Abstract
There is evidence for a negative correlation between green tea consumption and cardiovascular diseases. The aim of the present study was to examine whether green tea extract (GTE) given before regional myocardial ischemia could improve depression of myocardial contractility by preventing cytosolic Ca2+ overload. Regional ischemia–reperfusion (IR) was induced in rats by ligating the left anterior descending branch for 20 min, then releasing the ligature. Ligation induced ventricular arrhythmias in rats without GTE pretreatment, but decreased arrhythmogenesis was seen in rats pretreated 30 min earlier with GTE (400 mg/kg). During reperfusion, arrhythmias only occurred during the initial 5 min, and GTE pretreatment had no effect. After overnight recovery, serum cTnI levels were greatly increased in control post-IR rats but only slightly elevated in GTE-pretreated post-IR rats. Myocardial contractility measured by echocardiography was still depressed after 3 days in control post-IR rats, but not in GTE-pretreated post-IR rats. No myocardial ischemic injury was seen in post-IR rats with or without GTE pretreatment. Using freshly isolated single heart myocytes, GTE was found to attenuate the post-IR injury-associated cytosolic Ca2+ overload and modulate changes in the levels and distribution of myofibril, adherens junction, and gap junction proteins. In summary, GTE pretreatment protects cardiomyocytes from IR injury by preventing cytosolic Ca2+ overload, myofibril disruption, and alterations in adherens and gap junction protein expression and distribution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies on myocardial ischemia–reperfusion (IR) have suggested that the myocardial protective effect of green tea polyphenols [e.g., catechin and (−)-epigallocatechin-3-gallate (EGCg)] is associated with their antioxidant properties of scavenging active oxygen radicals, modulating the expression of redox-sensitive transcription factors (e.g., NFκB, AP-1), reducing STAT-1 activation and Fas receptor expression, and increasing NO production [1, 10, 29, 31]. Recently, we suggested a novel mechanism in which EGCg acts by binding to the C-lobe of troponin C (cTnC) and altering Ca2+-dependent protein–protein interactions between other regulatory molecules in the thin filament of cardiac muscle [18]. This mechanistic action is supported by results in a rat model of myocardial infarction (MI) induced by left anterior descending coronary ligation (LAD ligation), showing that green tea polyphenols protect the ischemic myocardium by modulating myofilament Ca2+ sensitivity in post-MI rats [28]. In addition, NMR spectroscopy has recently shown that EGCg binds to the C-lobe of cTnC near the region involved in the interaction with the N-terminal helix of cTnI [24].
Green tea polyphenols have been shown to have a positive inotropic effect on myocardial contractility without an increase of intracellular Ca2+ [10], presumably via activation of Na+–H+ exchange (NHE) and the reverse mode of the Na+–Ca2+ exchange (NCX) [12] or a protein kinase-Cε-dependent signaling pathway [16]. Transient ischemia followed by reperfusion can result in cellular Ca2+ overload by cellular Na+ overload due to inhibition of sarcolemmal Na+–K+–ATPase and acidosis resulting in NHE [20]. The NHE is known to facilitate the heart to adapt to intracellular acidosis during ischemia and recovery from acidosis after reperfusion [12]. Na+ overload would lead to Ca2+ overload via increased NCX. This increase in cellular Ca2+ activates calpains to cause selective proteolysis of myofibrils, thus altering myocardial structure and contractility after IR injury [11, 12, 16–19].
There is evidence suggesting that remodeling of intercellular junctional complexes in the intercalated discs plays an important role in the modulation of functional recovery of myocardial contractility after global IR injury [30]. The intercalated disc consists of adherens junctions, which provide cell–cell adhesion, and gap junctions, which allow direct communication between adjacent cells [11, 14, 30, 37]. N-cadherin, a member of the cadherin family of Ca2+-dependent homophilic cell adhesion molecules, plays a critical role in maintaining the integrity of the intercalated disc and transmitting cell–cell adhesion signals to the actin cytoskeleton by interacting with β-catenin [13, 15, 37]. Connexin 43 (Cx43), the major ventricular gap junction protein, is involved in the connections between adjacent myocytes to preserve synchronization of excitation of the heart [2, 17, 22]. Structural disruption of the intercalated disc and subsequent alterations in adherens and gap junction proteins have been implicated in the increased conduction heterogeneity and intercellular electrical uncoupling seen in diseased hearts [25, 26, 30].
In this study, we used a surgical IR model in the rat involving transient LAD ligation for 20 min (ischemia), followed by subsequent release of ligation (reperfusion), to examine whether supplementation with green tea extract (GTE) 30 min prior to surgery could reduce the risk for regional myocardial IR injury. To further examine the effects of GTE on IR-associated changes in cellular Ca2+ levels, myofibril structures, and adherens and gap junction protein expression and subcellular distribution, single heart cardiomyocytes were freshly isolated from the animals and the results showed that GTE prevented post-IR injury when administered before regional ischemia by lowering intracellular Ca2+ levels, maintaining the structural integrity of cardiomyocytes and modulating the expression and distribution of adherens and gap junction proteins in the intercalated disc.
Materials and methods
Chemicals and reagents
Green tea extract (GTE) (Sunphenon 90DCF-T) was purchased from Taiyo Kagaku Co., Ltd. (Tokyo, Japan). According to the manufacturer's information, the GTE used in this study contained 100.44% polyphenols, of which 91.75% were catechins, 47.02% EGCg, and 0.73% caffeine. Pure EGCg was purchased from Sigma (St. Louis, MO) and was prepared as a 10-mM stock solution.
