yang F, Samokhvalov V, El-Sikhry HE, Daniel EE, Seubert JM (2013) Effect of ischemia reperfusion injury and epoxyeicosatrienoic acids on caveolin expression in mouse myocardium. J Cardiovasc Pharmacol 61:258–263. doi:10.1097/FJC.0b013e31827afcee
Chung TH, Wang SM, Liang JY, Yang SH, Wu JC (2009) The interaction of estrogen receptor alpha and caveolin-3 regulates connexin43 phosphorylation in metabolic inhibition-treated rat cardiomyocytes. Int J Biochem Cell Biol 41:2323–2333. doi:10.1016/j.biocel.2009.06.001
Das M, Cui J, Das DK (2007) Generation of survival signal by differential interaction of p38MAPKalpha and p38MAPKbeta with caveolin-1 and caveolin-3 in the adapted heart. J Mol Cell Cardiol 42:206–213. doi:10.1016/j.yjmcc.2006.08.118
Das M, Das S, Lekli I, Das DK (2012) Caveolin induces cardioprotection through epigenetic regulation. J Cell Mol Med 16:888–895. doi:10.1111/j.1582-4934.2011.01372.x
Das M, Das S, Wang P, Powell SR, Das DK (2008) Caveolin and proteasome in tocotrienol mediated myocardial protection. Cell Physiol Biochem 22:287–294. doi:10.1159/000149807
Das M, Gherghiceanu M, Lekli I, Mukherjee S, Popescu LM, Das DK (2008) Essential role of lipid raft in ischemic preconditioning. Cell Physiol Biochem 21:325–334. doi:10.1159/000129391
de Marco MC, Kremer L, Albar JP, Martinez-Menarguez JA, Ballesta J, Garcia-Lopez MA, Marazuela M, Puertollano R, Alonso MA (2001) BENE, a novel raft-associated protein of the MAL proteolipid family, interacts with caveolin-1 in human endothelial-like ECV304 cells. J Biol Chem 276:23009–23017. doi:10.1074/jbc.M009739200
Der P, Cui J, Das DK (2006) Role of lipid rafts in ceramide and nitric oxide signaling in the ischemic and preconditioned hearts. J Mol Cell Cardiol 40:313–320. doi:10.1016/j.yjmcc.2005.10.005
Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV (2001) Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293:2449–2452. doi:10.1126/science.1062688
Feron O, Balligand JL (2006) Caveolins and the regulation of endothelial nitric oxide synthase in the heart. Cardiovasc Res 69:788–797. doi:10.1016/j.cardiores.2005.12.014
Fiedler K, Parton RG, Kellner R, Etzold T, Simons K (1994) VIP36, a novel component of glycolipid rafts and exocytic carrier vesicles in epithelial cells. EMBO J 13:1729–1740
Fridolfsson HN, Kawaraguchi Y, Ali SS, Panneerselvam M, Niesman IR, Finley JC, Kellerhals SE, Migita MY, Okada H, Moreno AL, Jennings M, Kidd MW, Bonds JA, Balijepalli RC, Ross RS, Patel PM, Miyanohara A, Chen Q, Lesnefsky EJ, Head BP, Roth DM, Insel PA, Patel HH (2012) Mitochondria-localized caveolin in adaptation to cellular stress and injury. FASEB J 26:4637–4649. doi:10.1096/fj.12-215798
Gandolfini MP, Coupaye M, Bouaziz E, Dehoux M, Hajage D, Lacorte JM, Ledoux S (2015) Cardiovascular changes after gastric bypass surgery: involvement of increased secretions of glucagon-like peptide-1 and brain natriuretic peptide. Obes Surg. doi:10.1007/s11695-015-1643-5
Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC (1997) Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem 272:25437–25440. doi:10.1074/jbc.272.41.25437
Gazzerro E, Sotgia F, Bruno C, Lisanti MP, Minetti C (2010) Caveolinopathies: from the biology of caveolin-3 to human diseases. Eur J Hum Genet 18:137–145. doi:10.1038/ejhg.2009.103
