Abstract
Purpose
Low intake of dietary fibre is associated with the development of type 2 diabetes. Dyslipidaemia plays a key role in the pathogenesis of type 2 diabetes. Knowledge of the impact of dietary fibres on postprandial lipaemia is, however, sparse. This study aimed in subjects with metabolic syndrome to assess the impact on postprandial lipaemia and features of the metabolic syndrome of a healthy carbohydrate diet (HCD) rich in cereal fibre, arabinoxylan and resistant starch compared to a refined-carbohydrate western-style diet (WSD).
Methods
Nineteen subjects completed the randomised, crossover study with HCD and WCD for 4-week. Postprandial metabolism was evaluated by a meal-challenge test and insulin sensitivity was assessed by HOMA-IR and Matsuda index. Furthermore, fasting cholesterols, serum-fructosamine, circulating inflammatory markers, ambulatory blood pressure and intrahepatic lipid content were measured.
Results
We found no diet effects on postprandial lipaemia. However, there was a significant diet × statin interaction on total cholesterol (P = 0.02) and LDL cholesterol (P = 0.002). HCD decreased total cholesterol (−0.72 mmol/l, 95% CI (−1.29; −0.14) P = 0.03) and LDL cholesterol (−0.61 mmol/l, 95% CI (−0.86; −0.36) P = 0.002) compared with WSD in subjects on but not without statin treatment. We detected no other significant diet effects.
Conclusions
In subjects with metabolic syndrome on statins a 4-week diet rich in arabinoxylan and resistant starch improved fasting LDL and total cholesterol compared to subjects not being on statins. However, we observed no diet related impact on postprandial lipaemia or features of the metabolic syndrome. The dietary fibre x statin interaction deserves further elucidation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic syndrome (MetS) is closely associated with the development of type 2 diabetes (T2D) and cardiovascular diseases (CVD) [1]. These life-style diseases are among the greatest challenges facing modern society [2]. They appear to be linked to easy access to processed energy-rich low-fibre food that impairs glucose and lipid metabolism [3]. Conversely, a high dietary fibre (DF) intake rich in cereal fibres and resistant starch (RS) lowers the risk of developing T2D [4, 5] and CVD [6]. DF is characterised by resistance to digestion in the small bowel; however, the underlying mechanisms in relation to the development of T2D and CVD is poorly understood.
An increased circulating triglyceride (TG) level is a characteristic feature of MetS. Prospective cohort studies showed that the association with CVD is stronger for non-fasting than for fasting TG levels [7, 8]. Interestingly, the postprandial TG response is increased in abdominally obese despite normal fasting TG [9]. The postprandial TG response is influenced by hormone secretion e.g., from the gastrointestinal tract. Thus, glucagon-like peptide 1 (GLP-1) decreases [10] and glucagon-like peptide 2 (GLP-2) [11, 12] increases the chylomicron production. GLP-1 and GLP-2 are co-secreted from the L-cells of the perfused porcine ileum [13] but little information is available on the temporal aspects of the concomitant secretion of GLP-1 and GLP-2 in humans [14, 15]. The TG concentration is modifiable by dietary factors [16] and it has been shown that RS increases GLP-1 secretion in animals [17, 18] which may be due to fermentation products of DFs i.e., short-chain fatty acids [19]. Butyrate has previously been detected as an important short-chain fatty acid with potential beneficial effects on metabolism [20]. Consequently, we have chosen to focus on major DFs i.e., arabinoxylan (AX) and RS which increase colonic butyrate production [21].
We hypothesised that a diet enriched with AX and RS would decrease postprandial lipaemia and have a positive influence on the features of MetS in subjects with MetS. The two types of DF, the cereal fibres AX and RS, were incorporated in a mixed healthy carbohydrate diet (HCD) which was compared with a western-style diet rich in refined carbohydrates (WSD). The primary endpoint was postprandial lipaemia. Secondary endpoints were postprandial glucose, insulin, glucagon, GLP-1 and GLP-2. Furthermore, we assessed insulin sensitivity, s-fructosamine, fasting cholesterols, intrahepatic lipid content (IHLC), inflammatory markers, 24-h ambulatory blood pressure and selected gene expressions in subcutaneous abdominal adipose tissue.
Methods
Study design
This study was part of an open-label, crossover study with two 4-week dietary intervention periods separated by a 4- to 6-week wash-out period in subjects with Mets. The design has been reported in detail previously [22]. The participants were randomly assigned to the sequence of intervention diets. The study was performed at Aarhus University Hospital, Aarhus, Denmark, from May 2012 to April 2013. Before and at the end of the interventions, the participants completed three test days; day 1: 24-h ambulatory blood pressure; day 2: OGTT and MR-spectroscopy; day 3: meal tolerance test.
Participants
Twenty-two participants (7 women, 15 men) with MetS [23] were included. Unchanged cholesterol-lowering and antihypertensive medications were allowed during the study. Seven participants were treated with cholesterol-lowering statins, and seven participants were treated with antihypertensive drugs. They were allowed to continue their medication to resemble the general MetS population. An exclusion criterion was fasting glucose >7 mmol/and/or HbA1c >48 mmol/mol. The participants were instructed to maintain their habitual life style during the study in terms of physical activity, smoking, medication and alcohol habits. All participants gave written informed consent before enrolment in the study. The study was conducted according to the Declaration of Helsinki. The protocol was approved by the Central Denmark Region Committees on Biomedical Research Ethics (Journal No. 1-10-72-122-12) and registered at ClinicalTrials.gov (NCT01584427).