Experimental animals
Male Sprague–Dawley rats (200–250 g), aged 8–9 weeks, were randomly divided into three groups; the control group, the LAD ligation without GTE pretreatment group, and the LAD ligation with GTE pretreatment group, with 20–30 animals per group. Chen et al. [4] has determined the plasma levels of green tea polyphenols after intragastric administration of GTE (200 mg/kg) to rats. According to their data, the time to maximum concentration of green tea polyphenols absorbed in plasma was 54.6–74.4 min. In this study, GTE was given intragastrically as a 400 mg/kg dose, 30 min before ligation, assuming that would get the similar efficiency of GTE absorption to their finding. The animals were housed in small groups in a temperature- (24 ± 1°C), humidity- (55 ± 5%), and light- (12 h light: 12 h dark) controlled room until the study. During this period, the rats had access to standard rat chow and distilled water ad libitum.
Left coronary artery ligation
The anesthesia protocol has been described previously [28]. Once the rat was anesthetized, the heart was exposed via a left thoracotomy and a 6–0 polypropylene suture tied on the LAD coronary artery 3 mm distal to the inferior margin of the left atrium and left for 20 min, then removed, and the chest wall closed in layers. All experimental procedures conformed to the “Guidelines for Proper Conduct of Animal Experiments” approved by the Animal Care and use Committee of Taichung Veterans General Hospital and National Chung-Hsing University.
Pseudo-ECG recordings
A pseudo-ECG recording was obtained using widely spaced bipoles, one at the apex of the left ventricle (LV) and the other at the high lateral wall of the right ventricle. The signals were filtered from 0.05 to 100 Hz and digitized using an AxoScope with a sampling rate of 1 kHz [32–34]. The pseudo-ECG recording was used to determine the rhythm of the ventricles. The data were continuously acquired during the 20 min of ligation and 20 min of reperfusion. The number of episodes with ventricular arrhythmias was then determined.
Measurement of cTnI levels in serum and histopathological analysis
Blood samples were centrifuged at 3,000×g for 10 min to obtain serum. Serum cTnI levels were measured by automated immunochemiluminescence, as described previously [28, 36].
To determine myocardial ischemic injury, the hearts were sectioned from the apex to the base into five 3 mm thick slices, each of which was incubated for 10 min at 32°C in 1% triphenyltetrazolium chloride (TTC, Alfa Aesar, Ward Hill, MA) in phosphate-buffered saline (PBS), pH 7.4, then examined by light microcopy. The non-injured myocardium was stained red, while the ischemic myocardium appeared white. The slices were photographed using a digital camera, and the images were analyzed using the computerized Image-Pro Plus software (Media Cybernetics Inc, Silver Spring, MD, USA). Non-infarcted myocardium was determined as a ratio dividing total TTC-stained areas to whole heart mass.
Echocardiography
The rats were anesthetized with intraperitoneal ketamine (80 mg/kg), and echocardiographic images were taken as described previously [28]. End diastole was measured at the time of maximal LV diastolic dimension. End systole was measured at the time of the most anterior systolic excursion of the posterior wall. The diastolic inter-ventricular septum thickness (IVSd), diastolic left ventricular inner diameter (LVIDd), end diastolic left ventricular thickness (EDLV), and systolic LV inner diameter (LVIDs) were measured by M-mode echocardiography. The LV fractional shortening (FS) and LV ejection fraction (EF) were determined using the equations:
Cardiomyocyte isolation
The heart was removed from a pentobarbital sodium-anesthetized (50 mg/kg) rat and the aorta cannulated and the heart perfused successively with 1.8 mM Ca2+ HEPES-Tyrode solution (in millimolar, 137 NaCl, 5.4 KCl, 1.8 CaCl2, 0.5 MgCl2, 0.33 NaH2PO4, 5 HEPES, 5 glucose, pH 7.4) for 5 min, Ca2+-free HEPES-Tyrode solution for 4 min, and Ca2+-free HEPES-Tyrode solution containing 0.2 mg/ml collagenase (Sigma) and 0.04 mg/ml type XIV protease (Sigma) for 4 min, as described previously [35]. The LV was then cut into small pieces and gently stirred in 0.2 mM Ca2+ HEPES-Tyrode solution for 10 min at 37°C, and ventricular myocytes were collected by centrifugation of the cell suspension and resuspended in 1.8 mM Ca2+ HEPES-Tyrode solution containing 1% bovine serum albumin (Sigma).
Western blots
Cardiac tissues were homogenized with the denaturing solution (8 M urea, 2 M thiourea, 50 mM Tris–OH, pH 6.8, 75 mM dithiothreitol, 3% SDS), and the homogenate was centrifuged at 13,200×g (4°C) for 15 min. The supernatant was diluted twice with sample buffer (125 mM Tris–HCl, pH 6.8, 4% SDS, 20% glycerol, 10% 2-mercaptoethanol) and incubated in a water bath (100°C) for 10 min. Electrophoresis was carried out on an acrylamide gradient (5–15%) gel at a constant current of 25 mA for about 90 min in a cold room, then the proteins were electro-transferred onto PVDF membranes at constant current (210 mA) for 3 h. After blocking for 1 h at 4°C with 5% non-fat milk in buffer A (20 mM Tris, pH 7.5, 500 mM NaCl, 0.05% Tween–20), the blots were incubated for 1 h at room temperature with primary antibodies diluted in buffer A; the primary antibodies were mouse monoclonal antibodies against bovine cTnI (Mab1691, Chemicon, Minipore; 1:5,000 dilution), human N-cadherin (H-63) (sc-7939), human-β-catenin (9F2) (sc-47752), or rat-nCx43(D-7)(sc-13558) (all from Santa Cruz and all 1:1,000 dilution), human Tm (all forms) (TM311, Sigma; 1:1,000 dilution), or chicken Tm (Sarcomeric) (CH1, Sigma; 1:1,000 dilution), rabbit polyclonal anti-rat Cx43 antibodies (71-0700) (Zymed, Invitrogen; 1:1,000 dilution), and rabbit monoclonal anti-mouse-glyceraldehyde 3-phosphate dehydrogenase antibody (G9545, Sigma; 1:10,000 dilution). After 3 × 10 min washes with PBS containing 0.05% Tween-20, the membrane was incubated for 2 h at 4°C with alkaline phosphatase-conjugated goat anti-rabbit or rabbit-anti-mouse IgG antibodies (Santa Cruz; 1:5,000 in buffer A) and the bound antibody detected using 5-bromo-4-chloro-3-indolyl phosphate-nitro blue tetrazolium.