Giusti B, Marini M, Rossi L, Lapini I, Magi A, Capalbo A, Lapalombella R, di Tullio S, Samaja M, Esposito F, Margonato V, Boddi M, Abbate R, Veicsteinas A (2009) Gene expression profile of rat left ventricles reveals persisting changes following chronic mild exercise protocol: implications for cardioprotection. BMC Genom 10:342. doi:10.1186/1471-2164-10-342
Glenney JR Jr, Zokas L (1989) Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J Cell Biol 108:2401–2408. doi:10.1083/jcb.108.6.2401
Hagiwara Y, Sasaoka T, Araishi K, Imamura M, Yorifuji H, Nonaka I, Ozawa E, Kikuchi T (2000) Caveolin-3 deficiency causes muscle degeneration in mice. Hum Mol Genet 9:3047–3054. doi:10.1093/hmg/9.20.3047
Halestrap AP (2009) What is the mitochondrial permeability transition pore? J Mol Cell Cardiol 46:821–831. doi:10.1016/j.yjmcc.2009.02.021
Hausenloy DJ, Tsang A, Yellon DM (2005) The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc Med 15:69–75. doi:10.1016/j.tcm.2005.03.001
Hausenloy DJ, Yellon DM (2011) The therapeutic potential of ischemic conditioning: an update. Nat Rev Cardiol 8:619–629. doi:10.1038/nrcardio.2011.85
Hayashi T, Arimura T, Ueda K, Shibata H, Hohda S, Takahashi M, Hori H, Koga Y, Oka N, Imaizumi T, Yasunami M, Kimura A (2004) Identification and functional analysis of a caveolin-3 mutation associated with familial hypertrophic cardiomyopathy. Biochem Biophys Res Commun 313:178–184. doi:10.1016/j.bbrc.2003.11.101
Head BP, Patel HH, Roth DM, Lai NC, Niesman IR, Farquhar MG, Insel PA (2005) G-protein-coupled receptor signaling components localize in both sarcolemmal and intracellular caveolin-3-associated microdomains in adult cardiac myocytes. J Biol Chem 280:31036–31044. doi:10.1074/jbc.M502540200
Hernandez-Resendiz S, Zazueta C (2014) PHO-ERK1/2 interaction with mitochondria regulates the permeability transition pore in cardioprotective signaling. Life Sci 108:13–21. doi:10.1016/j.lfs.2014.04.037
Heusch G (2015) Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res 116:674–699. doi:10.1161/circresaha.116.305348
Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D (2015) Remote ischemic conditioning. J Am Coll Cardiol 65:177–195. doi:10.1016/j.jacc.2014.10.031
Hnasko R, Lisanti MP (2003) The biology of caveolae: lessons from caveolin knockout mice and implications for human disease. Mol Interv 3:445–464. doi:10.1124/mi.3.8.445
Horikawa YT, Panneerselvam M, Kawaraguchi Y, Tsutsumi YM, Ali SS, Balijepalli RC, Murray F, Head BP, Niesman IR, Rieg T, Vallon V, Insel PA, Patel HH, Roth DM (2011) Cardiac-specific overexpression of caveolin-3 attenuates cardiac hypertrophy and increases natriuretic peptide expression and signaling. J Am Coll Cardiol 57:2273–2283. doi:10.1016/j.jacc.2010.12.032
Horikawa YT, Patel HH, Tsutsumi YM, Jennings MM, Kidd MW, Hagiwara Y, Ishikawa Y, Insel PA, Roth DM (2008) Caveolin-3 expression and caveolae are required for isoflurane-induced cardiac protection from hypoxia and ischemia/reperfusion injury. J Mol Cell Cardiol 44:123–130. doi:10.1016/j.yjmcc.2007.10.003
Hsieh SR, Hsu CS, Lu CH, Chen WC, Chiu CH, Liou YM (2013) Epigallocatechin-3-gallate-mediated cardioprotection by Akt/GSK-3beta/caveolin signalling in H9c2 rat cardiomyoblasts. J Biomed Sci 20:86. doi:10.1186/1423-0127-20-86
