Abstract
Purpose
In our previous study, we showed that Lycium chinense Miller fruit extract (LFE) exerted hepatoprotective effects in mice. In the current study, we examined the effect of LFE on liver enzyme levels in subjects with mild hepatic dysfunction.
Methods
A total of 90 subjects, aged 19 to 70 years old, with abnormal alanine aminotransferase (ALT) levels, were randomly placed into either an LFE (n = 45) treatment group or a placebo group (n = 45). During the 12-week clinical trial, subjects in each group received either LFE or placebo capsules, and were instructed to take four tablets per day (1760 mg/day). The primary outcome of the study was the changes of ALT and γ-glutamyltransferase (GGT) levels in each subject. The safety of LFE supplementation was assessed and adverse events were recorded.
Results
LFE supplementation for 12 weeks resulted in a significant reduction of ALT (P = 0.0498) and GGT (P = 0.0368) levels in comparison to the placebo. No clinically significant changes were observed in any safety parameters.
Conclusion
These results suggest that LFE can be applied to subjects with mild hepatic dysfunction with no possible side effects.
Trial registration
This study was registered at the Clinical Research Information Service (CRIS) as no. KCT0003985.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The liver is the biggest glandular organ and it is responsible for metabolizing lipids and carbohydrates, and detoxifying alcohol, toxins, and a wide range of drugs [1]. Hepatic dysfunction, which is associated with hepatitis and non-alcoholic fatty liver disease (NAFLD), chronic alcohol consumption, and frequent use of antibiotics, can affect the regenerative capacity of hepatocytes [2]. However, there are no clear clinical symptoms that enable early detection of hepatic dysfunction which often leads to further liver injury and severe liver disease. Currently, early detection of hepatic dysfunction is accomplished using liver enzyme assays. Alanine aminotransferase (ALT) is a widely used serum marker, and even a minor elevation of ALT is an accurate predictor of mortality from liver diseases [3, 4]. ALT is also found in skeletal muscle, heart tissue, and the brain; however, the concentration of ALT in these organs is much lower than that in the liver [5, 6]. For this reason, ALT is generally considered to be among the most precise markers of liver injury. ALT screening tests can also detect otherwise unapparent and often undiagnosed liver diseases, such as NAFLD [7, 8]. In addition, γ-glutamyltransferase (GGT) is a sensitive but non-specific indicator of a primary liver disease [9]. In particular, the highest levels of GGT activity are found in cases of biliary obstruction, carcinoma metastatic to the liver, cirrhosis, and chronic alcoholism [10]. Early detection of abnormal liver enzyme serum levels could benefit individuals experiencing hepatic dysfunction.
Lycium chinense Miller (Solanaceae) fruit is widely consumed in northeast Asia and is cultivated in countries with temperate and subtropical climates such as Korea and Japan, as well as southeast Asian and European countries [11, 12]. The fruits have traditionally been used as a tonic, and have been reported to possess neuroprotective [13], immunomodulatory [14], anti-obesity [15], anti-tumor [16], and anti-oxidative properties [17]. A number of studies have reported that oral administration of L. chinense fruit in hepatotoxicity models has a potent hepatoprotective effect [17,18,19,20]. These hepatoprotective properties can be attributed to the various nutraceuticals and phytochemicals of L. chinense fruit, such as carotenoids, phenolic compounds, carbohydrates, and amino acids [11, 20,21,22,23,24]. Previous studies have reported that betaine in L. chinense fruit mitigates carbon tetrachloride (CCl4)-induced hepatic injury by increasing anti-oxidative activity and decreasing inflammatory mediators such as nitric oxide and prostanoids [18]. In our previous study, methionine choline-deficient (MCD) mice that had been fed a diet supplemented with L. chinense fruit extract (LFE) exhibited attenuated hepatic oxidative stress, decreased cytokines release, and reduced liver enzyme serum levels [25].
Although various studies have suggested that L. chinense fruit can produce a hepatoprotective effect in animals, there is a lack of clinical data on its potential effect on human hepatic dysfunction. We designed a randomized, double-blind, placebo-controlled clinical trial to evaluate the effects of LFE supplementation on liver enzyme levels in human subjects experiencing mild hepatic dysfunction.
