Abstract
Purpose
Resistant starch (RS) content has exhibited beneficial effects on glycemic control; however, few studies have investigated the effects of this substance on postprandial responses and appetite in subjects with type 2 diabetes (T2D). Here, we aimed to examine the effects of RS from two sources on glycemic response (GR), postprandial lipemia, and appetite in subjects with T2D.
Methods
In a randomized and crossover study, 17 subjects with T2D consumed native banana starch (NBS), high-amylose maize starch (HMS) or digestible maize starch (DMS) for 4 days. On day 5, a 6-h oral meal tolerance test (MTT) was performed to evaluate glycemic and insulinemic responses as well as postprandial lipemia. Besides, subjective appetite assessment was measured using a visual analogue scale.
Results
NBS induced a reduction on fasting glycemia, glycemia peak and insulinemic response during MTT. However, no modifications on postprandial lipemia were observed after RS treatments. Both NBS and HMS reduced hunger and increased satiety.
Conclusion
NBS supplementation induced more beneficial effects on glycemic metabolism than HMS even when all interventions were matched for digestible starch content. RS intake did not modify postprandial lipemia, however, positively affected subjective appetite rates.
Trial registration: This trial was retrospectively registered at www.anzctr.org.au (ACTRN12621001382864) on October 11, 2021.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The growing global prevalence of type 2 diabetes (T2D) confirms this disease to be a significant global health challenge, being responsible for 6.7 million deaths in 2021. Nowadays, it is recognized that postprandial glycemia and lipemia in patients with T2D are better predictors of cardiovascular disease (CVD) risk than fasting concentrations of these substances [1, 2]. Due to the increasing number of individuals with obesity or diabetes, there is an intense request for healthy diets and alimentary supplements able to improve glucose metabolism and other related dysfunctions [3]. In this line, the dietary fiber content has been considered an important factor for modulating the rate of absorption and modifying the postprandial metabolism in these patients [4, 5].
Resistant starch (RS) is considered a fermentable fiber that can resist digestion in the stomach and small intestine and reaches the large intestine where is fermented by the intestinal microbiota. RS can be divided into five types [6]. Particularly, resistant starch type 2 (RS2) is a naturally occurring form of starch that is indigestible to intestinal enzymes due to the inaccessibility of its granular structure. Some sources of RS2 include high-amylose maize and wheat, green bananas, raw potatoes, and some legumes [7].
Most studies on the beneficial effects of RS2 have been conducted using high-amylose maize starch (Hi-Maize), which is isolated from a special hybrid of corn that is naturally high in amylose content [6]. Many studies have demonstrated that this substance reduces postprandial glycemia, reducing the risk of chronic diseases [8, 9]. In addition, native banana starch (NBS) obtained from unripe ‘Dwarf Cavendish’ bananas (Musa, AAA Group), is well known as a food with low glycemic index, non-manufactured, attractive, inexpensive, and containing a high-RS content [10]. Long-term NBS supplementation showed having beneficial effects on body weight, glycemia, and insulin sensitivity in subjects with obesity [11, 12]. Even when all the different types of RS2 refer to starch molecules with type B or C crystalline forms, the differences in amylose/amylopectin ratio and in the granules structure confer distinct physiological properties such as solubility, viscosity and fermentability which have implications on their clinical effects [13].
In humans, most studies have focused on the fasting parameters, which have shown that long-term RS supplementation induces a reduction in total cholesterol and LDL cholesterol mainly in healthy or with obesity subjects [14,15,16,17,18]. Additionally, RS consumption for a few days was shown to reduce food intake, although the appetite sensations were not modified in healthy young subjects [19]. Conversely, other studies report that RS consumption did not improve lipemic metabolism or appetite neither in healthy individuals nor in individuals with metabolic alterations [20,21,22,23]. Recently, we reported that a single acute NBS supplementation did not improve postprandial lipemia or subjective appetite measures in subjects with overweight or obesity [24]. Nevertheless, it is unknown whether this substance influences postprandial metabolism or affects appetite sensations in patients with T2D.
The aim of this randomized, crossover, single-blind study was to examine the effects of ingesting RS from two sources on glycemic response, postprandial lipemia and appetite in subjects with T2D. We hypothesized that RS consumption would reduce postprandial glycemia and lipemia and have a positive influence on subjective appetite scores.