Diets and dietary assessments
The participants were provided with a fixed amount of key foods comprising breakfast cereals, bread, pasta and pancakes. A dietician instructed the participants regarding the substitution of a part of their habitual diet with the key foods according to their calculated energy requirements. The participants handed in 3-day food records before (run-in) and half-way through each diet intervention. Macronutrient composition of foods, other than the key foods, was estimated by Master Dietist System version 1.235 (2007). The composition of the key foods was determined by chemical analysis which has previously been described [22]. Table 1 is adapted from our previous report [22] providing the estimated macronutrient intake from run-in periods, during the diet interventions and in the key food compositions. HCD key foods contained 64 g DF per day originating primarily from AX (16 g/day) and RS (21 g/day). AX was derived from whole-grain rye and enzyme-treated wheat bran, whereas RS was provided as heat-resistant high-amylose maize starch and raw potato-starch. The WSD was characterised by key foods made of primarily refined wheat flour, i.e. with a total DF content of 18 g/day, hereof 4 g from AX and 3 g from RS.
Meal-challenge test
The study participants attended the research facility for the meal-challenge test after 12-h of fasting. Baseline blood samples were drawn (at time 0 min), whereupon the test meal was consumed within the next 20 min.
The test meal consisted of 50 g whole grain rye bread, 30 g white sandwich bread, 30 g butter, 45 g scrambled egg, 40 g cheese, 18 g fried bacon, 40 g salami, 100 mL decaffeinated coffee and 150 mL semi-skimmed milk. The test meal provided 3911 kJ (935 kcal) distributed as 17 E% protein, 15 E% carbohydrate and 68 E% fat and contained 7.1 g DF.
During the following 6 h, blood samples were drawn for analyses of TG and FFA at timepoints 0, 60, 120, 180, 240 and 360 min; for GLP-1, GLP-2, insulin, glucose and glucagon at 0, 15, 30, 60, 120, 240, and 360 min; and for apoB-48 at 0, 120, 240 and 360 min.
The participants were instructed not to perform strenuous exercise the day before the meal tests and to abstain from alcohol and use of non-steroidal anti-inflammatory drugs for 2 days prior to the test day. Smoking was not allowed during the study visits.
Oral glucose tolerance test (OGTT)
The OGTT was performed in the morning after 12 h fasting. Blood samples were drawn at −15, −10 and 0 min. Subsequently, the subjects consumed 75 g glucose within 5 min. Thereafter, blood samples were drawn at 30, 60 and 120 min for the determination of plasma glucose and insulin. A mean fasting glucose and insulin level was calculated as the mean of the three fasting values.
Blood analyses
Blood samples for plasma analyses were immediately centrifuged at 2000g for 15 min at 4 °C. Blood samples for serum analyses were kept at room temperature for 30–60 min and subsequently centrifuged for 10 min at 2000g. All samples were stored at −80 °C until analysis. Serum apoB-48 measurements were performed using a human apoB-48 ELISA kit (Code: AKHB48; Shibayagi Co. Ltd, Ishihara, Shibukawa, Gunma Pref., Japan) as described by Bohl et al. [24]. Plasma TG cholesterols and FFA were analysed with enzymatic colorimetric assays on the COBAS c111 system (TG, code 04657594190; total cholesterol, code 4718917190, HDL, code 5401488190; LDL, code 5401682190, all Roche Diagnostics Gmbh, Mannheim, Germany; FFA, code 270-7700, Wako Chemicals Gmbh, Neuss, Germany).
Plasma glucose was measured by the glucose oxidase method (GOD-PAP glucose kit, code11491253 216; Roche Diagnostics Gmbh, Mannheim, Germany). Plasma insulin was measured with ELISA using a DAKO insulin kit (Code: K6219; Dako, Glostrup, Denmark). Plasma glucagon was measured by radioimmunoassay (Code: # GL-32K, Millipore Corporation, MA, USA).
Plasma GLP-1 was measured using a radioimmunoassay specific for the C-terminal of the GLP-1 molecule (antibody code 89390) described by Orskov et al. [25] Thus, it reacts equally with intact GLP-1 and the primary (N-terminally truncated) metabolite. Intact GLP-2 was measured using a radioimmunoassay (antibody code 92160) originally described by Hartmann et al. [14]. All samples were extracted in a final concentration of 70% ethanol before GLP-1 measurements and 75% ethanol before GLP-2 measurements. Sensitivity for both assays was below 2 pmol/l, and intra-assay coefficient of variation below 6%.
Plasma IL-6 and IL-1ra were measured by ELISA using human Quantikine® kits (IL-6, code HS600B; IL-1RA, code DRA00B, R&D Systems, Minneapolis, MN, USA).
Measurement of adiponectin was performed with ELISA (code UM100101, B-Bridge International Inc., Santa Clara, CA, USA).
Serum hs-CRP was determined with a highly sensitive human ELISA kit (code EIA3954, DRG Diagnostics GmbH, Marburg, Germany).
Serum fructosamine was measured at Unilabs A/S (Copenhagen, Denmark).
Ambulatory blood pressure
Twenty-four hour ambulatory blood pressure and heart rate was monitored with a portable calibrated Spacelabs 90207 monitor (Spacelabs Inc., Redmond, WA, USA) as described by Brader et al. [26].
Intrahepatic lipid content (IHLC)
IHLC was assessed by a non-invasive 1H-magnetic resonance spectroscopy using a Signa Excite 1.5 T Twin Speed scanner (GE Medical Systems, Milwaukee, USA). The scan procedure was carried out as previously described [27] except that the subjects were allowed to eat and drink before the scan.
Adipose tissue biopsies
Abdominal subcutaneous adipose tissue biopsies were obtained at the end of WSD and HCD on the meal-challenge test days. Sampling was performed in the fasting state immediately before the test meal was served. Another sample was taken 4 h after the meal ingestion at a new site on the abdomen. The adipose tissue was collected as described by Pietraszek et al. [28].