Materials and methods
A randomized, double-blind, placebo-controlled clinical trial was conducted from June 2018 to November 2019 in the Clinical Trial Center for Functional Foods (CTCF2) at the Jeonbuk National University Hospital. The study adhered to the Consolidated Standards of Reporting Trials (CONSORT) guidelines and was conducted in accordance with the World Medical Association’s Helsinki Declaration (2013). Written informed consent was obtained from each subject. The study protocol was approved by the Institutional Review Board of Jeonbuk National University Hospital (approval no. 2018-03-013) and was registered with the Clinical Research Information Service (registration no. KCT0003985).
Subjects
A total of 205 subjects visited CTCF2, and 90 subjects met the inclusion criteria, which were defined as follows: (1) between 19 and 70 years of age; (2) abnormal serum ALT levels (35–105 IU/l); (3) voluntary agreement to participate in the clinical trial and provision of informed consent. Subjects were excluded from participating in the study if they met any of the following conditions: (1) aspartate aminotransferase (AST) or GGT level > 3 times the normal range; (2) alcohol abuse (> 210 g/weeks for males and > 140 g/weeks for females); (3) acute or chronic hepatitis or carriers of viral hepatitis (type B or C); (4) history of underlying cirrhosis or liver cancer; (5) history of underlying biliary diseases such as jaundice or gallstones; (6) major medical illnesses such as cardiovascular, neurologic, hepatic, musculoskeletal, psychiatric, endocrine, immune, renal, pulmonary diseases, or malignant tumors; (7) intake of medications within the previous 4 weeks that affect liver function, adrenal cortex hormones, or sex hormones (such as: antiviral drugs, antituberculosis drugs, antiseizure drugs, arthrifuge, antidepressants, lipid-lowering agents, anesthetic agents, hepatoprotective agents, and non-steroidal anti-inflammatory drugs); (8) intake of supplements to improve liver function within the previous 2 weeks (such as milk thistle, oriental medicine, or over-the-counter medication); (9) allergy or hypersensitivity to drugs or compounds used in the study (L. chinense, microcrystalline cellulose); (10) history of substance abuse; (11) history of gastrointestinal disease or gastrointestinal surgery that could interfere with the study or impede their absorption; (12) participation in other clinical trials within the previous 2 months; (13) pregnant, planning to become pregnant, or breast feeding; (14) female subjects of childbearing potential who were not willing to use contraception (exception: surgery for female infertility); (15) serum creatinine level > 2.0 mg/dl; (16) laboratory test results and/or medical/psychological condition that may prevent successful participation in the study. Potential participants also underwent an abdominal ultrasonography, a viral hepatitis test (hepatitis B and hepatitis C), and analyses of thyroid stimulating hormone and alpha-fetoprotein at screening. Potential participants in whom clinically significant liver disease was identified were excluded from the study.
Study material
LFE was provided by the Cheongyang-Gun Rich Farm Support Center (Chungyang, Korea). LFE was prepared from the dried fruits of L. chinense, as described previously [25]. LFE was supplied as a powder in capsule form. A LFE capsules was 440 mg and contained 437.5 mg of LFE powder. Nutrition composition of LFE powder is summarized in Table 1. The main constituents of the LFE is polysaccharides, and the betaine content is 7.62 mg/g. During the 12-week clinical trial, subjects received a supply of either LFE or placebo capsules at 6-week intervals, and were instructed to take four capsules per day (1760 mg/day). The placebo was composed primarily of microcrystalline cellulose, and the flavor, color, appearance, and dosage were identical to the LFE supplement.
Study design
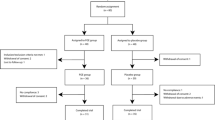
Subject disposition throughout the study is presented in the CONSORT flow diagram (Fig. 1). Subjects (n = 90) were placed into LFE-treatment or placebo groups via a computer-generated random sequence. The subjects were advised to maintain their usual lifestyle during the study, including diet and physical activity. Throughout the trial, the subjects attended follow-up visits to assess compliance and adherence to the study protocol, and to record potential adverse events. The primary outcome of the study was the alteration of ALT and GGT levels. The secondary outcomes included changes to AST, alkaline phosphatase (ALP), and total bilirubin concentrations, as well as controlled attenuation parameter (CAP) score, liver stiffness, lipid parameters (total cholesterol, triglyceride, HDL-cholesterol, and LDL-cholesterol), total antioxidant status (TAS), high sensitivity C-reactive protein (hs-CRP) level, and multidimensional fatigue scale (MFS) score. Vital signs, electrocardiogram (ECG) tests, laboratory lab, and reported adverse events were used as parameters to evaluate the safety of LFE supplementation.