Materials and methods
Ethics statement
This study was approved by the Ethical Committee of the Instituto Mexicano del Seguro Social (IMSS) (Approval No. 2015-2701-17) and followed the ethical principles and guidelines of the Declaration of Helsinki. This trial was registered at www.anzctr.org.au (Identifier Code: ACTRN12621001382864). Written informed consent was obtained from each participant prior to the start of the testing procedures.
Study population and eligibility criteria
Seventeen subjects of both genders with previous diagnosis of T2D from a health care provider of the IMSS, glycosylated hemoglobin (HbA1c) values > 6.5%, younger than 65 years old, and under medical treatment with diet and exercise, or using metformin and/or glibenclamide for glycemic control were included in the study. Exclusion criteria include the presence of cardiovascular, renal, hepatic, or gastrointestinal diseases; medication with insulin, glitazones, DPP4 inhibitors, GLP-1 analogues, or other drugs that alter the absorption of monosaccharides such as acarbose, and current practice of physical exercise for more than 4 h a week. Alcohol or smoking intake, pregnancy, and subjects who had non-permeable veins were additional exclusion criteria.
Study design and protocol
This study was part of a randomized, crossover, single-blind study in which participants were subjected to three treatment phases, each with a duration of 4 days, and 9-day washout periods between treatments. The design has been reported in detail previously [25]. During each treatment phase, subjects were asked to consume digestible maize starch (DMS), Hi-Maize Starch (HMS) or Native banana starch (NBS) as a supplement. During days 1 to 4, each participant consumed two daily beverages containing one of three different supplements for the randomly assigned treatment. In each treatment phase, fasting blood samples were obtained before and after interventions to evaluate effects on fasting parameters. Moreover, on day 5 of each phase, a 6 h meal tolerance test (MTT) was carried out to estimate glycemic and insulinemic responses, postprandial lipemia, and appetite sensations.
Starch sources
Green bananas (Musa acuminata, AAA Group, ‘Dwarf Cavendish’) were purchased from a packing plant in Villahermosa in the State of Tabasco, Mexico. The isolation procedure for banana starch was performed according to Waliszewski et al. with slight modifications [25, 26]. RS content of NBS was 70.5% according to Megazyme commercial kit K-DSTRS 11/2019 (Megazyme Ltd Co, Wicklow, Ireland). Hi-Maize® 260 (HMS) containing 60% RS and 40% rapidly digestible starch (RDS) and Amioca® containing 0% RS and 100% RDS were purchased from Ingredion Mexico, S.A. de C.V.
Dietary monitoring
A clinical nutritionist instructed detailed dietary advice to each participant a week before the experimental period. To ensure dietary adherence, a daily food record format was provided to the participants on the first day of each treatment phase to record the type and amount of consumed food during days 1 to 4. Moreover, medications ingestion was also recorded along with the specific hour of its intake. In the first treatment phase, participants were asked to keep their usual dietary intake while restricting their dietary fiber consumption (under 15 g/day, without considering the treatments fiber content). During the second and third treatment phases, subjects maintained the same food intake pattern to achieve a similar caloric intake in all treatment phases. To manage adherence to recommendations, daily phone calls were performed to the participants. The daily energy intake, macronutrients and dietary fiber were calculated according to the Mexican System of Food Equivalents [27].
Treatments
The DMS group received 26.6 g/day of Amioca® (100% RDS). The HMS group received 66.6 g/day of Hi-Maize® 260 (60% RS and 40% DS) to provide 26.6 g of DS and 40 g of RS. The NBS group received a combination of 57.2 g/day of NBS and 20.92 g/day of Amioca® to provide 26.6 g of DS and 40 g of RS, based in the RS content of 70.5% RS and 10% DS. Thus, all supplements were matched in terms of digestible carbohydrate content (available carbohydrates) and the RS content was similar in both HMS and NBS groups. The total daily dose of each treatment was divided into two portions and provided to the participants in ready-to-use sachets to be mixed with a favorite drink and ingest them twice a day, one at breakfast (7:00–9:00 a.m.) and one at lunchtime (01:00–03:00 p.m.) during the first 4 days of each treatment phase. To avoid the risk of bias, participants were assigned to all treatments according to a computer-based online random sequence generator (random.org).