Gene transcription analysis
RNA was isolated from 250 mg adipose tissue with TriZol Reagent (Gibco BRL, Life Technologies, Roskilde, Denmark). The gene transcription analyses were performed by AROS Applied Biotechnology A/S (Aarhus, Denmark) using Fluidigm BioMark real-time reverse transcriptase-polymerase chain reaction system. Predesigned primers and TaqMan Gene Expression Assay probes (Applied Biosystems, Life Technologies, California, USA) were used. Cycle threshold (C T) values were measured in duplicates. C T values were normalised to the mean of two reference genes; Beta-2-microglobulin and RNA polymerase II. The mean fold change in transcription of target genes is given relative to the value obtained the fasting state after the WSD diet using the 2−∆∆CT method [29].
The following target genes involved in lipid metabolism were investigated: Lipoprotein lipase (LPL), G protein-coupled receptor 120 (GPR120), fatty acid translocase (CD36); fatty acid binding protease (FABP4), fatty acid synthase (FAS). The glucose metabolism was evaluated from transcripts of: glucose transporter type 4 (SLC2A4), insulin receptor (INSR), insulin receptor substrate (IRS), peroxisome proliferator-activated receptor gamma (PPARG), uncoupling protein 3 (UCP3) and glycogen synthase (GYS 2).
Statistical analysis
The power calculation was based on the primary effect parameter, TG incremental area under the curve (iAUC), i.e., the area above baseline for 360 min. The number of participants needed to obtain a statistical power of 80% (β = 0.2) at a level of P < 0.05 (α = 0.05) was calculated as 16 with a minimal relevant difference in iAUC TG of 20% between the diets [30]. The anticipated dropout was set to 20%. This power calculation was based on a study that was not completely comparable with this study and, therefore, the number of participants was increased to 22. Descriptive statistics are presented as means ± standard deviations (SD) of the variation between study participants. Figures are presented as means ± SEM. Results are presented as means with 95% confidence intervals (CIs). A log-transformation was performed when data were not normally distributed as evaluated by qq-plots, and in such cases, descriptive statistics are presented as medians with interquartile range and results as medians with 95% CI. Except for FFAs, the postprandial responses are given as iAUC. FFA responses were calculated as total area under the curve (tAUC). Plasma glucose concentrations reached baseline in 120 min, thus iAUC is given for 120 min. ANOVA was used to compare the effect of diet including a random participant and period within participant effect. The period effect was taken as nested within the participant effect thus taking into account variation both between participants and between periods within the participants. This implies that the treatment-time interaction is tested against the residual error representing the variation between time points in a given period for a given participant. Additionally, we checked for the interaction between diet and statin as well as diet and antihypertensive treatment.
Differences at individual postprandial time points were assessed by ANOVA for repeated measurements examining the effect of diet and time on the postprandial responses using participant, test day and order as random variables and baseline as systematic variable. BMI, gender and age were tested as covariates, but were not included in the final analysis since they did not have a significant effect on the results. Model validation for ANOVA was performed by inspecting the probability plot of the residuals. The random coefficient model was used to perform linear regression analysis between GLP-1 and GLP-2 concentrations for each of the four visit days. Since there were no significant differences between the slopes for each visit day, we performed a concerted linear regression on all measurements from all four visits. Model validation was performed by a scatterplot of the standardised residuals against the fitted values and a probability plot of the residuals. Spearman’s rank order correlation was used to test if there was a correlation between baseline variables.
A P value <0.05 was considered significant. Statistical analyses were performed using STATA/IC 12.1 (Statacorp, College Station, TX, USA). Artworks were created with GraphPad Prism 6.04 (Graph Pad Software, Inc., La Jolla, California, USA).
Results
Nineteen participants (5 females and 14 males) with a mean age of 58 ± 11 (total range 39–75) years completed the study. One subject withdrew from the study during the first week of HCD due to abdominal discomfort, and two subjects discontinued for reasons unrelated to the study. Anthropometric and fasting metabolic parameters of the participants are given in Table 2.
We observed no significant differences in anthropometric or fasting metabolic parameters during the run-in period before the dietary interventions (Table 2). However, there was significantly higher total cholesterol (P = 0.02) and LDL cholesterol (P = 0.01) as well as fructosamine (P = 0.01) at run-in in period 2 compared to period 1. In the comparison of HCD and WSD, there was significant interaction between diet and statin treatment for total cholesterol (P = 0.02) and LDL cholesterol (P = 0.002) but not for HDL cholesterol (P = 0.64), body weight (P = 0.61) or other fasting metabolic parameters (data not shown). Thus, statin treated subjects had significant lower total cholesterol (−0.72 mmol/l, 95% CI (−1.29; −0.14) P = 0.03) and LDL cholesterol (−0.61 mmol/l, 95% CI (−0.86; −0.36) P = 0.002) after HCD compared with WSD. In contrast, there was no diet effect on total cholesterol (P = 0.91) or LDL cholesterol (P = 0.84) for subjects who did not receive lipid lowering treatment (Table 2). Additional statistical analyses showed that there were diet x period interaction on total cholesterol (P = 0.05) and LDL cholesterol (P = 0.02) but not fructosamine (P = 0.19).
Postprandial lipaemia
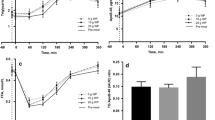
The postprandial responses of TG, apoB-48 and FFA (Table 3; Fig. 1) were not significantly different between the diets. There was no significant statin × diet interaction for TG iAUC (P = 0.81), apoB-48 iAUC (P = 0.9) or FFA tAUC (P = 1.0).
Postprandial plasma responses of a triglyceride, b ApoB-48 and c free fatty acids (FFA) after the healthy carbohydrate diet (HCD) and the western style diet (WSD). Data are presented as means ± SEM, n = 19. No significant time × diet interactions were found for triglyceride (P = 0.74), apoB-48 (P = 0.14) or FFA (P = 0.83)
Postprandial glucose, insulin, glucagon, GLP-1 and GLP-2 responses to meal test
Results of iAUC to the meal test for glucose, insulin and glucagon are shown in Table 3. In the analysis of variance for the concentrations at the individual time points, there was no significant time × diet interaction for glucose (P = 0.64), insulin (P = 0.97) or glucagon (P = 0.93).