Biochemical measurements
Blood samples were taken from the participants after fasting for 12 h. Biochemical testing was performed at baseline, 6 weeks, and 12 weeks to determine serum concentration of ALT, GGT, AST, ALP, and total bilirubin using an ADVIA® 2400 chemistry system (Siemens, Bayern, Germany). At baseline and 12 weeks, additional testing was conducted to determine levels of lipid parameters, TAS, and hs-CRP. Lipid parameters (total cholesterol, triglyceride, HDL-C and LDL-C) were measured using a Hitachi 7600-110® analyzer (Hitachi High-Technologies, Tokyo, Japan). Serum TAS was measured using a TAS kit (Rel Assay Company, Gaziantep, Turkey) and a Cobas 800 auto-analyzer (Roche Diagnostics GmbH, Mannheim, Germany) via a colorimetric method. All of the biochemical analyses were performed using a centralized laboratory setup.
Measurement of CAP and liver stiffness
CAP and liver stiffness were measured using a FibroScan system (Echosens, Paris, France) and a trained operator at baseline and 12 weeks. The measurements were performed using a 3.5 MHz standard probe on the right hepatic lobe through the intercostal spaces while the subject laid supine. Final CAP and liver stiffness were recorded as the median values of all measurements and were expressed in dB/m and kPa, respectively [26].
Dietary intake and physical activity assessment
Information on food intake was self-reported by the subjects, and consisted of their diet over 3 days (two non-consecutive weekdays and one weekend) at baseline, 6 weeks, and 12 weeks. Three-day averages of dietary intake were analyzed using Can-Pro 4.0 software (The Korean Nutrition Society, Seoul, Korea). Physical activity level was assessed using the Global Physical Activity Questionnaire (GPAQ).
Sample size estimation and statistical analysis
We decided on our sample size based on a study conducted by Fried et al. [27]. Assuming a difference of 10 IU/L, with an estimated standard deviation of 15 IU/L in ALT level between the groups, with 95% confidence and a power of 80%, we calculated that the sample size should be at least 36 cases in each group. The sample size was increased to 45 cases in each group to account for a possible dropout rate of 20%. The analyses were performed based on the per-protocol approach with SAS version 9.4 software (SAS Institute Inc., Cary, NC, USA). Categorical variables were summarized by frequency and proportions, and continuous variables by mean and standard deviation (SD) or median and interquartile range (IQR), depending on the normality of their distribution. For between-group comparisons, the χ2 or Fisher’s exact test was used for categorical variables, and the independent samples t test or Mann–Whitney U test was used for differences between continuous variables. Statistical significance was set at P < 0.05.
Results
Subjects characteristics
A total of 90 subjects were randomly assigned into two groups and received either LFE (n = 45) or a placebo (n = 45). After being assigned to groups, 15 subjects were removed from the clinical trial due to: withdrawn consent (n = 7), judgment of the investigator (n = 1), lost to follow-up (n = 1), poor compliance (n = 2), ingestion of medication(s) listed in the exclusion criteria (n = 2), and major protocol violation (n = 2). Therefore, the per-protocol set included a total of 75 subjects (38 subjects in the LFE group and 37 subjects in the placebo group). Baseline characteristics of the subjects, obtained prior to the removal of the aforementioned 15 subjects, are shown in Table 2. At baseline, there were no significant differences between the groups based on the variables of age, sex, anthropometric measurements (weight and BMI), current drinkers, alcohol consumption, current smokers, amount of smoking, metabolic equivalent of task (MET), energy intake, vital signs (SBP, DBP, and pulse), liver enzyme (ALT, GGT, AST, and total bilirubin), and severity of fatty liver (P > 0.05). ALP level was the sole exception (P = 0.0097). Forty-six subjects (51.1%) were newly diagnosed with liver function abnormalities, and forty-four subjects (48.9%) were diagnosed with chronic abnormalities based on a liver function test. There were no significant differences between groups at baseline (P = 0.2058). The compliance rate of subjects in the two groups was more than 93% and there was no significant difference between the groups (data not shown). No significant changes were observed between groups in terms of dietary composition and physical activity at baseline, 6 weeks, and 12 weeks (Table 3). Body weight, BMI, glucose metabolism, and parameters of metabolic syndrome were constant in both groups throughout the 12-week clinical trial (Supplementary Table 1, 2, and 3).