Meal tolerance test
A 6-h MTT was performed to evaluate all the outcomes. Prior to the MTT days, subjects were advised not to ingest alcohol and to avoid vigorous exercise for 24 h. Subjects were also recommended to have a supper with low-fat (< 30% E) and low fiber (< 15 g/day) content the evening prior to the MTTs. Participants arrived at the research center at 7:00 a.m. after 12 h of fasting. Appetite sensation was immediately assessed using a visual analogue scale (VAS) (time point 0). An IV catheter was then inserted into the antecubital vein of each subject, and a fasting blood sample was taken. Subsequently, the subjects ate a standardized breakfast that consisted of a ham and cheese sandwich with 200 mL of semi-skimmed milk in which the test starches were diluted. During this test, half of the daily dose of the supplements previously used was given (20 g). The food content and energy composition of the test meal are shown in Table 1.
Blood samples were collected before breakfast (time 0), then the test meal was consumed within 15 min and additional samples were obtained at 30, 60, 90, 120, 150, 180, 240, 300, and 360 min after meal. The VAS scores were recorded every 30 min after the meal for 6 h and 2 min prior to each sampling. Glucose and insulin concentrations were determined through the indicated time points. During the test period, subjects remained at the research center and could read, use their computer, watch television, or talk. At the end of the 6-h MTT, the catheter was removed, and subjects began the washout period before receiving the alternative treatment.
Appetite assessment
To assess subjective appetite sensation, a validated VAS was used [28]. These were 100 mm in length and anchored with words at each end stating the most positive and most negative ratings of apetite sensations. Hunger, satiety, fullness, and prospective food consumption were assessed. Questions were asked as follows: (1) How hungry do you feel? (2) How satisfied do you feel? (3) How full do you feel? (4) How much food do you think you could eat? Hunger is defined as a feeling that drives the desire to eat or indicates the need for food. Satiety is the perception of not having an immediate need for food intake between meals, which refers to the state of inhibition of eating, and fullness is the sensation of the degree heaviness of stomach that leads to stop eating.
Biochemical determinations
Fasting blood samples were collected before (day 1) and after interventions (day 5) to determine glucose, cholesterol, triglycerides, GLP-1, and insulin concentrations. Other blood samples were collected during the MTT time points to determine glucose and insulin. On all occasions, samples that were not immediately analyzed were stored at −70 °C until further analysis.
Glucose, cholesterol, and triglycerides determinations were performed using Abbott’s Architect c8000 Clinical Chemistry Autoanalyzer. GLP-1 was determined by an enzymatic-linked immunosorbent assay (Millipore Corporation Pharmaceuticals, St. Charles, MO, USA). Insulin was measured by immunoassay of chemiluminescent microparticles (CMIA) using an INMULITE 1000 System (Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA). HbA1c was determined using the D-10 Hemoglobin Testing System from Bio-Rad. Insulin resistance (IR) at fasting was estimated according to the homeostatic model assessment (HOMA-IR), calculated as the product of fasting glucose (mg/dL) and insulin (μU/mL) divided by 405 [29].
Statistical analysis
The sample size calculation in this study was originally estimated in 10 subjects to detect a difference in 10% of 24 h mean blood glucose (MBG) as a primary outcome [22]. However, in this secondary study our primary variable was postprandial triglycerides, for this reason we estimated a total of 13 participants to obtain a power of 0.8 at a level of α = 0.05, to detect a difference of 30% in the primary variable plasma triglycerides [21]. Data were expressed as mean ± standard error of the mean (SEM) or unless otherwise specified. The D’Agostino-Pearson normality test was performed to assess whether the data were consistent with a Gaussian distribution. One-way analysis of variance (ANOVA) in combination with Dunnett’s test or only Student’s t-tests were used to determine differences in fasting biochemical parameter changes from the baseline. Time-course data were analyzed by repeated measures (RM) two-way ANOVA to assess the effects of treatment, time, and the interaction of treatment and time. Increases in concentration of triglycerides at given points in time (Δ-TG) were calculated by subtracting concentrations at time = 0 from those at following time points. To compare the results of the VAS scores between treatments, the data were expressed as absolute changes (mm VAS) from the baseline (0 min). Differences were considered statistically significant at p < 0.05. Data were analyzed using the GraphPad Prism Software version 7.0 (San Diego, CA, USA).
Results
Subject characteristics
The baseline characteristics of the participants are displayed in Table 2. Twenty subjects were recruited; however, three of them withdrew from the study because of lack of adherence to treatments. Seventeen subjects (9 men and 8 women) completed the study for baseline parameters and the postprandial response evaluations. All of them exhibited uncontrolled glycemia with HbA1c levels over 6.5%. Most of the patients (76.4%) had overweight or obesity, according to World Health Organization (WHO) criteria (BMI values > 25). Approximately half of participants were under metformin treatment, and the others were under combined metformin and glibenclamide treatment.