Immunocytochemistry and confocal fluorescence scanning microscopy
The cellular distribution of N-cadherin, β-catenin, connexin 43, cTnI, Tm, and F-actin was studied in single cardiomyocytes isolated from the myocardium of the three groups. The myocytes were fixed in 4% paraformaldehyde for 15 min, permeabilized with 0.1% Triton X-100 in PBS for 1 min, and blocked with 2% bovine serum albumin in PBS for 30 min (all at room temperature). For the first five proteins, the fixed cells were incubated for 1 h at 4°C with the same primary antibodies as in the Western blot section (1:200 in PBS), then for 30 min at room temperature with secondary antibodies conjugated to the fluorescence probe Alexa Fluor® 488 (1:700 in PBS) (Molecular Probes, Invitrogen Life Sciences, Carlsbad, CA), then F-actin was labeled by incubating the cells for 5 min at room temperature with Alexa Fluor® 568 phalloidin (1:1,000 in PBS) (Molecular Probes), and the nucleus was labeled with DAPI (1:1,000) (Molecular Probes) for 1 min. Fluorescence images were observed using an Olympus FluoView™ FV1000 confocal fluorescence scanning microscope (Olympus Co., Tokyo, Japan). A series of optical sections at 0.5 μm intervals was prepared from the bottom to the top of the cells using a computerized motor. Image analysis was performed as described previously [27].
Determination of cellular Ca2+ levels
Fura 2-tetra-acetoxymethyl ester (fura 2-AM; Molecular Probes, Eugene, OR) was used as the fluorescent indicator. Cardiomyocytes were harvested and washed in 1.8 mM Ca2+ HEPES-Tyrode solution containing 1% bovine serum albumin, dispersed in PBS containing 2 mM fura 2-AM and incubated for 45 min at room temperature, then for 30 min at 37°C, during which time the fura 2-AM was trapped intracellularly by esterase cleavage. The cardiomyocytes were then washed twice with PBS and brought to a density of 2 × 106 cells per milliliter in PBS. Recordings were made on a 3-ml sample in a thermostatically controlled (25°C) stirred cuvette in a Perkin-Elmer LS 50B spectrofluorometer equipped with an accessory to measure Ca2+ (Beaconsfield, Buckinghamshire, England). The dye trapped inside the cells was excited every second by exposure to alternating 340 and 380 nm light beams, and the intensity of light emission at 510 nm was measured, allowing monitoring of the light intensity and the 340 nm fluorescence/380 nm ratio (F340/F380). EGCg was added to the cuvette using an at least 100-fold concentrated stock solution to avoid large volume variations [27]. The 340/380 ratio (R) was calculated and converted to the corresponding levels of [Ca2+] i as described by Grynkiewicz et al. [8], using a Kd of 0.14 μM:
where R min and R max are the ratios measured by releasing the intracellular dye with 2 mM EGTA in 0.1% Triton X-100 (R min), then adding 2.1 mM Ca2+ (R max), and Sf2/Sb2 is the ratio of the 380-nm signals in Ca2+-free and Ca2+-repletion solution, respectively.
Statistical analysis
Quantitative values are presented as the mean and standard error of the mean (mean ± SEM). A difference was considered to be statistically significant when the P value was less than 0.05.
Results
GTE pretreatment prevents the ventricular arrhythmias associated with IR
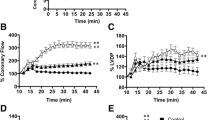
Using pseudo-ECG recording, we first defined six different ventricular arrhythmias patterns ranging in severity from premature ventricular contraction (PVC, patterns 1 and 2) through ventricular tachycardia (VT, patterns 3 and 4) to ventricular fibrillation (VF, patterns 5 and 6) in rats after transient ligation of left anterior descending (LAD) coronary artery with or without GTE pretreatment 30 min before ligation (Fig. 1a). The less severe patterns 1 and 2 were grouped as group 1 (Gr 1), and the remaining four patterns as group 2 (Gr 2). Clinically, Gr 2 arrhythmias are high-risk and life-threatening ventricular arrhythmias as compared to Gr 1. In the case of Gr 1, GTE pretreatment resulted in significant suppression of genesis of arrhythmia at 5–10 min after LAD ligation, but not at other time periods (0–5, 10–15, and 15–20 min) (Fig. 1b, left panel). In the case of group 2 (Gr 2), GTE pretreatment caused a significant decrease in occurrence at 5–10 min and 10–15 min after LAD ligation (Fig. 1b, right panel). Apparently, GTE pretreatment prevents arrhythmogenesis more effectively for Gr 2 than for Gr 1. In contrast, in both groups, during the 20 min of reperfusion, ventricular arrhythmias only occurred during the initial 5 min, and there was no significant effect of GTE on arrhythmogenesis (Fig. 1c).