Fasting and postprandial GLP-1 and GLP-2 (Table 3; Fig. 2a, b) were unaffected by the diets. GLP-1 and GLP-2 concentrations were linearly correlated r = 0.86 (P < 0.0001) (Fig. 2c). A concerted slope was calculated for all test days taking all 532 measurements (19 participants × 4 test days × 7 time points) into account: [GLP-2]pM = 1.75 (1.65–1.85; P < 0.0001) × [GLP-1]pM + k.
Postprandial responses of a GLP-1 and b GLP-2 after the healthy carbohydrate diet (HCD) and the western style diet (WSD). No significant time × diet interaction for GLP-1 (P = 0.82) or GLP-2 (P = 0.21). c Illustrates all 532 measurements of GLP-2 concentrations plotted against GLP-1 concentrations. Data are presented as means ± SEM, n = 19
Glucose tolerance and insulin sensitivity
Glucose and insulin responses to OGTT after the diets are shown in Fig. 3. There was no interaction between diet and time for glucose (P = 0.52) and insulin (P = 0.85) responses and no diet effect on the Matsuda index (P = 0.98). Table 2 presents data on insulin sensitivity assessed by HOMA-IR. Additional statistical analyses showed no gender × diet interaction for HOMA-IR (P = 0.89) or the Matsuda index (P = 0.83).
Responses to the oral glucose tolerance for plasma glucose a and insulin b after the healthy carbohydrate diet (HCD) and western style diet (WSD). Data are presented as geometric means with 95% CI, n = 19. No significant time × diet interactions for glucose (P = 0.52) and insulin (P = 0.85) were found
Inflammatory markers
hs-CRP, IL-6, IL-1Ra and adiponectin were not significantly changed by diet (Table 1).
Intrahepatic lipid content (IHLC)
One subject was unable to enter the MR-scanner due to claustrophobia. Thus, MR-spectroscopy data include 18 subjects. At baseline (first run-in period) 89% (n = 16/18) of the subjects had an IHLC above 5.56% which is considered the cut-off value for a normal intrahepatic lipid fraction estimated by MR-spectroscopy [31]. Baseline IHLC correlated significantly with baseline HOMA-IR (r = 0.55, P = 0.01) as well as with baseline BMI (r = 0.51, P = 0.03). IHLC was unchanged by the diets (Table 2).
Ambulatory blood pressure and heart rate
Blood pressure results are presented in Table 2. Diet effects were not significant.
Gene transcription
Transcription of target genes related to lipid and carbohydrate metabolism in the abdominal adipose tissue was not significantly changed by diet and time, Table 4.
Discussion
This study in subjects with MetS compared the impact of a 4-week HCD with a median DF content of 68 g/day (based on AX and RS) with a WSD rich in refined carbohydrates (median DF content of 21 g/day) on postprandial lipaemia (primary endpoint) as an important and sparsely investigated marker of CVD risk.
The postprandial lipid responses after the two dietary interventions were similar due to a non-significant diet effect. As intended, the study design secured a small within-participant variation. However, the levels of apoB-48 did not reach baseline and tended to be lower to HCD after 6 h although not statistically significant. Only few groups have investigated postprandial lipaemia in response to AX [32, 33] and RS-rich diets [34–36]. Giacco et al. [32] reported lower 3-h postprandial TG responses after 3 months of supplementation with whole-grain wheat and rye than with refined cereal products despite unchanged fasting TG [32]. However, their meal test differed from ours in several ways. Most importantly, Giacco et al. [32] chose test meals similar to their two intervention diets. In this study, we used identical test meals before and after each diet intervention. A standard test meal made us able to discriminate more accurately the longer term effects of the two diets. Potential acute TG lowering effects of HCD key foods could have been weakened by the overnight fast. However, Garcia et al. [33] found decreased 4-h postprandial TG response after 6-week intervention with 15 g/day of concentrated wheat AX supplementation compared with placebo. They used a standardised liquid meal without DF after 12 h fasting for the meal test. In contrast, we used a mixed test meal with 7 g DF. In addition, higher solubility or other different physicochemical properties of AX derived from wheat concentrate [33] compared to rye may influence the results. The longer duration of the intervention periods in the two AX studies [32, 33] compared to ours may also contribute to the different outcomes.
Two recent intervention studies supplementing with 40 g RS/day from high-amylose maize showed no differences in the postprandial TG responses compared with placebo in insulin resistant subjects [34, 35]. Furthermore, Bodingham et al. [35] reported that RS increased fasting TG while Robertson et al. [34] found no impact of RS on fasting TG. However, in both studies RS induced a clear suppression of FFA [34, 35] suggesting decreased lipolysis in the adipose tissue in response to increased peripheral insulin sensitivity [34]. In this study, we used only 21 g RS per day, which may not be enough to affect FFA.
Surprisingly, we found that the participants who received cholesterol lowering statin treatment significantly decreased their fasting LDL and total cholesterol to HCD compared with WSD, whereas this was not seen in subjects without cholesterol-lowering medication. The reason for the differential effect is not known, however, it can be speculated if AX and RS in combination may further enhance the statin-induced competitive inhibition of the hepatic HMG-CoA reductase that leads to an exaggerated lowering of total and LDL-cholesterol. Others have reported lowered LDL-cholesterol and total cholesterol after 3–4 weeks of AX-rich diets in healthy [37] and hypercholesterolaemic subjects [38]. However, none of the latter study populations received cholesterol-lowering medication. Previously, soluble DFs including AX have been found to lower plasma cholesterol through increased cholesterol excretion with the bile [39] and soluble fibres may counteract an increased cholesterol absorption in statin treated individuals [40]. However, the combination of RS and AX in HCD makes it impossible to distinguish between separate effects of RS and AX. It should be noted that RS has not previously been associated with improved fasting cholesterols [41–43]. Furthermore, the diet × period interaction may have biased the results since the statin and non-statin groups were not balanced for diet order. Although our results should be taken with caution due to the low number of participants they may be of potential clinical significance.