Caloric intake
Table 3 shows the average daily dietary intake in patients during the three treatment phases of the experimental period. Data were obtained from the daily food record. No significant differences in daily caloric intake and macronutrients were observed between treatments.
Effects of treatments on fasting biochemical parameters
NBS intake for four days resulted in a lower fasting glucose concentration compared with DMS (p < 0.05). However, no modifications were observed in other parameters such as fasting insulin, triglycerides, cholesterol, GLP-1 or HOMA-IR (Table 4).
Effects of treatments on postprandial responses during MTT
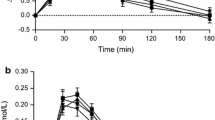
Figure 1 displays the effects of the treatments on different postprandial responses over time during the MTT. No significant differences in the GR were observed between treatments or for the interaction of time and treatment. However, after NBS intake, the GR peak was lower than that following DMS at 60 min, and when compared to HMS group at 90 min and 120 min (p < 0.05). In the HMS group, GR was higher than DMS at 360 min (p < 0.05) (Fig. 1A).
Postprandial responses of glycemia (A), insulin (B), cholesterol (C), and triglycerides (D) obtained during a 6-h MTT in subjects with T2D. Data are expressed as means only or media ± SEM (n = 17). All comparisons are based on two-way ANOVA the Dunnett´s post-hoc test. # p < 0.05 HMS vs DMS; $ p < 0.05 NBS vs DMS; * p < 0.05 HMS vs NBS. DMS, Digestible Maize Starch; HMS, Hi-Maize Starch; NBS, Native Banana Starch
Regarding the insulin response, there was a significant difference between treatments (p = 0.038) but not in the interaction time-treatment (p = 0.24). NBS supplementation induced lower levels of insulin at 60 min and 120 min time points compared to the DMS or HMS groups (p < 0.05) and at 360 min compared to HMS. At 360 min, insulin values remained higher after the HMS treatment compared to DMS or NBS treatments (p < 0.05) (Fig. 1B). No differences in insulin sensitivity, as assessed by the Matsuda index, were observed between treatments: [DMS 4.81 (3.72, 10.66); HMS 6.09 (3.99, 11.54); NBS 6.09 (3.64, 16.87)] where median and 25th and 75th percentiles are shown. Postprandial cholesterol and triglycerides profiles did not differ between treatments (Fig. 1 C, D).
Effects of treatments on subjective appetite measures
Hunger and satiety time courses during the MTT are depicted in Fig. 2A, B. The subjects reported less hunger sensation during resistant starch treatments (HMS and NBS) compared to the control group (DMS) and respect to the time-treatment interaction (p < 0.05). Particularly, hunger perception was lower after NBS intake compared to the control from 30 to 120 min (p < 0.01) and 240 min to 360 min (p < 0.01). HMS induced less hunger from 60 to 120 min (p < 0.01), 210 to 240 min (p < 0.001), and 330 to 360 min (p < 0.01) after intake compared to DMS. Satiety significantly increased after NBS and HMS intake (between treatments, p < 0.05; time-treatment interaction, p < 0.001). Furthermore, the NBS satiety response increased over the total time course (6 h) in comparison with DMS. On the other hand, a sharp reduction in satiety following HMS intake was observed during the last two hours (180–360 min). No significant differences between treatments were found in fullness and prospective consumption temporal profiles (Fig. 2C, D).
Effects of different treatments on feelings of hunger (A), satiety (B), fullness (C), and prospective consumption (D) estimated by VAS in subjects with T2D. Data are presented as changes from baseline and expressed as mean ± SEM (n = 17). Comparisons are based on two-way ANOVA and the Dunnett´s post-hoc test. # p < 0.05 HMS vs DMS; $ p < 0.05 NBS vs DMS; * p < 0.05 HMS vs NBS. DMS, Digestible Maize Starch; HMS, Hi-Maize Starch; NBS, Native Banana Starch
Discussion
In this randomized, crossover dietary intervention study of subjects with uncontrolled type 2 diabetes, we compared the impact of a 4-day supplementation with 40 g of RS from high-amylose maize or native banana starch on glycemic response, postprandial lipemia and subjective appetite.