Hsieh SR, Tsai DC, Chen JY, Tsai SW, Liou YM (2009) Green tea extract protects rats against myocardial infarction associated with left anterior descending coronary artery ligation. Pflugers Arch 458:631–642. doi:10.1007/s00424-009-0655-1
Jasmin JF, Rengo G, Lymperopoulos A, Gupta R, Eaton GJ, Quann K, Gonzales DM, Mercier I, Koch WJ, Lisanti MP (2011) Caveolin-1 deficiency exacerbates cardiac dysfunction and reduces survival in mice with myocardial infarction. Am J Physiol Heart Circ Physiol 300:H1274–H1281. doi:10.1152/ajpheart.01173.2010
Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K (2006) Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116:1784–1792. doi:10.1172/jci29126
Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC (1997) Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: reduction of myocardial infarct size with an acute memory phase. Anesthesiology 87:361–370. doi:10.1097/00000542-199708000-00024
Kim HA, Kim KH, Lee RA (2006) Expression of caveolin-1 is correlated with Akt-1 in colorectal cancer tissues. Exp Mol Pathol 80:165–170. doi:10.1016/j.yexmp.2005.09.001
Koneru S, Penumathsa SV, Thirunavukkarasu M, Samuel SM, Zhan L, Han Z, Maulik G, Das DK, Maulik N (2007) Redox regulation of ischemic preconditioning is mediated by the differential activation of caveolins and their association with eNOS and GLUT-4. Am J Physiol Heart Circ Physiol 292:H2060–H2072. doi:10.1152/ajpheart.01169.2006
Krajewska WM, Maslowska I (2004) Caveolins: structure and function in signal transduction. Cell Mol Biol Lett 9:195–220
Lacerda L, Somers S, Opie LH, Lecour S (2009) Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res 84:201–208. doi:10.1093/cvr/cvp274
Lamont KT, Somers S, Lacerda L, Opie LH, Lecour S (2011) Is red wine a SAFE sip away from cardioprotection? Mechanisms involved in resveratrol- and melatonin-induced cardioprotection. J Pineal Res 50:374–380. doi:10.1111/j.1600-079X.2010.00853.x
Lang XE, Wang X, Zhang KR, Lv JY, Jin JH, Li QS (2013) Isoflurane preconditioning confers cardioprotection by activation of ALDH2. PLoS One 8:e52469. doi:10.1371/journal.pone.0052469
Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, Nishimoto I, Lisanti MP (1995) Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem 270:15693–15701
Liou YM, Hsieh SR, Wu TJ, Chen JY (2010) Green tea extract given before regional myocardial ischemia–reperfusion in rats improves myocardial contractility by attenuating calcium overload. Pflugers Arch 460:1003–1014. doi:10.1007/s00424-010-0881-6
Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M (1994) Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol 126:111–126. doi:10.1083/jcb.126.1.111
Lochner A, Huisamen B, Nduhirabandi F (2013) Cardioprotective effect of melatonin against ischaemia/reperfusion damage. Front Biosci (Elite Ed) 5:305–315
Lu Q, Yi X, Cheng X, Sun X, Yang X (2014) Melatonin protects against myocardial hypertrophy induced by lipopolysaccharide. In Vitro Cell Dev Biol Anim 51:353–360. doi:10.1007/s11626-014-9844-0
Mayor S, Rao M (2004) Rafts: scale-dependent, active lipid organization at the cell surface. Traffic 5:231–240. doi:10.1111/j.1600-0854.2004.00172.x