Insulin sensitivity and postprandial glucose metabolism were unchanged by diet which is in concordance with previous studies showing unchanged insulin sensitivity after AX-containing wholegrain diets [44–46]. Others have demonstrated lowered postprandial insulin [32, 47] and glucose responses [33]. The reason why we did not detect postprandial improvements may rely on differences in the design of the meal-challenge tests as mentioned above. We cannot exclude that the adipose tissue sampling in relation to the meal tests may have induced metabolic stress responses influencing the metabolic responses during the meal tests. However, the randomised crossover design should minimise the risk of bias.
Observational studies emphasise that the beneficial effect of DF on MetS is highly correlated to lower BMI [48] and that people who have a high DF intake are more physically active and smoke less [49]. To eliminate the potential effect of body weight changes, we instructed our participants to maintain stable body weight and habitual physical activity throughout the study. However, weight stability may be the reason why we and other groups did not detect significant changes in insulin sensitivity to AX-rich whole grain [38, 45, 46, 50], whereas Peirera et al. [47] found that whole grains increased insulin sensitivity concomitantly with a weight loss tendency. Using a more sensitive technique than the present techniques (HOMA-IR and Matsuda index), i.e., the euglycaemic hyperinsulinaemic clamp, intervention studies with 40 g RS/day have demonstrated increased peripheral insulin sensitivity [34, 36, 41]. This improvement in insulin sensitivity was apparently not influenced by changes in body weight. It should be stressed that we also applied a 40 g difference in DFs per day. Although our methods for evaluating insulin sensitivity may not be very sensitive it is noteworthy, that the Matsuda index has been found to correlate well with the glucose disposal during euglycaemic hyperinsulinaemic clamp [51]. A study by Maki et al. [43] reported improved insulin sensitivity in overweight men, but not in overweight women, after 4-week intervention with RS. We did not detect any gender difference in insulin sensitivity. Interestingly, we did find a tendency for fructosamine to decrease to HCD suggesting improved glycaemic control in line with the response to AX reported by Lu et al. [52]. This may be related to increased peripheral insulin sensitivity or acute effects on the intestinal glucose uptake mediated by frequent intake of RS [53, 54] and AX [52]. It should be noted, that our power calculation was carried out for the primary end point, postprandial lipaemia, and not for insulin sensitivity. Consequently, the study may have been underpowered to allow detection of a difference in insulin sensitivity between the two diets.
There was no diet effect on glucose, insulin and glucagon. In accordance with this, the diets had no influence on GLP-1 or GLP-2 responses as opposed to observations in animal models [17, 19]. Our results are in line with GLP-1 results from another 12-week human study using AX-rich whole grain [32]. In a 12-week human intervention study with RS, GLP-1 was reduced during fasting but increased postprandially [35]. Another intervention study suggested that it may take 9–12 months to stimulate increased GLP-1 secretion in response to wheat bran [55]. We observed a linear relationship between GLP-1 and GLP-2 concentrations corroborating previous human studies [14, 15]. GLP-1 and GLP-2 are both degraded by dipeptidyl peptidase-4 (DPP-4) and different elimination rates probably explain why there is a twofold increase in GLP-1 and a threefold rise in GLP-2 postprandially [56, 57]. It is noteworthy, that DPP-4 inhibitors (increasing both GLP-1 and GLP-2 levels) can reduce postprandial lipaemia in T2D [58].
We found that HOMA-IR as well as BMI correlated significantly with the IHLC at baseline as previously reported [59]. The unchanged IHLC was in agreement with two RS studies showing no effect of 40 g RS per day in 12-weeks versus placebo in subjects with insulin resistance [41] and T2D [35]. Interestingly, Musso et al. reported that people with non-alcoholic steatohepatitis had a lower intake of DF than healthy controls [60]. Furthermore, a recent study reported that whole grain consumption is associated with decreased progression from non-alcoholic fatty liver disease to non-alcoholic steatohepatitis [61]. However, other observational studies did not confirm the protective effects of DF [62, 63]. On the other hand, refined carbohydrates in the diet may exert damaging effects on liver fat [64]. We cannot exclude that an increased intake of digestible carbohydrates along with DF during HCD may have biased our results.
We did not find any change or difference in the inflammatory markers investigated. As previously mentioned, the fact that these markers were secondary outcome measures may have caused the study to be under-powered for this purpose. Previously, hs-CRP has been observed to be downregulated in a 5-week intervention study where AX-rich rye was combined with oat and sugar beet fibre [44]. In contrast, other intervention studies substituting with whole grains [46, 65] and supplementing with RS [34, 41] have not been able to demonstrate beneficial effects on hs-CRP [41, 46, 65], IL-6 [34, 41, 46, 65], IL-1Ra [46] or adiponectin [34, 41].
A recent meta-analysis [66] has raised the question if the contrast between high- and low-fibre contents in diet intervention studies is too small to cause differences. In this study, there was almost a fourfold difference in DF between the key foods of the two diets and the subjects were advised to reduce DF from other food sources during the interventions. The amount of DF during run-in periods and WSD was not statistically different and was below the recommended daily intake of 25–30 g of DF. Furthermore, it was comparable to the mean habitual DF intake of 22 g DF/day in Danish adults [67]. Nevertheless, the study was conducted in a free living setting which may have diminished the intended differences between the diets.
In concordance with other studies [41, 45, 46, 68] we found no effect of the interventions on the 24 h blood pressure.
The expression of selected genes involved in lipid and carbohydrate metabolism did not change which corroborates findings in another RS study where adipose tissue samples were collected from the abdominal region [36]. In another study, RS was reported to induce upregulation of LPL transcription in subcutaneous adipose tissue from the upper buttock region [34]. This could suggest that the metabolic activity in the subcutaneous adipose tissue in the abdominal region might differ from that from the upper buttock region [34].