Results demonstrated that after 40 g/d of RS administration for 4 days there were some differences between RS from banana and Hi-Maize. While NBS induced a reduction on fasting glycemia, postprandial glycemia peak and insulin response, HMS did not have any effect on these markers. In contrast, HMS unexpectedly exhibited a trend to increase glycemic and insulin excursions during MTT with significant values with respect to DMS at 360 min time point. The beneficial effect of NBS on these variables is very important because postprandial hyperglycemia is known to contribute significantly to the development of cardiovascular complications, by enhancing injury in both the macro- and microvascular systems [30]. Postprandial hyperglycemia may promote the accumulation of atherogenic LDL cholesterol through several pathways: accelerating the glycation of LDL cholesterol which impairs the receptor-mediated catabolism by the liver, enhancing LDL cholesterol oxidation, inducing epigenetic changes in cholesterol receptors of the liver, promoting the generation of reactive oxygen species, and disrupting the coronary flow reserve in patients with T2D [31].
The beneficial effect of NBS on glycemic metabolism has also been observed in previous studies with different designs conducted by our group. In subjects with obesity and poor-controlled diabetes, the administration of 24 g/d of NBS for 4 weeks induced a reduction in body weight, BMI, fasting insulin and HOMA-IR, however, no changes in fasting glycemia were observed [11]. In another study with female participants with obesity, the administration of 30 g of NBS for 4 weeks induced a reduction in fasting glycemia, and HOMA-IR comparable to metformin 850 mg/d treatment administered during the same period [12]. Other authors have informed similar effects of RS to cause a reduction on insulin response [32, 33]. Interestingly, a recent review shows convincing evidence of the beneficial effects of RS consumption on glycemic metabolism [34].
The observed effects of NBS on glycemia peak after NBS differ with the results reported in ten of the participants during the continuous glucose monitoring (CGM) along the 4-day intervention where RS did not modify glycemic control or glycemic variability, but rather increased them on some days [25]. We do not have an exact explanation for this discrepancy, however, the dietary control could have affected. During the CGM period, participants were under free-living conditions in which the meal quality and eating timings were not strictly controlled, meanwhile during the MTT performance, the ingested meal quality and timings were rigorously controlled wich makes this latter data interpretation more reliable.
The differences between HMS and NBS effects on glycemic and insulinemic responses in this study could not be explained by variations in digestibility since all treatments were matched by digestible starch content and thus the rate of digestion by the gastrointestinal enzymes was expected to be similar. However, it is known that these substances have intrinsic chemical structures even when both exhibit type B or C crystalline forms. These differences can directly impact on some properties such as intestinal microbiota modulation, appetite hormones production, viscosity, or gastric emptying, which in turn can influence glycemic and insulinemic responses [7, 35].
Interestingly, in recent years it has been recognized that most of the beneficial effects of RS are mediated by the fermentation process caused by members of the gut microbiota. As a result, some important short-chain fatty acids (SCFA) such as acetic, propionic, and butyric acids are produced. These substances in turn stimulate L-cells to produce gastrointestinal peptides such as PYY and GLP-1 which contribute on modulating glycemic metabolism and appetite [36]. In this line, a recent study has recognized that individual microbiomes influence in the amount and the range of SCFAs produced, playing a determining role in the beneficial outcomes of RS consumption [37, 38]. Although in the present study, we did not carry out studies on the microbiome composition, it was observed that NBS did not modify fasting GLP-1 values in comparison with DMS.
In the present study, no modifications of postprandial lipemia were observed after RS treatments. This negative result is consistent with a previous one-day acute intervention study in subjects with overweight or obesity during a MTT using 20 g of RS from Hi-Maize or from NBS [24] and other chronic studies providing 40 g RS from high-amylose maize/day for 8 or 12 weeks to subjects with insulin resistance [9], after administering a diet rich in arabinoxylan (16 g/d) and RS (21 g/d) to patients with metabolic syndrome for 4 weeks [39], or after supplementation with 75 g or slowly digestible starch for 14 days to healthy female volunteers [40]. Contrariwise, the findings from animal models provide strong evidence that RS feeding can reduce the blood concentration of fasting and postprandial lipemia [34].
In relation to subjective appetite estimation, our results showed that RS and HMS induced beneficial effects such as reduced hunger and increased satiety in comparison with the DMS group. These findings partially differ from another study conducted by our group in young subjects with overweight and obesity but without diabetes [24] where the same doses and types of RS were used in an acute one-day crossover study resulting in an improvement caused by HMS but not by NBS on all measurements of subjective appetite. These studies, however, differ with this study in length of intervention and subject characteristics; the latter study was conducted in an acute one-day crossover design in overweight young subjects, while in this study, RS was given during four days to poor metabolically controlled subjects with T2D. From six other studies where subjective appetite after RS was evaluated through VAS score, only two of them exhibited beneficial effects; one reported an increase in fullness sensation and the other one a reduction on both appetite and fullness sensation. Although there are studies supporting the beneficial effects of RS on inducing satiety and reducing energy intake, more research is needed before a conclusion can be reached [34].