Efficacy
Changes in both groups from baseline ALT and GGT levels are shown in Fig. 2. Compared with the baseline, ALT (54.0 ± 16.9 IU/L to 44.0 ± 20.3 IU/L, P < 0.0001) and GGT (72.3 ± 53.0 IU/L to 61.7 ± 51.5 IU/L, P = 0.0205) levels were significantly reduced after 12 weeks of LFE supplementation; however, there was no significant reduction in ALT (52.3 ± 15.7 IU/L to 51.2 ± 26.3 IU/L, P = 0.5320) and GGT (55.9 ± 41.2 IU/L to 56.1 ± 41.7 IU/L, P = 0.5629) levels in the placebo group (Fig. 2A, B). The values from baseline were significantly different between groups at the end of the study (Fig. 2C, D). ALT and GGT levels were reduced to − 10.0 ± 13.1 IU/L (P = 0.0498) and − 10.5 ± 33.4 IU/L (P = 0.0368), respectively, from baseline after 12 weeks of LFE supplementation, whereas the placebo group were remained similar to baseline at 12 weeks. There were no significant changes between the groups in AST (− 5.5 ± 9.4 IU/L vs. − 1.5 ± 9.4 IU/L, P = 0.1398) and total bilirubin levels (0.1 ± 0.4 mg/dL vs. 0.0 ± 0.3 mg/dL, P = 0.5179) after 12 weeks (Fig. 3A, B). ALP levels, after adjustment for baseline, were not significantly changed by LFE supplementation compared to the placebo (− 1.3 ± 11.2 IU/L vs. 1.3 ± 12.0 IU/L, P = 0.7337) after 12 weeks (Fig. 3C). CAP and liver stiffness in both groups are detailed in Table 4. The mean decrease in CAP level, from its baseline value to the time after 12 weeks of LFE supplementation, was − 8.6 ± 50.2 dB/m, which was not significantly different than the placebo group (1.6 ± 45.8 dB/m, P = 0.3605). There was no significant change in liver stiffness (− 0.1 ± 2.9 kPa vs. − 0.4 ± 1.8, P = 0.6258). Lipid profiles, hs-CRP level, TAS, and MFS score were not significantly changed by LFE supplementation (data not shown).
Safety
Nine moderate adverse events (AEs) occurred during the run-in period, none of which were serious (Table 5). The proportion of subjects who reported an adverse event was similar in each group (LFE, n = 3; placebo, n = 4). There was no statistically significant difference in the rate of AEs between the study groups (P = 0.5148). Other safety parameters (vital signs, ECG readings, and laboratory test results) in the LFE group did not significantly change during the study period.
Discussion
In this clinical trial, daily consumption of an LFE supplement for 12 weeks resulted in a significant reduction in ALT and GGT levels. ALT is an enzyme that catalyzes the transfer of amino groups to form the hepatic metabolite oxaloacetate, which is found in abundance in the cytosol of hepatocytes [28]. ALT is the most precise indicator of hepatocellular injury because it is observed exclusively in the liver, whereas AST occurs to some extent in the heart, skeletal muscle, kidneys, the pancreas, the brain, erythrocytes, and leukocytes [29, 30]. GGT, another liver enzyme, is located on the plasma membranes of most cells and organ tissues, but more commonly in hepatocytes, and is routinely used in clinical practice to identify liver injury [31]. GGT plays an essential role in the extracellular catabolism of glutathione, which is the major antioxidant in mammalian cells and is a general marker of oxidative stress [10, 32]. Recently, ALT and GGT (but not AST) have been shown in cross-sectional studies to be associated with the presence of fatty liver, observed using ultrasonography or magnetic resonance imaging spectroscopy [33, 34]. Therefore, early detection of changes in ALT and GGT levels is extremely valuable as a biomarker of hepatic dysfunction. In addition, ALT and GGT level changes have attracted interest as potential indicators of a variety of extrahepatic conditions. A number of studies have shown that these enzymes are associated with obesity, type 2 diabetes, metabolic syndrome, and overall mortality [35,36,37,38,39]. Therefore, supplements that lower ALT and GGT may improve overall health and prevent liver diseases.