GTE pretreatment prevents ventricular arrhythmias triggered by IR. a Six different patterns of ventricular arrhythmias in IR-operated rats with or without GTE pretreatment were defined and classified into two groups, group 1 (patterns 1 and 2) and group 2 (patterns 3–6). VT ventricular tachycardia, VF ventricular fibrillation, PVC premature ventricular contraction. b Effect of GTE pretreatment on genesis of arrhythmia at different times after LAD ligation on group 1 (Gr 1, left panel) and group 2 (Gr 2, right panel). c Lack of effect of GTE pretreatment on arrhythmias during reperfusion for group1 (left) and group 2 (right). The data are the mean ± SD for n = 10. Asterisk, significant difference between LAD-operated rats with or without GTE pretreatment
GTE pretreatment reduces serum cTnI levels, myocardial injury, and cardiac performance in post-IR rats
It has been suggested that reperfusion after brief myocardial ischemia plays a dual role in preserving cell viability and leading to depression of myocardial contractility [3, 5–7, 9, 12, 20]. In this study, serum cTnI levels after overnight recovery were significantly increased in post-IR rats without GTE pretreatment (1.24 ± 0.36 ng/ml) but showed a much lower increase in the GTE-pretreated post-IR rats (0.07 ± 0.03 ng/ml) compared to controls (<0.006 ng/ml) (Fig. 2a). This indicates that regional myocardial IR injury causes myofibril proteolysis and/or sarcomere dissociation and that GTE pretreatment protects against this effect. After 3 days of recovery from IR, transthoracic echocardiography showed a 34% decrease in fractional shortening (FS) (Fig. 2b, left lower panel) and a 26% decrease in the ejection fraction (EF) (Fig. 2b, right lower panel) in post-IR rats without GTE pretreatment, but no changes in the EF (P = 0.19) and FS (P = 0.15) in GTE-pretreated post-IR rats compared to controls. However, TTC staining (Fig. 2c, left panel) with quantitative analyses (Fig. 2c, right panel) detected no irreversible myocardial ischemic injury at 3 days post-IR in rats with or without GTE pretreatment. This suggests that regional myocardial IR causes depression of myocardial contractility and that GTE given 30 min before the ischemia improves the functional recovery.
Effect of GTE pretreatment on myocardial injury-associated changes in serum cTnI levels, contractility, and infarction in post-IR rats. a Serum cTnI levels after overnight recovery in post-IR rats with or without GTE pretreatment. The data are the mean ± SEM for n = 10. Asterisk, significant difference between controls and IR rats. b Echocardiogram showing a decreased fractional shortening (FS) and ejection fraction (EF) in rats 3 days post-IR with or without GTE pretreatment. The data are the mean ± SEM for n = 10. Asterisk, significant difference between controls and IR-operated rats with and without GTE pretreatment. c TTC staining showed no irreversible myocardial injury in control and post-IR rats with or without GTE pretreatment after 3 days of recovery. The calibration bar is 1 cm (left panel). Non-infarcted myocardium was determined by a ratio dividing total TTC-stained areas to whole heart mass (right panel). The data are the mean ± SEM for n = 16
Effect of EGCg on cytosolic Ca2+ levels in isolated cardiomyocytes from controls and in 3 day post-IR rats with or without GTE pretreatment
Decreases in myocardial functional recovery after myocardial IR have been shown to alter myocardial structures involving selective proteolysis of myofibril proteins by the generation of oxygen-derived free radicals and/or by a transient Ca2+ overload and the activation of Ca2+-dependent proteases [3, 5–7, 9, 12, 20]. To determine whether GTE pretreatment could attenuate the cytosolic Ca2+ overload associated with regional myocardial IR injury, cellular Ca2+ levels were measured using the fura 2 fluorescence ratio (F340/F380) in cardiomyocytes from the three groups after 3 days of recovery, then during the addition of increasing concentrations of ECGg for 3-min periods. Figure 3a shows a typical example of the F340/F380 ratio, and Fig. 3b shows the mean corresponding Ca2+ concentration for more than 20 experiments. The cellular Ca2+ concentration in the untreated cell suspension (2 × 106 cells) was 67.53 ± 6.06 nM in controls (N = 20 animals), 132.43 ± 13.25 nM in post-IR rats without GTE pretreatment (N = 20), and 94.45 ± 9.20 nM in post-IR rats with GTE pretreatment (N = 23). This indicates that brief IR increases intracellular Ca2+ levels and that GTE pretreatment reduces this cytosolic Ca2+ overload. When EGCg was added at increasing concentrations from 25 to 200 μM, the lowest concentration causing a significant reduction in intracellular Ca2+ levels was 200 and 25 μM in post-IR rats with or without GTE pretreatment, respectively. EGCg had no effect on intracellular Ca2+ levels in the controls (Fig. 3). Clearly, additional EGCg can act on cardiomyocytes of 3-day post-IR rats with or without GTE pretreatment to attenuate the Ca2+ overload.