Minetti C, Bado M, Broda P, Sotgia F, Bruno C, Galbiati F, Volonte D, Lucania G, Pavan A, Bonilla E, Lisanti MP, Cordone G (2002) Impairment of caveolae formation and T-system disorganization in human muscular dystrophy with caveolin-3 deficiency. Am J Pathol 160:265–270. doi:10.1016/s0002-9440(10)64370-2
Mora R, Bonilha VL, Marmorstein A, Scherer PE, Brown D, Lisanti MP, Rodriguez-Boulan E (1999) Caveolin-2 localizes to the golgi complex but redistributes to plasma membrane, caveolae, and rafts when co-expressed with caveolin-1. J Biol Chem 274:25708–25717. doi:10.1074/jbc.274.36.25708
Ohsawa Y, Toko H, Katsura M, Morimoto K, Yamada H, Ichikawa Y, Murakami T, Ohkuma S, Komuro I, Sunada Y (2004) Overexpression of P104L mutant caveolin-3 in mice develops hypertrophic cardiomyopathy with enhanced contractility in association with increased endothelial nitric oxide synthase activity. Hum Mol Genet 13:151–157. doi:10.1093/hmg/ddh014
Oka N, Yamamoto M, Schwencke C, Kawabe J, Ebina T, Ohno S, Couet J, Lisanti MP, Ishikawa Y (1997) Caveolin interaction with protein kinase C. Isoenzyme-dependent regulation of kinase activity by the caveolin scaffolding domain peptide. J Biol Chem 272:33416–33421. doi:10.1074/jbc.272.52.33416
Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T, Shirouzu M, Ogata H, Tokuyama K, Ueki K, Nagano T, Tanaka A, Yokoyama S, Kadowaki T (2013) A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 503:493–499. doi:10.1038/nature12656
Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia-Dorado D, Hausenloy DJ, Heusch G, Vinten-Johansen J, Yellon DM, Schulz R (2010) Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res 87:406–423. doi:10.1093/cvr/cvq129
Panneerselvam M, Patel HH, Roth DM (2012) Caveolins and heart diseases. Adv Exp Med Biol 729:145–156. doi:10.1007/978-1-4614-1222-9_10
Park DS, Woodman SE, Schubert W, Cohen AW, Frank PG, Chandra M, Shirani J, Razani B, Tang B, Jelicks LA, Factor SM, Weiss LM, Tanowitz HB, Lisanti MP (2002) Caveolin-1/3 double-knockout mice are viable, but lack both muscle and non-muscle caveolae, and develop a severe cardiomyopathic phenotype. Am J Pathol 160:2207–2217. doi:10.1016/s0002-9440(10)61168-6
Parolini I, Sargiacomo M, Galbiati F, Rizzo G, Grignani F, Engelman JA, Okamoto T, Ikezu T, Scherer PE, Mora R, Rodriguez-Boulan E, Peschle C, Lisanti MP (1999) Expression of caveolin-1 is required for the transport of caveolin-2 to the plasma membrane. Retention of caveolin-2 at the level of the Golgi complex. J Biol Chem 274:25718–25725. doi:10.1074/jbc.274.36.25718
Parton RG, Simons K (2007) The multiple faces of caveolae. Nat Rev Mol Cell Biol 8:185–194. doi:10.1038/nrm2122
Patel HH, Head BP, Petersen HN, Niesman IR, Huang D, Gross GJ, Insel PA, Roth DM (2006) Protection of adult rat cardiac myocytes from ischemic cell death: role of caveolar microdomains and delta-opioid receptors. Am J Physiol Heart Circ Physiol 291:H344–H350. doi:10.1152/ajpheart.01100.2005
Patel HH, Murray F, Insel PA (2008) Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol 48:359–391. doi:10.1146/annurev.pharmtox.48.121506.124841
Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M, Horikawa Y, Huang D, Moreno AL, Patel PM, Insel PA, Roth DM (2007) Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. FASEB J 21:1565–1574. doi:10.1096/fj.06-7719com
Pawson T, Scott JD (1997) Signaling through scaffold, anchoring, and adaptor proteins. Science 278:2075–2080. doi:10.1126/science.278.5346.2075
Peart JN, Gross ER, Gross GJ (2005) Opioid-induced preconditioning: recent advances and future perspectives. Vascul Pharmacol 42:211–218. doi:10.1016/j.vph.2005.02.003
Peart JN, Pepe S, Reichelt ME, Beckett N, See Hoe L, Ozberk V, Niesman IR, Patel HH, Headrick JP (2014) Dysfunctional survival-signaling and stress-intolerance in aged murine and human myocardium. Exp Gerontol 50:72–81. doi:10.1016/j.exger.2013.11.015
Raikar LS, Vallejo J, Lloyd PG, Hardin CD (2006) Overexpression of caveolin-1 results in increased plasma membrane targeting of glycolytic enzymes: the structural basis for a membrane associated metabolic compartment. J Cell Biochem 98:861–871. doi:10.1002/jcb.20732
Rassaf T, Schulz R (2015) Mitochondrias’ sense of SNO-pathway to cardioprotection in ischemic preconditioning. Cardiovasc Res 106:182–183. doi:10.1093/cvr/cvv115
Ratajczak P, Damy T, Heymes C, Oliviero P, Marotte F, Robidel E, Sercombe R, Boczkowski J, Rappaport L, Samuel JL (2003) Caveolin-1 and -3 dissociations from caveolae to cytosol in the heart during aging and after myocardial infarction in rat. Cardiovasc Res 57:358–369. doi:10.1016/S0008-6363(02)00660-0
Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, Kneitz B, Hou H Jr, Christ GJ, Edelmann W, Lisanti MP (2002) Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol 22:2329–2344. doi:10.1128/MCB.22.7.2329-2344.2002
Razani B, Woodman SE, Lisanti MP (2002) Caveolae: from cell biology to animal physiology. Pharmacol Rev 54:431–467. doi:10.1124/pr.54.3.431
Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG (1992) Caveolin, a protein component of caveolae membrane coats. Cell 68:673–682. doi:10.1016/0092-8674(92)90143-Z
Ruiz-Meana M, Nunez E, Miro-Casas E, Martinez-Acedo P, Barba I, Rodriguez-Sinovas A, Inserte J, Fernandez-Sanz C, Hernando V, Vazquez J, Garcia-Dorado D (2014) Ischemic preconditioning protects cardiomyocyte mitochondria through mechanisms independent of cytosol. J Mol Cell Cardiol 68:79–88. doi:10.1016/j.yjmcc.2014.01.001
Salzer U, Prohaska R (2001) Stomatin, flotillin-1, and flotillin-2 are major integral proteins of erythrocyte lipid rafts. Blood 97:1141–1143. doi:10.1182/blood.V97.4.1141
Sanguinetti AR, Cao H, Corley Mastick C (2003) Fyn is required for oxidative- and hyperosmotic-stress-induced tyrosine phosphorylation of caveolin-1. Biochem J 376:159–168. doi:10.1042/bj20030336
Sanguinetti AR, Mastick CC (2003) c-Abl is required for oxidative stress-induced phosphorylation of caveolin-1 on tyrosine 14. Cell Signal 15:289–298. doi:10.1016/S0898-6568(02)00090-6
Sanon VP, Sawaki D, Mjaatvedt CH, Jourdan-Le Saux C (2015) Myocardial tissue caveolae. Compr Physiol 5:871–886. doi:10.1002/cphy.c140050
Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP (1996) Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci USA 93:131–135. doi:10.1073/pnas.93.1.131