Oxidative stress and inflammation are the most prevalent pathogeneses of liver diseases [40]. The liver is a major organ and can be attacked by reactive oxygen species (ROS) [41]. While causing liver damage, ROS can also induce the generation of pro-inflammatory genes. Overexpression of pro-inflammatory genes provokes an intracellular signaling cascade that produces more ROS, resulting in a vicious cycle, where increased oxidative stress and inflammatory lesions promote the pathogenesis of liver diseases [40, 42]. Thus, antioxidant and anti-inflammatory therapies may have beneficial effects in those experiencing hepatic dysfunctions. L. chinense Miller fruit has been reported to exert an antioxidant effect in CCl4-exposed rats [18, 19]. These antioxidant properties can be attributed to phenolic compounds, such as glycolipid, pyrrole derivatives, cerebroside, zeaxanthin dipalmitate, and betaine [20,21,22,23, 43]. Among these bioactive components, zeaxanthin dipalmitate has been reported to inhibit hepatic fibrosis in rats, due to, at least in-part, its anti-oxidative activity [22]. Betaine, derived from the oxidation of dietary sources of choline, improves the condition of those suffering from NAFLD by reducing oxidative stress [44] and suppressing inflammatory pathways [45,46,47,48,49]. However, the clinical trial performed by Abdelmalek et al. found that even high doses of betaine supplementation does not improve markers associated with liver damage [50], suggesting that the beneficial effects of LFE may be due to the synergistic effect of various active components in L. chinense Miller fruit.
In our previous study [25], we reported that LFE supplementation demonstrated hepatoprotective effects in mice, due to increased antioxidant enzyme activity and modulated inflammation signaling. Mice fed an LFE-supplemented diet exhibited increased GSH concentrations, decreased malondialdehyde levels, and increased protein levels of antioxidant enzymes such as superoxide dismutase and catalase. In addition, LFE supplementation inhibits ROS-induced c-Jun N-terminal kinases (JNK) activation and significantly enhances the level of phosphorylated extracellular signal-regulated kinases (ERK), which promotes cellular proliferation after injury [51]. Moreover, LFE effectively prevents macrophage infiltration and the release of cytokines such as TNF-α, IL-6, and IL-1β [25]. Thus, we hypothesized that the antioxidant and anti-inflammatory activity of LFE supplementation could decrease ALT and GGT levels in subjects experiencing mild hepatic dysfunction. In this study, we did not observe that LFE supplementation improved other parameters that may indicate hepatic dysfunction, such as CAP, TAS, MFS, and lipid profiles. One possible explanation is the severity of hepatic dysfunction can vary widely from mild to severe. Differences in nutritional status, genetic background, and other environmental factors may have also affected overall outcomes [52, 53].
This study has some limitations. First, while the sample size was relatively small, the study had sufficient statistical power to detect the change of variables. Second, we did not consider other biomarkers when evaluating the effects of LFE on hepatic dysfunction. Additional types of diagnostics, such as hepatic ultra-sonographic scans, computed tomography scans, magnetic resonance imaging, or liver biopsies, may have been helpful in evaluating the effect of LFE on hepatic dysfunction. Third, we did not measure bacterial components like lipopolysaccharide (LPS) and inflammatory cytokines, which may be linked to the development and progression of liver disease [54, 55]. These markers should be evaluated in future studies.