Cytosolic Ca2+ levels in cardiomyocytes from control rats and 3-day post-IR rats with and without GTE pretreatment and effect of EGCg addition. a Cytosolic Ca2+ levels in cardiomyocytes isolated from the three groups and the dose effect of added EGCg. Ca2+ levels were measured as the fura-2 F340/F380 fluorescence ratio in freshly isolated cardiomyocytes, then different concentrations of ECGg (25, 50, 75, 100, and 200 μM) were added for 3-min periods and the F340/F380 continuously monitored. b Quantitative analysis of the EGCg-induced decrease in cytosolic Ca2+ levels in cardiomyocytes from the three groups. The values are the mean ± SEM, with asterisk indicating a significant difference compared to the controls. In all groups, at least 20 animals were sacrificed for preparation of cardiomyocytes and for the measurement of cytosolic Ca2+ levels in the cell suspensions
Effects of IR and GTE pretreatment on the content and subcellular distribution of the myofibril proteins cTnI and Tm
Depressed myocardial contractility after brief IR is thought to be a result of sarcomere disruption initiated by oxidative stress [3, 9, 20]. To examine changes in all Tm isoforms in cardiomyocytes, sarcomeric Tm isoforms were measured using antibody CH1, and both the sarcomeric and cytoskeletal isoforms of Tm were measured using antibody TM311 (both from Sigma). Western blot analysis showed that levels of Tm (34 kDa) relative to GADPH were increased by 68% using antibody TM311 and by 41% using antibody CH1 in cardiomyocytes from day 3 post-IR rats without GTE pretreatment (lane 2) but were unchanged in myocytes from post-IR rats with GTE pretreatment (lane 3) compared to controls (lane 1) (Fig. 4a). In contrast, cTnI levels were decreased by 56% in cardiomyocytes of post-IR rats without GTE pretreatment (lane 2), but only by 36% with GTE pretreatment (lane 3) compared to controls (lane 1) (Fig. 4a). In addition, confocal fluorescence microscopy was used to study spatial changes of cTnI (Fig. 4b) and Tm (Fig. 4c) associated with F-actin in cardiomyocytes from the three groups. In cardiomyocytes, cTnI or Tm was labeled with immunofluorescence as green, while F-actin was labeled with rhodamine phalloidin as red. Quantitatively, the cTnI staining in cardiomyocytes was decreased by 58% in post-IR rats without GTE pretreatment compared to controls, but only by 20% in post-IR rats with GTE pretreatment (Fig. 4d). Note that no difference in F-actin staining of cardiomyocytes was seen in the different groups (Fig. 4d). Sarcomeric and cytoskeletal Tm staining using antibody TM311 relative to F-actin fluorescence in myocytes was increased by about twofold in post-IR rats without GTE pretreatment compared to controls, but was only slightly increased by 24% in GTE-pretreated post-IR rats (Fig. 4e). This suggests that Tm might serve a compensatory role in augmenting contractile recovery during IR periods of myocardial injury [23].
Effects of IR and GTE pretreatment on the content and cellular distribution of cTnI and Tm. a Western blotting and quantitative analyses of Tm and cTnI levels in the myocardium of controls (lane 1) or 3-day post-IR rats with (lane 3) or without GTE (lane 2) pretreatment. Two different antibodies, CH1 (Sigma) and TM311 (Sigma), were used to detect the sarcomeric and cytoskeletal isoforms of Tm in cardiomyocytes as indicated. GADPH was used as the loading control for data analyses. b, c Confocal immunocytofluorescence images showing fluorescence staining of cTnI (b) and Tm (c) in cardiomyocytes from the three groups. d, e Quantitative analyses of the results from the experiments shown in (b) and (c) for the ratio of the fluorescence density to the labeled area for F-actin and cTnI (d) or the Tm fluorescence relative to that of F-actin (e) in the three groups. Fluorescence labeling was performed as described previously [34]; cTnI or Tm is labeled green, F-actin red, and the nucleus blue. In (a), (d), and (e), each value is the mean ± SEM, with n = 8 in (a) and 20 in (d) and (e). Asterisk indicates a significant difference compared to the controls
Effects of IR and GTE pretreatment on the content and subcellular distribution of the adherens junction proteins N-Cad and β-catenin and the ventricular gap junction protein Cx43
To examine whether GTE has a protective effect on changes in adherens and gap junction proteins in the intercalated disc after regional myocardial IR, we studied the differential expression of the adhesion molecules N-cadherin (N-Cad) and β-catenin and the ventricular gap junction protein connexin 43 (Cx43) in cardiomyocytes from the three groups. Western blotting analyses for N-cadherin (N-Cad) levels in the myocardium (Fig. 5a) showed a 30% decrease in protein content (P = 0.013) in cardiomyocytes from post-IR rats without GTE pretreatment (lane 2) compared to controls (lane 1), but not in GTE-pretreated post-IR rats (lane 3) (P = 0.89). Spatial changes in Ν-Cad were examined by confocal immunofluorescence microscopy (Fig. 5b, top panel). N-Cad is labeled green, F-actin red, and the nucleus blue. The fluorescence of N-Cad labeling relative to that of F-actin labeling (Fig. 5b, bottom panel) indicated a 43% decrease in subcellular distribution (P = 0.003) in cardiomyocytes from post-IR rats without GTE pretreatment compared to controls, but not in GTE-pretreated post-IR rats (P = 0.33).
Effects of IR and GTE pretreatment on levels and cellular distribution of N-cadherin and β-catenin. a Western blotting with quantitative analyses of N-cadherin (N-Cad) levels in the myocardium of controls (lane 1) or 3-day post-IR rats with (lane 3) or without (lane 2) GTE pretreatment. b Spatial changes in Ν-Cad examined by confocal immunofluorescence microscopy (top panel) and quantitative analyses of the fluorescence of N-Cad labeling relative to that of F-actin labeling (bottom panel). N-Cad is labeled green, F-actin red, and the nucleus blue. c Western blotting with quantitative analyses of β-catenin levels in the myocardium of controls (lane 1) or 3-day post-IR rats with (lane 3) or without (lane 2) GTE pretreatment. d Top panel micrographs showing the same optical section of fluorescence images with β-catenin fluorescence (left column), F-actin fluorescence (middle column), and the merged fluorescence (right column) in cardiomyocytes from the three groups. Bottom panel quantification by measuring the relative fluorescence of β-catenin labeling to that of F-actin labeling. Each value is the mean ± SEM, with n = 8 in (a) and (c) and 20 in (b) and (d). Asterisk indicates a significant difference compared to the controls
For β-catenin levels in the myocardium, Western blotting analyses revealed a 39% decrease in protein content (P = 0.04; Fig. 5c) in cardiomyocytes from post-IR rats without GTE pretreatment (lane 2) compared to controls (lane 1), but no significant changes in post-IR rats with GTE pretreatment (lane 3) (P = 0.48). Using confocal immunofluorescence microscopy (Fig. 5d), images showing the same optical section of β-catenin staining (left column) and F-actin fluorescence (middle column) were taken for data analyses. The relative fluorescence of β-catenin labeling to that of F-actin labeling showed a 25% decrease in cytosolic distribution (P = 0.0001) in cardiomyocytes from post-IR rats without GTE pretreatment compared to controls, but no significant changes compared to controls in post-IR rats with GTE pretreatment (P = 0.97). It should be noted that N-Cad and β-catenin labeling was significantly increased at the two ends of the cells in GTE-pretreated post-IR rats compared to post-IR rats with no GTE pretreatment (Fig. 5b, d). Thus, it appears that GTE pretreatment overcomes IR-induced stress and maintains the proper expression level and subcellular distribution of N-Cad and β-catenin in cardiomyocytes to maintain the structural integrity of the cell–cell adhesion junction.