The strengths of the present study include the crossover design in which subjects act as their own control, which decreased the within-participant variation and the extended MTT period of study (6 h) which allowed the observation of changes during a longer period. Some limitations of this study include the lack of an ad libitum test meal to determine caloric intake, not measuring other appetite-regulating hormones, and not including microbiota analysis.
Conclusions
Our results indicate that in patients with poorly controlled diabetes, NBS supplementation induced more beneficial effects on reducting fasting glucose, glycemia peak, and insulin response when compared to HMS. Although both RS treatments positively impacted subjective appetite rates, no effects on postprandial lipemia were observed. Further studies are required to elucidate the mechanisms involved in these findings.
Data availability
The dataset used in this publication is available from the corresponding author on reasonable request.
References
Ansar S, Koska J, Reaven PD (2011) Postprandial hyperlipidemia, endothelial dysfunction and cardiovascular risk: focus on incretins. Cardiovasc Diabetol 10(1):61. https://doi.org/10.1186/1475-2840-10-61
Hanssen NMJ, Kraakman MJ, Flynn MC, Nagareddy PR, Schalkwijk CG, Murphy AJ (2020) Postprandial glucose spikes, an important contributor to cardiovascular disease in diabetes? Front Cardiovasc Med. https://doi.org/10.3389/fcvm.2020.570553
Lotfollahi Z, Mello APdQ, Costa ES, Oliveira CLP, Damasceno NRT, Izar MC et al (2020) Green-banana biomass consumption by diabetic patients improves plasma low-density lipoprotein particle functionality. Sci Rep 10(1):12269. https://doi.org/10.1038/s41598-020-69288-1
Kristensen M, Savorani F, Christensen S, Engelsen SB, Bugel S, Toubro S et al (2013) Flaxseed dietary fibers suppress postprandial lipemia and appetite sensation in young men. Nutr Metab Cardiovasc Dis 23(2):136–143. https://doi.org/10.1016/j.numecd.2011.03.004
Khossousi A, Binns CW, Dhaliwal SS, Pal S (2008) The acute effects of psyllium on postprandial lipaemia and thermogenesis in overweight and obese men. Br J Nutr 99(5):1068–1075. https://doi.org/10.1017/S0007114507864804
Lockyer S, Nugent AP (2017) Health effects of resistant starch. Nutr Bull 42(1):10–41. https://doi.org/10.1111/nbu.12244
Sajilata MG, Singhal RS, Kulkarni PR (2006) Resistant starch—a review. Compr Rev Food Sci Food Saf 5(1):1–17. https://doi.org/10.1111/j.1541-4337.2006.tb00076.x
Maki KC, Pelkman CL, Finocchiaro ET, Kelley KM, Lawless AL, Schild AL et al (2012) Resistant starch from high-amylose maize increases insulin sensitivity in overweight and obese men. J Nutr 142(4):717–723. https://doi.org/10.3945/jn.111.152975
Robertson MD, Wright JW, Loizon E, Debard C, Vidal H, Shojaee-Moradie F et al (2012) Insulin-sensitizing effects on muscle and adipose tissue after dietary fiber intake in men and women with metabolic syndrome. J Clin Endocrinol Metab 97(9):3326–3332. https://doi.org/10.1210/jc.2012-1513
Dhull SB, Malik T, Kaur R, Kumar P, Kaushal N, Singh A (2021) Banana starch: properties illustration and food applications—a review. Starch-Stärke 73(1–2):2000085. https://doi.org/10.1002/star.202000085
Ble-Castillo JL, Aparicio-Trapala MA, Francisco-Luria MU, Cordova-Uscanga R, Rodriguez-Hernandez A, Mendez JD et al (2010) Effects of native banana starch supplementation on body weight and insulin sensitivity in obese type 2 diabetics. Int J Environ Res Public Health 7(5):1953–1962. https://doi.org/10.3390/ijerph7051953
Ble-Castillo JL, Aparicio-Trápala MA, Gómez-Vázquez A, Rodríguez-Hernández A, Mendez JD, Juárez-Rojop IE et al (2012) Potential beneficial effects of native banana starch on glycemia and insulin resistance in obese non-diabetic women. Interciencia 37(6):470–476