Schilling JM, Horikawa YT, Zemljic-Harpf AE, Vincent KP, Tyan L, Yu JK, McCulloch AD, Balijepalli RC, Patel HH, Roth DM (2016) Electrophysiology and metabolism of caveolin-3-overexpressing mice. Basic Res Cardiol 111:28. doi:10.1007/s00395-016-0542-9
Schilling JM, Roth DM, Patel HH (2015) Caveolins in cardioprotection—translatability and mechanisms. Br J Pharmacol 172:2114–2125. doi:10.1111/bph.13009
Schultz JE, Hsu AK, Gross GJ (1998) Ischemic preconditioning in the intact rat heart is mediated by delta 1- but not mu- or kappa-opioid receptors. Circulation 97:1282–1289. doi:10.1161/01.CIR.97.13.1282
See Hoe LE, Schilling JM, Tarbit E, Kiessling CJ, Busija AR, Niesman IR, Du Toit E, Ashton KJ, Roth DM, Headrick JP, Patel HH, Peart JN (2014) Sarcolemmal cholesterol and caveolin-3 dependence of cardiac function, ischemic tolerance, and opioidergic cardioprotection. Am J Physiol Heart Circ Physiol 307:H895–H903. doi:10.1152/ajpheart.00081.2014
Sehirli AO, Koyun D, Tetik S, Ozsavci D, Yiginer O, Cetinel S, Tok OE, Kaya Z, Akkiprik M, Kilic E, Sener G (2013) Melatonin protects against ischemic heart failure in rats. J Pineal Res 55:138–148. doi:10.1111/jpi.12054
Shahabi P, Siest G, Visvikis-siest S (2014) Influence of inflammation on cardiovascular protective effects of cytochrome P450 epoxygenase-derived epoxyeicosatrienoic acids. Drug Metab Rev 46:33–56. doi:10.3109/03602532.2013.837916
Shi Y, Pritchard KA Jr, Holman P, Rafiee P, Griffith OW, Kalyanaraman B, Baker JE (2000) Chronic myocardial hypoxia increases nitric oxide synthase and decreases caveolin-3. Free Radic Biol Med 29:695–703. doi:10.1016/S0891-5849(00)00364-6
Shimizu T, Suzuki S, Sato A, Nakamura Y, Ikeda K, Saitoh SI, Misaka S, Shishido T, Kubota I, Takeishi Y (2015) Cardio-protective effects of pentraxin 3 produced from bone marrow-derived cells against ischemia/reperfusion injury. J Mol Cell Cardiol 89:306–313. doi:10.1016/j.yjmcc.2015.10.013
Shiomi M, Miyamae M, Takemura G, Kaneda K, Inamura Y, Onishi A, Koshinuma S, Momota Y, Minami T, Figueredo VM (2014) Induction of autophagy restores the loss of sevoflurane cardiac preconditioning seen with prolonged ischemic insult. Eur J Pharmacol 724:58–66. doi:10.1016/j.ejphar.2013.12.027
Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP (1999) Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol 19:7289–7304
Sonne DP, Engstrom T, Treiman M (2008) Protective effects of GLP-1 analogues exendin-4 and GLP-1(9-36) amide against ischemia–reperfusion injury in rat heart. Regul Pept 146:243–249. doi:10.1016/j.regpep.2007.10.001
Sotgia F, Martinez-Outschoorn UE, Howell A, Pestell RG, Pavlides S, Lisanti MP (2012) Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annu Rev Pathol 7:423–467. doi:10.1146/annurev-pathol-011811-120856
Sun J, Nguyen T, Aponte AM, Menazza S, Kohr MJ, Roth DM, Patel HH, Murphy E, Steenbergen C (2015) Ischaemic preconditioning preferentially increases protein S-nitrosylation in subsarcolemmal mitochondria. Cardiovasc Res 106:227–236. doi:10.1093/cvr/cvv044
Swaney JS, Patel HH, Yokoyama U, Head BP, Roth DM, Insel PA (2006) Focal adhesions in (myo)fibroblasts scaffold adenylyl cyclase with phosphorylated caveolin. J Biol Chem 281:17173–17179. doi:10.1074/jbc.M513097200
Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP (1996) Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem 271:2255–2261