To measure levels of phosphorylated and nonphosphorylated Cx43 in cardiomyocytes, two different antibodies were used. Mouse monoclonal anti-rat-Cx43 antibody from Santa Cruz (sc-13558) labeled one band with a molecular weight of 43 kDa (Fig. 6a), and the intensity of this band was reduced in cardiomyocytes from post-IR rats by 24% with (lane 3) (P = 0.08) or by 30% without (lane 2) (P = 0.02) GTE pretreatment compared to controls (lane 1) (Fig. 6a). Rabbit polyclonal anti-Cx43 antibodies from Zymed (71-0700, Zymed, Invitrogen), known to recognize both phosphorylated (pCx43) (43 kDa) and nonphosphorylated (nCx43) (41 kDa) Cx43 isoforms on polyacrylamide gels [17, 22, 30], labeled two bands with the expected molecular weights (Fig. 6b), and the intensity of the 43 kDa band was reduced in cardiomyocytes from post-IR rats by 37% with (lane 3) (P = 0.02) or by 50% without (lane 2) (P = 0.006) GTE pretreatment compared to controls (lane 1) (Fig. 6a, b), whereas that of the 41 kDa band was similar in controls (lane 1) and 3-day post-IR rats with (lane 3) (P = 0.15) or without (lane 2) (P = 0.06) GTE pretreatment. Fluorescence confocal scanning microscopy of cardiomyocytes labeling with mouse monoclonal anti-rat-Cx43 antibody (sc-13558) showed that Cx43 was mainly located at the two ends of the cells (Fig. 6c, top panels). The Cx43/F-actin labeling ratio was decreased in post-IR rats without GTE pretreatment (0.39 ± 0.01 n = 11), but less so in GTE-pretreated post-IR rats (0.57 ± 0.06, n = 14) compared to controls (0.73 ± 0.02, n = 7) (Fig. 6c, bottom panel). Clearly, IR injury alters the protein levels and subcellular distribution of phosphorylated pCx43 in cardiomyocytes, and GTE pretreatment has a beneficial effect on restoring spatial changes in the gap junction protein, which might protect cardiomyocytes from disruption.
Effects of IR and GTE pretreatment on levels and subcellular distribution of Cx43. (a) Top Cx43 levels on western blots using mouse monoclonal anti-rat Cx43 antibody (sc-13558) in the myocardium of controls (lane 1) or 3-day post-IR rats with (lane 3) or without (lane 2) GTE pretreatment. Bottom quantitative analyses using GADPH as the loading control. b Western blot analyses of Cx43 using rabbit polyclonal antibodies (71-0700) that recognize both the phosphorylated (pCx43) and nonphosphorylated (nCx43) Cx43 isoforms. c Top micrographs showing merged fluorescence images for cardiomyocytes stained for Cx43 using the mouse anti-rat Cx43 antibody (green), F-actin (red), and the nucleus (blue) in controls, post-IR rats with and without GTE pretreatment. Bottom quantification by measuring the Cx43/F-actin fluorescence ratio. In (a), (b), and (c), each value is the mean ± SEM, with asterisk indicating a significant difference compared to the controls. For all the groups, n = 8 in (a) and (b), and n = 20 in (c)
Discussion
Evidence suggests that contractile recovery of the ischemic area can occur after brief myocardial IR [3, 5–7, 9, 12, 20]. However, recovery in experimental IR animals depends on the extent of the loss of contractility due to irreversible injury and delayed recovery of viable myocardium due to stunning [3, 9, 20]. The present study in a transient surgical rat model of IR showed that brief regional ischemia for 20 min followed by subsequent reperfusion can induce two possible outcomes; one preserving cell viability with no severe myocardial injury (Figs. 1c and 2b) and the other leading to depression of myocardial contractility (Fig. 2a, c). In addition, the present study showed that GTE pretreatment could protect post-IR rats against myocardial damage and enhance recovery of myocardial contractility (Figs. 1 and 2).
Decreases in myocardial functional recovery after myocardial IR have been attributed to the production of reactive oxygen species and Ca2+ overload in cardiomyocytes [3, 9, 20]. The Na+/H+ exchanger (NHE) is a major factor in helping the heart adapt to intracellular acidosis during ischemia and recovery from acidosis after reperfusion [10]. During myocardial ischemia, depleting intracellular ATP stores, subsequent acidosis activates the NHE and causes an increase in intracellular Na+ [3, 5–7, 9, 12, 20]. This Na+ overload then activates the reverse mode of the Na+/Ca2+ exchanger (NCX) and produces Ca2+ overload. During reperfusion, increased ATP production restores SR Ca2+ release, resulting in cytosolic Ca2+ overload. Consequently, the Ca2+ overload activates calpains, ultimately leading to selective proteolysis of elements within the contractile apparatus (i.e., cTnI) and decreased Ca2+ responsiveness of myofilaments [3, 5–7, 9, 12, 20]. In the present study, 20 min of ischemia followed by 72 h of reperfusion induced a twofold increase in intracellular Ca2+ levels (Fig. 3) and a 60% decrease in cTnI levels in cardiomyocytes (Fig. 4). GTE pretreatment of the animals decreased intracellular Ca2+ overload by 1.6-fold and cTnI degradation by 40% in cardiomyocytes (Figs. 3 and 4). Clearly, complete recovery from decreased myocardial contractility requires the repair of damage to contractile proteins and Ca2+ overload in the IR-injured myocardium. GTE administered before ischemia evidently has myocardial protective effects in increasing recovery of myofibril organization and restoring Ca2+ homeostasis in cardiomyocytes.