Fuentes-Zaragoza E, Riquelme-Navarrete MJ, Sánchez-Zapata E, Pérez-Álvarez JA (2010) Resistant starch as functional ingredient: a review. Food Res Int 43(4):931–942. https://doi.org/10.1016/j.foodres.2010.02.004
Yuan HC, Meng Y, Bai H, Shen DQ, Wan BC, Chen LY (2018) Meta-analysis indicates that resistant starch lowers serum total cholesterol and low-density cholesterol. Nutr Res 54:1–11. https://doi.org/10.1016/j.nutres.2018.02.008
Park OJ, Ekang N, Chang MJ, Kim WK (2004) Resistant starch supplementation influences blood lipid concentrations and glucose control in overweight subjects. J Nutr Sci Vitaminol 50(2):93–99. https://doi.org/10.3177/jnsv.50.93
Nichenametla SN, Weidauer LA, Wey HE, Beare TM, Specker BL, Dey M (2014) Resistant starch type 4-enriched diet lowered blood cholesterols and improved body composition in a double blind controlled cross-over intervention. Mol Nutr Food Res 58(6):1365–1369. https://doi.org/10.1002/mnfr.201300829
de Oliveira Lomeu FLR, Vieira CR, Lucia FD, Veiga S, Martino HSD, Silva RR (2021) Cocoa and unripe banana flour beverages improve anthropometric and biochemical markers in overweight women: a randomised double-blind study. Int J Vitam Nutr Res 91(3–4):325–334. https://doi.org/10.1024/0300-9831/a000637
Dodevska MS, Sobajic SS, Djordjevic PB, Dimitrijevic-Sreckovic VS, Spasojevic-Kalimanovska VV, Djordjevic BI (2016) Effects of total fibre or resistant starch-rich diets within lifestyle intervention in obese prediabetic adults. Eur J Nutr 55(1):127–137. https://doi.org/10.1007/s00394-015-0831-3
Ble-Castillo JL, Juárez-Rojop IE, Tovilla-Zárate CA, García-Vázquez C, Servin-Cruz MZ, Rodríguez-Hernández A et al (2017) Acute consumption of resistant starch reduces food intake but has no effect on appetite ratings in healthy subjects. Nutrients. https://doi.org/10.3390/nu9070696
White U, Peterson CM, Beyl RA, Martin CK, Ravussin E (2020) Resistant starch has no effect on appetite and food intake in individuals with prediabetes. J Acad Nutr Diet 120(6):1034–1041. https://doi.org/10.1016/j.jand.2020.01.017
Emilien CH, Hsu WH, Hollis JH (2017) Effect of resistant wheat starch on subjective appetite and food intake in healthy adults. Nutrition 43–44:69–74. https://doi.org/10.1016/j.nut.2017.06.020
Al-Mana NM, Robertson MD (2018) Acute effect of resistant starch on food intake, appetite and satiety in overweight/obese males. Nutrients. https://doi.org/10.3390/nu10121993
Peterson CM, Beyl RA, Marlatt KL, Martin CK, Aryana KJ, Marco ML et al (2018) Effect of 12 wk of resistant starch supplementation on cardiometabolic risk factors in adults with prediabetes: a randomized controlled trial. Am J Clin Nutr 108(3):492–501. https://doi.org/10.1093/ajcn/nqy121
García-Vázquez C, Ble-Castillo JL, Arias-Córdova Y, Córdova-Uscanga R, Tovilla-Zárate CA, Juárez-Rojop IE et al (2019) Effects of resistant starch ingestion on postprandial lipemia and subjective appetite in overweight or obese subjects. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph16203827
Arias-Cordova Y, Ble-Castillo JL, Garcia-Vazquez C, Olvera-Hernandez V, Ramos-Garcia M, Navarrete-Cortes A et al (2021) Resistant starch consumption effects on glycemic control and glycemic variability in patients with type 2 diabetes: a randomized crossover study. Nutrients. https://doi.org/10.3390/nu13114052
Waliszewski KN, Aparicio MA, LsA B, Monroy JA (2003) Changes of banana starch by chemical and physical modification. Carbohyd Polym 52(3):237–242. https://doi.org/10.1016/S0144-8617(02)00270-9
Pérez-Lizaur AB, Castro-Becerra AL (2008) Sistema Mexicano de Alimentos Equivalentes, 3rd edn. Fomento de Nutrición y Salud, México
Flint A, Raben A, Blundell JE, Astrup A (2000) Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 24(1):38–48. https://doi.org/10.1038/sj.ijo.0801083
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419. https://doi.org/10.1007/BF00280883