Tonkovic-Capin M, Gross GJ, Bosnjak ZJ, Tweddell JS, Fitzpatrick CM, Baker JE (2002) Delayed cardioprotection by isoflurane: role of K(ATP) channels. Am J Physiol Heart Circ Physiol 283:H61–H68. doi:10.1152/ajpheart.01040.2001
Tsutsumi YM, Horikawa YT, Jennings MM, Kidd MW, Niesman IR, Yokoyama U, Head BP, Hagiwara Y, Ishikawa Y, Miyanohara A, Patel PM, Insel PA, Patel HH, Roth DM (2008) Cardiac-specific overexpression of caveolin-3 induces endogenous cardiac protection by mimicking ischemic preconditioning. Circulation 118:1979–1988. doi:10.1161/circulationaha.108.788331
Tsutsumi YM, Kawaraguchi Y, Horikawa YT, Niesman IR, Kidd MW, Chin-Lee B, Head BP, Patel PM, Roth DM, Patel HH (2010) Role of caveolin-3 and glucose transporter-4 in isoflurane-induced delayed cardiac protection. Anesthesiology 112:1136–1145. doi:10.1097/ALN.0b013e3181d3d624
Tsutsumi YM, Kawaraguchi Y, Niesman IR, Patel HH, Roth DM (2010) Opioid-induced preconditioning is dependent on caveolin-3 expression. Anesth Analg 111:1117–1121. doi:10.1213/ANE.0b013e3181f3351a
Tsutsumi YM, Tsutsumi R, Hamaguchi E, Sakai Y, Kasai A, Ishikawa Y, Yokoyama U, Tanaka K (2014) Exendin-4 ameliorates cardiac ischemia/reperfusion injury via caveolae and caveolins-3. Cardiovasc Diabetol 13:132. doi:10.1186/s12933-014-0132-9
Tsutsumi YM, Tsutsumi R, Horikawa YT, Sakai Y, Hamaguchi E, Ishikawa Y, Yokoyama U, Kasai A, Kambe N, Tanaka K (2014) Geranylgeranylacetone protects the heart via caveolae and caveolin-3. Life Sci 101:43–48. doi:10.1016/j.lfs.2014.02.019
Tsutsumi YM, Tsutsumi R, Horikawa YT, Sakai Y, Hamaguchi E, Kitahata H, Kasai A, Kambe N, Tanaka K (2014) Geranylgeranylacetone and volatile anesthetic-induced cardiac protection synergism is dependent on caveolae and caveolin-3. J Anesth 28:733–739. doi:10.1007/s00540-014-1816-8
Umegaki E, Kuramoto T, Kojima Y, Nouda S, Ishida K, Takeuchi T, Inoue T, Tokioka S, Higuchi K (2014) Geranylgeranylacetone, a gastromucoprotective drug, protects against NSAID-induced esophageal, gastroduodenal and small intestinal mucosal injury in healthy subjects: a prospective randomized study involving a comparison with famotidine. Intern Med 53:283–290. doi:10.2169/internalmedicine.53.1572
Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, Tester DJ, Balijepalli RC, Foell JD, Li Z, Kamp TJ, Towbin JA (2006) Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation 114:2104–2112. doi:10.1161/circulationaha.106.635268
Volonte D, Galbiati F, Li S, Nishiyama K, Okamoto T, Lisanti MP (1999) Flotillins/cavatellins are differentially expressed in cells and tissues and form a hetero-oligomeric complex with caveolins in vivo. Characterization and epitope-mapping of a novel flotillin-1 monoclonal antibody probe. J Biol Chem 274:12702–12709. doi:10.1074/jbc.274.18.12702
Wang J, Schilling JM, Niesman IR, Headrick JP, Finley JC, Kwan E, Patel PM, Head BP, Roth DM, Yue Y, Patel HH (2014) Cardioprotective trafficking of caveolin to mitochondria is Gi-protein dependent. Anesthesiology 121:538–548. doi:10.1097/aln.0000000000000295
Wang X, Yuan B, Dong W, Yang B, Yang Y, Lin X, Gong G (2014) Induction of heat-shock protein 70 expression by geranylgeranylacetone shows cytoprotective effects in cardiomyocytes of mice under humid heat stress. PLoS One 9:e93536. doi:10.1371/journal.pone.0093536