A recent study showed that green tea polyphenols regulate myocardial contractility in cardiomyocytes in isolated rat hearts via a PKCε-dependent signaling pathway, without increasing intracellular Ca2+ levels [16]. The positive inotropic effects of green tea polyphenols on the rat myocardium are also mediated, in part, by activation of the NHE and the reverse mode of the NCX [19]. In the present study, applying additional EGCg induced a significant reduction in intracellular Ca2+ levels in post-IR rats with or without GTE pretreatment but no effect in the controls (Fig. 3). This result is consistent with our recent report that GTE modulates myofilament Ca2+ sensitivity in a rat model of myocardial ischemia, while EGCg potentiates myofilament Ca2+ sensitivity for myocardial contractility under acidic conditions in vitro [28]. A recent study using isolated perfused working rat hearts subjected to global IR demonstrated that addition of a selective inhibitor of glycogen synthase kinase-3 (GSK-3) prior to ischemia or at the onset of reperfusion improves recovery of left ventricular work by reducing proton production and attenuating the intracellular Ca2+ overload [21]. Further studies are needed to determine whether green tea polyphenols exert cardioprotection by inhibition of GSK-3 during myocardial IR injury.
Although protective effects of green tea polyphenols on the cardiovascular system have been demonstrated [1, 10, 16, 18, 19, 28, 29, 31], little is known about effects of green tea polyphenols in maintaining myofibril structure and the integrity of intercellular junctional complexes organized in the intercalated disc after myocardial IR injury. A study using Langendorff-perfused rabbit hearts subjected to global IR showed that there are significant alterations in the structural integrity of the myocardium and gap and adherens junction protein expression with increasing global ischemia time [30]. Consistent with this, the present study, using a regional IR rat model, demonstrated changes in the expression and distribution of adherens junction proteins (i.e., N-Cad and β-catenin) (Fig. 5) and a gap junction protein (i.e., Cx43) (Fig. 6) organized in the intercalated disc after regional myocardial IR. As the adherens junction is involved in anchoring myofibrils at cell–cell attachments, the decreased expression of N-Cad and β-catenin has implications for disrupting cell–cell contact and, subsequently, uncoupling mechanical attachment between cardiomyocytes [37]. A study using induced deletion of N-Cad in the adult mouse myocardium showed that complete loss of N-Cad expression leads to decreased expression of intercalated disc components, such as Cx43, and the dissolution of the intercalated disc structure [13]. These findings suggest that N-Cad conveys signals that control the localization of Cx43. In addition, the data reported here showed that GTE pretreatment has a beneficial effect in preventing the IR-induced spatial changes in the intercellular assembly of adherens junctions (Fig. 5) and gap junctions (Fig. 6), which might protect cardiomyocytes from disruption. In summary, GTE pretreatment protects cardiomyocytes against cytosolic Ca2+ overload, myofibril disruption, and alterations in adherens and gap junction protein levels and distribution in regional myocardial IR injury.
References
Aneja R, Hake PW, Burroughs TJ, Denenberg AG, Wong HR, Zingarelli B (2004) Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol Med 10:55–62
Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, Kléber AG, Schuessler RB, Saffitz JE (2000) Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res 87:656–662
Bolli R, Marbán E (1999) Molecular and cellular mechanisms of myocardial stunning. Physiol Rev 79:609–634
Chen L, Lee MJ, Li H, Yang CS (1999) Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab Dispos 25:1045–1050
Corbucci GG, Perrino C, Donato G, Ricchi A, Lettieri B, Troncone G, Indolfi C, Chiariello M, Awedimento EV (2004) Transient and reversible deoxyribonucleic acid damage in human left ventricle under controlled ischemia and reperfusion. J Am Coll Cardiol 43:1992–1999
Depre C, Vatner SF (2007) Cardioprotection in stunned and hibernating myocardium. Heart Fail Rev 12:307–317
Ferdinandy P, Schulz R, Baxter GF (2007) Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev 59:418–458
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Heyndrickx GR, Millard RW, McRitchie RJ, Maroko PR, Vatner SF (1975) Regional myocardial functional and electrophysiological alterations after brief coronary artery occlusion in conscious dogs. J Clin Invest 56:978–985
Hotta Y, Huang L, Muto T, Yajima M, Miyazeki K, Ishikawa N, Fukuzawa Y, Wakida Y, Tushima H, Ando H, Nonogaki T (2006) Positive inotropic effect of purified green tea catechin derivative in guinea pig hearts: the measurements of cellular Ca2+ and nitric oxide release. Eur J Pharmacol 552:123–130
Huang XD, Sandusky GE, Zipes DP (1999) Heterogeneous loss of connexin43 protein in ischemic dog hearts. J Cardiovasc Electrophysiol 10:79–91
Karmazyn M, Gan XT, Humphreys RA, Yoshida H, Kusumoto K (1999) The myocardial Na(+)–H(+) exchange: structure, regulation, and its role in heart disease. Circ Res 85:777–786