Conclusion
Our results showed that LFE supplementation can significantly lower ALT and GGT levels in subjects with mild hepatic dysfunction. In addition, the dose of LFE provided during this study was generally well tolerated by the subjects and no difference of the number of adverse events was found between the LFE and placebo groups. Therefore, LFE could be suggested to subjects with hepatic dysfunction with no side effects.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Smilin Bell Aseervatham G, Arul Ananth D, Sivasudha T (2018) Chapter 20–The liver: oxidative stress and dietary antioxidants. In: Patel VB, Rajendram R, Preedy VR (eds) The liver. Academic Press, Boston, pp 239–246
Forbes SJ, Newsome PN (2016) Liver regeneration–mechanisms and models to clinical application. Nat Rev Gastroenterol Hepatol 13:473–485. https://doi.org/10.1038/nrgastro.2016.97
Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I (2004) Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ 328:983. https://doi.org/10.1136/bmj.38050.593634.63
West J, Brousil J, Gazis A, Jackson L, Mansell P, Bennett A, Aithal GP (2006) Elevated serum alanine transaminase in patients with type 1 or type 2 diabetes mellitus. QJM 99:871–876
Yanai I, Benjamin H, Shmoish M, Chalifa-Caspi V, Shklar M, Ophir R, Bar-Even A, Horn-Saban S, Safran M, Domany E, Lancet D, Shmueli O (2005) Genome-wide midrange transcription profiles reveal expression level relationships in human tissue specification. Bioinformatics 21:650–659. https://doi.org/10.1093/bioinformatics/bti042
Yang RZ, Park S, Reagan WJ, Goldstein R, Zhong S, Lawton M, Rajamohan F, Qian K, Liu L, Gong DW (2009) Alanine aminotransferase isoenzymes: molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatology 49:598–607. https://doi.org/10.1002/hep.22657
Martin-Rodriguez JL, Gonzalez-Cantero J, Gonzalez-Cantero A, Arrebola JP, Gonzalez-Calvin JL (2017) Diagnostic accuracy of serum alanine aminotransferase as biomarker for nonalcoholic fatty liver disease and insulin resistance in healthy subjects, using 3T MR spectroscopy. Medicine (Baltimore) 96:e6770. https://doi.org/10.1097/MD.0000000000006770
Verma S, Jensen D, Hart J, Mohanty SR (2013) Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int 33:1398–1405. https://doi.org/10.1111/liv.12226
Penn R, Worthington DJ (1983) Is serum gamma-glutamyltransferase a misleading test? Br Med J (Clin Res Ed) 286:531–535. https://doi.org/10.1136/bmj.286.6364.531
Whitfield JB (2001) Gamma glutamyl transferase. Crit Rev Clin Lab Sci 38:263–355. https://doi.org/10.1080/20014091084227
Potterat O (2010) Goji (Lycium barbarum and L. chinense): phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med 76:7–19. https://doi.org/10.1055/s-0029-1186218
Zhu Y-P (1988) Chinese Materia medica: chemistry, pharmacology, and applications. Harwood Academic Publishers, Netherlands
Olatunji OJ, Chen H, Zhou Y (2017) Neuroprotective effect of trans-N-caffeoyltyramine from Lycium chinense against H2O2 induced cytotoxicity in PC12 cells by attenuating oxidative stress. Biomed Pharmacother 93:895–902. https://doi.org/10.1016/j.biopha.2017.07.013
Kim N-H, Baek S-H (2014) Effects of Lycium chinense Miller fruit and its constituent betaine on immunomodulation in Balb/c mice. Korean J Environ Agric 33:189–193. https://doi.org/10.5338/KJEA.2014.33.3.189
Kim MH, Kim EJ, Choi YY, Hong J, Yang WM (2017) Lycium chinense improves post-menopausal obesity via regulation of PPAR-γ and estrogen receptor-alpha/beta expressions. Am J Chin Med 45:269–282. https://doi.org/10.1142/S0192415X17500173
Cui B, Chen Y, Liu S, Wang J, Li S, Wang Q, Li S, Chen M, Lin X (2012) Antitumour activity of Lycium chinensis polysaccharides in liver cancer rats. Int J Biol Macromol 51:314–318. https://doi.org/10.1016/j.ijbiomac.2012.05.004
Zhang R, Kang KA, Piao MJ, Kim KC, Kim AD, Chae S, Park JS, Youn UJ, Hyun JW (2010) Cytoprotective effect of the fruits of Lycium chinense Miller against oxidative stress-induced hepatotoxicity. J Ethnopharmacol 130:299–306. https://doi.org/10.1016/j.jep.2010.05.007
Ahn M, Park JS, Chae S, Kim S, Moon C, Hyun JW, Shin T (2014) Hepatoprotective effects of Lycium chinense Miller fruit and its constituent betaine in CCl4-induced hepatic damage in rats. Acta Histochem 116:1104–1112. https://doi.org/10.1016/j.acthis.2014.05.004