In conclusion, we did not confirm our hypothesis that a 4-week intervention in MetS with a diet enriched with AX from cereals and RS improved postprandial lipaemia or other features of the MetS. Interestingly, we found that participants on statin treatment improved their fasting total and LDL cholesterol on HCD. Additional research is needed to confirm this diet x statin interaction.
References
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr, International Diabetes Federation Task Force on Epidemiology and Prevention, Hational Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, International Association for the Study of Obesity (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120:1640–1645
International Diabetes Federation (2013) IDF Diabetes Atlas, 6th edn. International Diabetes Federation, Brussels. http://www.idf.org/diabetesatlas. Accessed 7 Jan 2017
Heidemann C, Schulze MB, Franco OH, van Dam RM, Mantzoros CS, Hu FB (2008) Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation 118:230–237
Maki KC, Phillips AK (2015) Dietary substitutions for refined carbohydrate that show promise for reducing risk of type 2 diabetes in men and women. J Nutr 145:159 S–163 S
Yao B, Fang H, Xu W, Yan Y, Xu H, Liu Y, Mo M, Zhang H, Zhao Y (2014) Dietary fiber intake and risk of type 2 diabetes: a dose-response analysis of prospective studies. Eur J Epidemiol 29:79–88
Threapleton DE, Greenwood DC, Evans CE, Cleghorn CL, Nykjaer C, Woodhead C, Cade JE, Gale CP, Burley VJ (2013) Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 347:f6879
Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A (2007) Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 298:299–308
Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM (2007) Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 298:309–316
Mekki N, Christofilis MA, Charbonnier M, Atlan-Gepner C, Defoort C, Juhel C, Borel P, Portugal H, Pauli AM, Vialettes B, Lairon D (1999) Influence of obesity and body fat distribution on postprandial lipemia and triglyceride-rich lipoproteins in adult women. J Clin Endocrinol Metab 84:184–191
Hermansen K, Baekdal TA, During M, Pietraszek A, Mortensen LS, Jorgensen H, Flint A (2013) Liraglutide suppresses postprandial triglyceride and apolipoprotein B48 elevations after a fat-rich meal in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, cross-over trial. Diabetes Obes Metab 15:1040–1048
Hein GJ, Baker C, Hsieh J, Farr S, Adeli K (2013) GLP-1 and GLP-2 as yin and yang of intestinal lipoprotein production: evidence for predominance of GLP-2-stimulated postprandial lipemia in normal and insulin-resistant states. Diabetes 62:373–381
Dash S, Xiao C, Morgantini C, Connelly PW, Patterson BW, Lewis GF (2014) Glucagon-like Peptide-2 regulates release of chylomicrons from the intestine. Gastroenterology 147:1275–1284
Orskov C, Holst JJ, Knuhtsen S, Baldissera FG, Poulsen SS, Nielsen OV (1986) Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology 119:1467–1475
Hartmann B, Johnsen AH, Orskov C, Adelhorst K, Thim L, Holst JJ (2000) Structure, measurement, and secretion of human glucagon-like peptide-2. Peptides 21:73–80
Lund MT, Dalby S, Hartmann B, Helge J, Holst JJ, Dela F (2014) The incretin effect does not differ in trained and untrained, young, healthy men. Acta Physiol (Oxf) 210:565–572
Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Sacks F, Murad MH, Stalenhoef AF, Endocrine society (2012) Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 97:2969–2989
Zhou J, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Shen L, Danna SC, Tripathy S, Hegsted M, Keenan MJ (2008) Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. doi:10.1152/ajpendo.90637.2008
Shen L, Keenan MJ, Raggio A, Williams C, Martin RJ (2011) Dietary-resistant starch improves maternal glycemic control in Goto?Kakizaki rat. Mol Nutr Food Res 55:1499–1508
Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM (2012) Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61:364–371
Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A (2011) Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol 17:1519–1528
Nielsen TS, Laerke HN, Theil PK, Sorensen JF, Saarinen M, Forssten S, Bach Knudsen KE (2014) Diets high in resistant starch and arabinoxylan modulate digestion processes and SCFA pool size in the large intestine and faecal microbial composition in pigs. Br J Nutr 112:1837–1849. doi:10.1017/S000711451400302X
Hald S, Schioldan AG, Moore ME, Dige A, Laerke HN, Agnholt J, Bach Knudsen KE, Hermansen K, Marco ML, Gregersen S, Dahlerup JF (2016) Effects of arabinoxylan and resistant starch on intestinal microbiota and short-chain fatty acids in subjects with metabolic syndrome: a randomised crossover study. PLoS One 11:e0159223
Alberti KG, Zimmet P, Shaw J (2006) Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 23:469–480
Bohl M, Bjornshave A, Rasmussen KV, Schioldan AG, Amer B, Larsen MK, Dalsgaard TK, Holst JJ, Herrmann A, O’Neill S, O’Driscoll L, Afman L, Jensen E, Christensen MM, Gregersen S, Hermansen K (2015) Dairy proteins, dairy lipids, and postprandial lipemia in persons with abdominal obesity (DairyHealth): a 12-week, randomized, parallel-controlled, double-blinded, diet intervention study. Am J Clin Nutr 101:870–878
Orskov C, Rabenhoj L, Wettergren A, Kofod H, Holst JJ (1994) Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes 43:535–539