Pappas C, Kandaraki EA, Tsirona S, Kountouras D, Kassi G, Diamanti-Kandarakis E (2016) Postprandial dysmetabolism: too early or too late? Hormones 15(3):321–344. https://doi.org/10.14310/horm.2002.1697
Cheng P-C, Kao C-H (2021) Postprandial plasma glucose excursion is associated with an atherogenic lipid profile in individuals with type 2 diabetes mellitus: a cross-sectional study. PLoS ONE 16(10):e0258771. https://doi.org/10.1371/journal.pone.0258771
Bodinham CL, Frost GS, Robertson MD (2010) Acute ingestion of resistant starch reduces food intake in healthy adults. Br J Nutr 103(6):917–922. https://doi.org/10.1017/S0007114509992534
Johnston KL, Thomas EL, Bell JD, Frost GS, Robertson MD (2010) Resistant starch improves insulin sensitivity in metabolic syndrome. Diabet Med 27(4):391–397. https://doi.org/10.1111/j.1464-5491.2010.02923.x
Guo J, Tan L, Kong L (2021) Impact of dietary intake of resistant starch on obesity and associated metabolic profiles in human: a systematic review of the literature. Crit Rev Food Sci Nutr 61(6):889–905. https://doi.org/10.1080/10408398.2020.1747391
Snelson M, Jong J, Manolas D, Kok S, Louise A, Stern R et al (2019) Metabolic effects of resistant starch type 2: a systematic literature review and meta-analysis of randomized controlled trials. Nutrients. https://doi.org/10.3390/nu11081833
Zhou J, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Shen L et al (2008) Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab 295(5):E1160–E1166. https://doi.org/10.1152/ajpendo.90637.2008
DeMartino P, Cockburn DW (2020) Resistant starch: impact on the gut microbiome and health. Curr Opin Biotechnol 61:66–71. https://doi.org/10.1016/j.copbio.2019.10.008
Cronin P, Joyce SA, O’Toole PW, O’Connor EM (2021) Dietary fibre modulates the gut microbiota. Nutrients 13(5):1655. https://doi.org/10.3390/nu13051655
Schioldan AG, Gregersen S, Hald S, Bjornshave A, Bohl M, Hartmann B et al (2018) Effects of a diet rich in arabinoxylan and resistant starch compared with a diet rich in refined carbohydrates on postprandial metabolism and features of the metabolic syndrome. Eur J Nutr 57(2):795–807. https://doi.org/10.1007/s00394-016-1369-8
Ells LJ, Seal CJ, Kettlitz B, Bal W, Mathers JC (2005) Postprandial glycaemic, lipaemic and haemostatic responses to ingestion of rapidly and slowly digested starches in healthy young women. Br J Nutr 94(6):948–955. https://doi.org/10.1079/bjn20051554
Acknowledgements
Special thanks are extended to all the volunteers participating in this study.
Funding
This study was funded by the Programa de Fomento a la Investigación of the Universidad Juárez Autónoma de Tabasco (UJAT-DACS 2015-IA-09).
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows—JLB-C, CG-V: contributed to the conceptualization of the manuscript; CG-V, YA-C, and GJ-D: contributed to the methodology; MCM-L, and CGG-P: contributed to the software; CG-V, and VO-H: contributed to the validation; CG-V, JLB-C, YA-C, and MR-G: contributed to the formal analysis; GJ-D, and MR-G: contributed to the investigation; JLB-C, CG-V, and CGG-P: contributed to the data curation; JLB-C, and MR-G: contributed to the writing—original draft preparation; JLB-C, CG-V, and JAH-B: contributed to the writing—review and editing. MCM-L, GJ-D, and JAH-B: contributed to the supervision. All authors have approved the final article for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethical Committee of the Instituto Mexicano del Seguro Social (Registration number 2015-2701-17, Approved on November, 2015).
Informed consent
Informed consent was obtained from all subjects involved in the study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
García-Vázquez, C., Ble-Castillo, J.L., Arias-Córdova, Y. et al. Effects of resistant starch on glycemic response, postprandial lipemia and appetite in subjects with type 2 diabetes. Eur J Nutr 62, 2269–2278 (2023). https://doi.org/10.1007/s00394-023-03154-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03154-4