Wang Y, Wang X, Jasmin JF, Lau WB, Li R, Yuan Y, Yi W, Chuprun K, Lisanti MP, Koch WJ, Gao E, Ma XL (2012) Essential role of caveolin-3 in adiponectin signalsome formation and adiponectin cardioprotection. Arterioscler Thromb Vasc Biol 32:934–942. doi:10.1161/atvbaha.111.242164
Way M, Parton RG (1995) M-caveolin, a muscle-specific caveolin-related protein. FEBS Lett 376:108–112. doi:10.1016/0014-5793(95)01256-7
Williams TM, Lisanti MP (2004) The caveolin proteins. Genome Biol 5:214. doi:10.1186/gb-2004-5-3-214
Yang G, Dong Z, Xu H, Wang C, Li H, Li Z, Li F (2015) Structural study of caveolin-1 intramembrane domain by circular dichroism and nuclear magnetic resonance. Biopolymers 104:11–20. doi:10.1002/bip.22597
Yang Y, Duan W, Jin Z, Yi W, Yan J, Zhang S, Wang N, Liang Z, Li Y, Chen W, Yi D, Yu S (2013) JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J Pineal Res 55:275–286. doi:10.1111/jpi.12070
Yao Y, Hong S, Zhou H, Yuan T, Zeng R, Liao K (2009) The differential protein and lipid compositions of noncaveolar lipid microdomains and caveolae. Cell Res 19:497–506. doi:10.1038/cr.2009.27
Young LH, Ikeda Y, Lefer AM (2001) Caveolin-1 peptide exerts cardioprotective effects in myocardial ischemia–reperfusion via nitric oxide mechanism. Am J Physiol Heart Circ Physiol 280:H2489–H2495
Yu H, Yang Z, Pan S, Yang Y, Tian J, Wang L, Sun W (2015) Hypoxic preconditioning promotes the translocation of protein kinase C epsilon binding with caveolin-3 at cell membrane not mitochondrial in rat heart. Cell Cycle 14:3557–3565. doi:10.1080/15384101.2015.1084446
Zhang J, Liem DA, Mueller M, Wang Y, Zong C, Deng N, Vondriska TM, Korge P, Drews O, Maclellan WR, Honda H, Weiss JN, Apweiler R, Ping P (2008) Altered proteome biology of cardiac mitochondria under stress conditions. J Proteome Res 7:2204–2214. doi:10.1021/pr070371f
Zhang Y, Lv F, Jin L, Peng W, Song R, Ma J, Cao CM, Xiao RP (2011) MG53 participates in ischaemic postconditioning through the RISK signalling pathway. Cardiovasc Res 91:108–115. doi:10.1093/cvr/cvr029
Zhao J, Wang F, Zhang Y, Jiao L, Lau WB, Wang L, Liu B, Gao E, Koch WJ, Ma XL, Wang Y (2013) Sevoflurane preconditioning attenuates myocardial ischemia/reperfusion injury via caveolin-3-dependent cyclooxygenase-2 inhibition. Circulation 128:S121–S129. doi:10.1161/circulationaha.112.000045
Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J (2003) Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 285:H579–H588. doi:10.1152/ajpheart.01064.2002
Zhu H, Hou J, Roe JL, Park KH, Tan T, Zheng Y, Li L, Zhang C, Liu J, Liu Z, Ma J, Walters TJ (2015) Amelioration of ischemia–reperfusion induced muscle injury by the recombinant human MG53 protein. Muscle Nerve 52:852–858. doi:10.1002/mus.24619
Author information
Authors and Affiliations
Corresponding authors
Additional information
Y. Yang and Z. Ma contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, Y., Ma, Z., Hu, W. et al. Caveolin-1/-3: therapeutic targets for myocardial ischemia/reperfusion injury. Basic Res Cardiol 111, 45 (2016). https://doi.org/10.1007/s00395-016-0561-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-016-0561-6