Brader L, Uusitupa M, Dragsted LO, Hermansen K (2014) Effects of an isocaloric healthy Nordic diet on ambulatory blood pressure in metabolic syndrome: a randomized SYSDIET sub-study. Eur J Clin Nutr 68:57–63
Moller L, Stodkilde-Jorgensen H, Jensen FT, Jorgensen JO (2008) Fasting in healthy subjects is associated with intrahepatic accumulation of lipids as assessed by 1H-magnetic resonance spectroscopy. Clin Sci (Lond) 114:547–552
Pietraszek A, Gregersen S, Pedersen SB, Holst JJ, Hermansen K (2014) Acute effects of monounsaturated fat on postprandial lipemia and gene expression in first-degree relatives of subjects with type 2 diabetes. Eur J Clin Nutr 68:1022–1028
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆∆CT method. Methods 25:402–408
Holmer-Jensen J, Mortensen LS, Astrup A, de Vrese M, Holst JJ, Thomsen C, Hermansen K (2013) Acute differential effects of dietary protein quality on postprandial lipemia in obese non-diabetic subjects. Nutr Res 33:34–40
Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL (2005) Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 288:E462–E468
Giacco R, Costabile G, Della Pepa G, Anniballi G, Griffo E, Mangione A, Cipriano P, Viscovo D, Clemente G, Landberg R, Pacini G, Rivellese AA, Riccardi G (2014) A whole-grain cereal-based diet lowers postprandial plasma insulin and triglyceride levels in individuals with metabolic syndrome. Nutr Metab Cardiovasc Dis 24:837–844
Garcia AL, Otto B, Reich SC, Weickert MO, Steiniger J, Machowetz A, Rudovich NN, Mohlig M, Katz N, Speth M, Meuser F, Doerfer J, Zunft HJ, Pfeiffer AH, Koebnick C (2007) Arabinoxylan consumption decreases postprandial serum glucose, serum insulin and plasma total ghrelin response in subjects with impaired glucose tolerance. Eur J Clin Nutr 61:334–341
Robertson MD, Wright JW, Loizon E, Debard C, Vidal H, Shojaee-Moradie F, Russell-Jones D, Umpleby AM (2012) Insulin-sensitizing effects on muscle and adipose tissue after dietary fiber intake in men and women with metabolic syndrome. J Clin Endocrinol Metab 97:3326–3332
Bodinham CL, Smith L, Thomas EL, Bell JD, Swann JR, Costabile A, Russell-Jones D, Umpleby AM, Robertson MD (2014) Efficacy of increased resistant starch consumption in human type 2 diabetes. Endocr Connect 3:75–84
Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN (2005) Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr 82:559–567
Giacco R, Clemente G, Cipriano D, Luongo D, Viscovo D, Patti L, Di Marino L, Giacco A, Naviglio D, Bianchi MA, Ciati R, Brighenti F, Rivellese AA, Riccardi G (2010) Effects of the regular consumption of wholemeal wheat foods on cardiovascular risk factors in healthy people. Nutr Metab Cardiovasc Dis 20:186–194
Leinonen KS, Poutanen KS, Mykkanen HM (2000) Rye bread decreases serum total and LDL cholesterol in men with moderately elevated serum cholesterol. J Nutr 130:164–170
Lundin EA, Zhang JX, Lairon D, Tidehag P, Aman P, Adlercreutz H, Hallmans G (2004) Effects of meal frequency and high-fibre rye-bread diet on glucose and lipid metabolism and ileal excretion of energy and sterols in ileostomy subjects. Eur J Clin Nutr 58:1410–1419
Hiramitsu S, Ishiguro Y, Matsuyama H, Yamada K, Kato K, Noba M, Uemura A, Yoshida S, Matsubara Y, Kani A, Hasegawa K, Hishida H, Ozaki Y (2010) The effects of ezetimibe on surrogate markers of cholesterol absorption and synthesis in Japanese patients with dyslipidemia. J Atheroscler Thromb 17:106–114
Johnston KL, Thomas EL, Bell JD, Frost GS, Robertson MD (2010) Resistant starch improves insulin sensitivity in metabolic syndrome. Diabet Med 27:391–397
Bodinham CL, Smith L, Wright J, Frost GS, Robertson MD (2012) Dietary fibre improves first-phase insulin secretion in overweight individuals. PLoS One 7:e40834
Maki KC, Pelkman CL, Finocchiaro ET, Kelley KM, Lawless AL, Schild AL, Rains TM (2012) Resistant starch from high-amylose maize increases insulin sensitivity in overweight and obese men. J Nutr 142:717–723. doi:10.3945/jn.111.152975
Johansson-Persson A, Ulmius M, Cloetens L, Karhu T, Herzig KH, Onning G (2014) A high intake of dietary fiber influences C-reactive protein and fibrinogen, but not glucose and lipid metabolism, in mildly hypercholesterolemic subjects. Eur J Nutr 53:39–48
Andersson A, Tengblad S, Karlstrom B, Kamal-Eldin A, Landberg R, Basu S, Aman P, Vessby B (2007) Whole-grain foods do not affect insulin sensitivity or markers of lipid peroxidation and inflammation in healthy, moderately overweight subjects. J Nutr 137:1401–1407
Giacco R, Lappi J, Costabile G, Kolehmainen M, Schwab U, Landberg R, Uusitupa M, Poutanen K, Pacini G, Rivellese AA, Riccardi G, Mykkanen H (2013) Effects of rye and whole wheat versus refined cereal foods on metabolic risk factors: a randomised controlled two-centre intervention study. Clin Nutr 32:941–949
Pereira MA, Jacobs DR Jr, Pins JJ, Raatz SK, Gross MD, Slavin JL, Seaquist ER (2002) Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr 75:848–855
Kuijsten A, Aune D, Schulze MB, Norat T, van Woudenbergh GJ, Beulens JW, Sluijs I, Spijkerman AM, van der A DL, Palli D, Kühn T, Wendt A, Buijsse B, Boeing H, Pala V, Amiano P, Buckland G, Huerta JM, Tjøonneland A, Kyrø C, Redondo ML, Sacerdote C, Sánchez MJ, Fagherazzi G, Balkau B, Lajous M, Panico S, Franks PW, Rolandsson O, Nilsson P, Orho-Melander M, Overvad K, Huybrechts I, Slimani N, Tumino R, Barricarte A, Key TJ, Feskens EJ, Langenberg C, Sharp S, Foroughi NG, Riboli E, Wareham NJ (2015) Dietary fibre and incidence of type 2 diabetes in eight European countries: the EPIC-InterAct Study and a meta-analysis of prospective studies. Diabetologia 58:1394–1408
Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H (2007) Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysis. Arch Intern Med 167:956–965
Juntunen KS, Niskanen LK, Liukkonen KH, Poutanen KS, Holst JJ, Mykkanen HM (2002) Postprandial glucose, insulin, and incretin responses to grain products in healthy subjects. Am J Clin Nutr 75:254–262
Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470
Lu ZX, Walker KZ, Muir JG, O’Dea K (2004) Arabinoxylan fibre improves metabolic control in people with type II diabetes. Eur J Clin Nutr 58:621–628
Behall KM, Hallfrisch J (2002) Plasma glucose and insulin reduction after consumption of breads varying in amylose content. Eur J Clin Nutr 56:913–920
Hoebler C, Karinthi A, Chiron H, Champ M, Barry JL (1999) Bioavailability of starch in bread rich in amylose: metabolic responses in healthy subjects and starch structure. Eur J Clin Nutr 53:360–366
Freeland KR, Wilson C, Wolever TM (2010) Adaptation of colonic fermentation and glucagon-like peptide-1 secretion with increased wheat fibre intake for 1 year in hyperinsulinaemic human subjects. Br J Nutr 103:82–90
Hartmann B, Harr MB, Jeppesen PB, Wojdemann M, Deacon CF, Mortensen PB, Holst JJ (2000) In vivo and in vitro degradation of glucagon-like peptide-2 in humans. J Clin Endocrinol Metab 85:2884–2888
Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ (1995) Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 44:1126–1131
Eliasson B, Moller-Goede D, Eeg-Olofsson K, Wilson C, Cederholm J, Fleck P, Diamant M, Taskinen MR, Smith U (2012) Lowering of postprandial lipids in individuals with type 2 diabetes treated with alogliptin and/or pioglitazone: a randomised double-blind placebo-controlled study. Diabetologia 55:915–925
Yatsuya H, Nihashi T, Li Y, Hotta Y, Matsushita K, Muramatsu T, Otsuka R, Matsunaga M, Yamashita K, Wang C, Uemura M, Harada A, Fukatsu H, Toyoshima H, Aoyama A, Tamakoshi K (2014) Independent association of liver fat accumulation with insulin resistance. Obes Res Clin Pract 8:e350–5
Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, Fagà E, Silli B, Pagano G (2003) Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 37:909–916
Georgoulis M, Kontogianni MD, Tileli N, Margariti A, Fragopoulou E, Tiniakos D, Zafiropoulou R, Papatheodoridis G (2014) The impact of cereal grain consumption on the development and severity of non-alcoholic fatty liver disease. Eur J Nutr 53:1727–1735
Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Blendis L, Halpern Z, Oren R (2007) Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study; 17850914. J Hepatol 47:711–717
Mollard RC, Senechal M, MacIntosh AC, Hay J, Wicklow BA, Wittmeier KD, Sellers EA, Dean HJ, Ryner L, Berard L, McGavock JM (2014) Dietary determinants of hepatic steatosis and visceral adiposity in overweight and obese youth at risk of type 2 diabetes. Am J Clin Nutr 99:804–812
Maersk M, Belza A, Stødkilde-Jørgensen H, Ringgaard S, Chabanova E, Thomsen H, Pedersen SB, Astrup A, Richelsen B (2012) Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr 95:283–289
Brownlee IA, Moore C, Chatfield M, Richardson DP, Ashby P, Kuznesof SA, Jebb SA, Seal CJ (2010) Markers of cardiovascular risk are not changed by increased whole-grain intake: the WHOLEheart study, a randomised, controlled dietary intervention. Br J Nutr 104:125–134
Buyken AE, Goletzke J, Joslowski G, Felbick A, Cheng G, Herder C, Brand-Miller JC (2014) Association between carbohydrate quality and inflammatory markers: systematic review of observational and interventional studies. Am J Clin Nutr 99:813–833
Pedersen AN, Christensen T, Matthiessen J, Knudsen VK, Rosenlund-Sørensen M, Biltoft-Jensen A, Hinsch H, Ygil KH, Kørup K, Saxholt E, Trolle E, Søndergaard AB, Fagt S (2015) Danskernes kostvaner 2011–2013. http://www.food.dtu.dk/english/publications/nutrition/danish-national-survey-of-dietary-habits-and-physical-activity. Accessed 7 Jan 2017
Ampatzoglou A, Atwal KK, Maidens CM, Williams CL, Ross AB, Thielecke F, Jonnalagadda SS, Kennedy OB, Yaqoob P (2015) Increased whole grain consumption does not affect blood biochemistry, body composition, or gut microbiology in healthy, low-habitual whole grain consumers. J Nutr 145:215–221
Acknowledgements
We thank Eva Molgaard Jensen, Lene Trudso and Tove Skrumsager for technical assistance and Kia Valum Rasmussen for dietetic assistance. Also, we thank professor Knud Erik Bach Knudsen and senior researcher Helle Nygaard Laerke from the Department of Animal Science at Aarhus University for carrying out the chemical analyses of the key foods. Associate professor Bo Martin Bibby and statistician Simon Bang Kristensen from the Department of Biostatistics at Aarhus University were consulted for statistical advice. The study was funded by The Danish Council for Strategic Research (DSF 10-093526). The following companies provided food items for the study participants: Lantmännen R&D; Ingredion Incorporated Inc.; DuPont Nutrition and Biosciences ApS; KMC AmbA; Lantmännen Cerealia A/S and Lantmännen Schulstad A/S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Schioldan, A.G., Gregersen, S., Hald, S. et al. Effects of a diet rich in arabinoxylan and resistant starch compared with a diet rich in refined carbohydrates on postprandial metabolism and features of the metabolic syndrome. Eur J Nutr 57, 795–807 (2018). https://doi.org/10.1007/s00394-016-1369-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1369-8