Kostetskii I, Li J, Xiong Y, Zhou R, Ferrari VA, Patel VV, Molkentin JD, Radice GL (2005) Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ Res 96:346–354
Lampe PD, Lau AF (2000) Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys 384:205–215
Li J, Levin MD, Xiong Y, Petrenko N, Patel VV, Radice GL (2008) N-cadherin haploinsufficiency affects cardiac gap junctions and arrhythmic susceptibility. J Mol Cell Cardiol 44:597–606
Li D, Yang C, Chen Y (2008) Identification of a PKCε-dependent regulation of myocardial contraction by epicatechin-3-gallate. Am J Physiol 294:345–353
Lindsey ML, Escobar GP, Mukherjee R, Goshorn DK, Sheats NJ, Bruce JA, Mains IM, Hendrick JK, Hewett KW, Gourdie RG, Matrisian LM, Spinale FG (2006) Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation 113:2919–2928
Liou YM, Kuo SC, Hsieh SR (2008) Differential effects of a green tea-derived polyphenol (−)-epigallocatechin-3-gallate on the acidosis-induced decrease in the Ca2+ sensitivity of cardiac and skeletal muscle. Pflugers Archiv 456:787–800
Lorenz M, Hellige N, Rieder P (2008) Positive inotropic effects of epigallocatechin-3-gallate (EGCG) involve activation of Na+/H+ and Na+/Ca2+ exchangers. Eur J Heart Fail 10:439–445
Murphy E, Steenbergen C (2008) Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 88:581–609
Omar MA, Wang L, Clanachan AS (2010) Cardioprotection by GSK-3 inhibition: role of enhanced glycogen synthesis and attenuation of calcium overload. Cardiovasc Res 86:478–486
Peters NS, Green CR, Poole-Wilson PA, Severs NJ (1993) Reduced content of connexin43 gap junctions in ventricular myocardium from hypertrophied and ischemic hearts. Circulation 88:664–675
Rao VS, La Bonte LR, Xu Y, Yang Z, French BA, Guilford WH (2007) Alterations to myofibrillar protein function in nonischemic regions of the heart early after myocardial infarction. Am J Physiol Heart Circ Physiol 293:H654–H659
Robertson IM, Li MX, Sykes BD (2009) Solution structure of human cardiac troponin C in complex with the green tea polyphenol, (−)-epigallocatechin 3-gallate. J Biol Chem 284:23012–23023
Severs NJ, Bruce AF, Dupont E, Rothery S (2008) Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res 80:9–19
Severs NJ, Coppen SR, Dupont E, Yeh HI, Ko YS, Matsushita T (2004) Gap junction alterations in human cardiac disease. Cardiovasc Res 62:368–377
Shieh DB, Li RY, Liao JM, Chen GD, Liou YM (2010) Effects of genistein on β-catenin signaling and subcellular distribution of actin-binding proteins in human umbilical CD105-positive stromal cells. J Cell Physiol 223:423–434
Shieh SR, Tsai DC, Chen JY, Tsai SW, Liou YM (2009) Green tea extract protects rats against myocardial infarction associated with left anterior descending coronary artery ligation. Pflugers Archiv 458:631–642
Stephanou A (2004) Role of STAT-1 and STAT-3 in ischaemia/reperfusion injury. J Cell Mol Med 8:519–525
Tansey EE, Kwaku KF, Hammer PE, Cowan DB, Federman M, Levitsky S, McCully JD (2006) Reduction and redistribution of gap and adherens junction proteins after ischemia and reperfusion. Ann Thorac Surg 82:1472–1479
Townsend PA, Scarabelli TM, Pasini E, Gitti G, Menegazzi M, Suzuki H, Knight RA, Latchman DS, Stephanou A (2004) Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis. FASEB J 18:1621–1623
Wu TJ, Lin SF, Baher A, Qu Z, Garfinkel A, Weiss JN, Ting CT, Chen PS (2004) Mother rotors and the mechanisms of D600-induced type 2 ventricular fibrillation. Circulation 110:2110–2118
Wu TJ, Lin SF, Hsieh YC, Ting CT, Chen PS (2006) Ventricular fibrillation during no-flow global ischemia in isolated rabbit hearts. J Cardiovasc Electrophysiol 17:1112–1120
Wu TJ, Lin SF, Weiss JN, Ting CT, Chen PS (2002) Two types of ventricular fibrillation in isolated rabbit hearts: importance of excitability and action potential duration restitution. Circulation 106:1859–1866
Yasuda SI, Sugiura S, Kobayakawa N, Fujita H, Yamashita H, Katoh K, Saeki Y, Kaneko H, Suda Y, Nagai R, Sugi H (2001) A novel method to study contraction characteristics of a single cardiac myocyte using carbon fibers. Am J Physiol 281:H1442–H1446
York M, Scudamore C, Brady S, Chen C, Wilson S, Curtis M, Evans G, Griffiths W, Whayman M, Williams T, Turton J (2007) Characterization of troponin responses in isoproterenol-induced cardiac injury in the Hanover Wistar rat. Toxicol Pathol 35:606–617
Zuppinger C, Eppenberger-Eberhardt M, Eppenberger HM (2000) N-cadherin: structure, function and importance in the formation of new intercalated disc-like cell contacts in cardiomyocytes. Heart Fail Rev 5:251–257
Acknowledgments
This work was supported by the National Science Council of Taiwan (grant NSC 98-2320-B-005-005 to Y-M L and grants NSC 98-2314-B-010-033-MY2 and 98-2314-B-075A-011-MY2 to T.J. W). The authors wish to express special thanks to Mrs. C.H. Chiu, Ms. D.C. Tsai, Mr. P.T. Tseng, and Mr. T.C. Chen for their technical assistance and animal care.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liou, YM., Hsieh, SR., Wu, TJ. et al. Green tea extract given before regional myocardial ischemia–reperfusion in rats improves myocardial contractility by attenuating calcium overload. Pflugers Arch - Eur J Physiol 460, 1003–1014 (2010). https://doi.org/10.1007/s00424-010-0881-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-010-0881-6