Ha KT, Yoon SJ, Choi DY, Kim DW, Kim JK, Kim CH (2005) Protective effect of Lycium chinense fruit on carbon tetrachloride-induced hepatotoxicity. J Ethnopharmacol 96:529–535. https://doi.org/10.1016/j.jep.2004.09.054
Jung K, Chin YW, Kim YC, Kim J (2005) Potentially hepatoprotective glycolipid constituents of Lycium chinense fruits. Arch Pharm Res 28:1381–1385. https://doi.org/10.1007/BF02977905
Chin YW, Lim SW, Kim SH, Shin DY, Suh YG, Kim YB, Kim YC, Kim J (2003) Hepatoprotective pyrrole derivatives of Lycium chinense fruits. Bioorg Med Chem Lett 13:79–81. https://doi.org/10.1016/s0960-894x(02)00846-6
Kim HP, Lee EJ, Kim YC, Kim J, Kim HK, Park JH, Kim SY, Kim YC (2002) Zeaxanthin dipalmitate from Lycium chinense fruit reduces experimentally induced hepatic fibrosis in rats. Biol Pharm Bull 25:390–392. https://doi.org/10.1248/bpb.25.390
Kim SK, Kim YC, Kim YC (1998) Effects of singly administered betaine on hepatotoxicity of chloroform in mice. Food Chem Toxicol 36:655–661. https://doi.org/10.1016/s0278-6915(98)00024-6
Kim SY, Lee EJ, Kim HP, Kim YC, Moon A, Kim YC (1999) A novel cerebroside from lycii fructus preserves the hepatic glutathione redox system in primary cultures of rat hepatocytes. Biol Pharm Bull 22:873–875. https://doi.org/10.1248/bpb.22.873
Bae U-J, Oh M-R, Park J, Park J-S, Bae E-Y, Chae S-W, Cho BH, Park B-H (2017) Supplementation with Lycium chinense fruit extract attenuates methionine choline-deficient diet-induced steatohepatitis in mice. J Funct Foods 31:1–8. https://doi.org/10.1016/j.jff.2017.01.032
Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, Beaugrand M, Palau R (2003) Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 29:1705–1713. https://doi.org/10.1016/j.ultrasmedbio.2003.07.001
Group CHS, Fried MW, Navarro VJ, Afdhal N, Belle SH, Wahed AS, Hawke RL, Doo E, Meyers CM, Reddy KR, Silymarin N (2012) Effect of silymarin (milk thistle) on liver disease in patients with chronic hepatitis C unsuccessfully treated with interferon therapy: a randomized controlled trial. JAMA 308:274–282. https://doi.org/10.1001/jama.2012.8265
Wright R (1979) Liverand biliary disease: pathophysiology, diagnosis, management. Saunders, Philadelphia
Rej R (1978) Aspartate aminotransferase activity and isoenzyme proportions in human liver tissues. Clin Chem 24:1971–1979. https://doi.org/10.1093/clinchem/24.11.1971
Kew MC (2000) Serum aminotransferase concentration as evidence of hepatocellular damage. Lancet 355:591–592. https://doi.org/10.1016/S0140-6736(99)00219-6
Kunutsor SK (2016) Gamma-glutamyltransferase-friend or foe within? Liver Int 36:1723–1734. https://doi.org/10.1111/liv.13221
Lee DH, Blomhoff R, Jacobs DR Jr (2004) Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res 38:535–539. https://doi.org/10.1080/10715760410001694026
Clark JM, Brancati FL, Diehl AM (2003) The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol 98:960–967. https://doi.org/10.1111/j.1572-0241.2003.07486.x
Westerbacka J, Corner A, Tiikkainen M, Tamminen M, Vehkavaara S, Hakkinen AM, Fredriksson J, Yki-Jarvinen H (2004) Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia 47:1360–1369. https://doi.org/10.1007/s00125-004-1460-1
Group DESIRs, Andre P, Balkau B, Born C, Charles MA, Eschwege E (2006) Three-year increase of gamma-glutamyltransferase level and development of type 2 diabetes in middle-aged men and women: the D.E.S.I.R. cohort. Diabetologia 49:2599–2603. https://doi.org/10.1007/s00125-006-0418-x
Insulin resistance atherosclerosis, Hanley AJ, Williams K, Festa A, Wagenknecht LE, D’Agostino RB Jr, Kempf J, Zinman B, Haffner SM (2004) Elevations in markers of liver injury and risk of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes 53:2623–2632. https://doi.org/10.2337/diabetes.53.10.2623
Lee DH, Silventoinen K, Jacobs DR Jr, Jousilahti P, Tuomileto J (2004) Gamma-Glutamyltransferase, obesity, and the risk of type 2 diabetes: observational cohort study among 20,158 middle-aged men and women. J Clin Endocrinol Metab 89:5410–5414. https://doi.org/10.1210/jc.2004-0505
Perry IJ, Wannamethee SG, Shaper AG (1998) Prospective study of serum gamma-glutamyltransferase and risk of NIDDM. Diabetes Care 21:732–737. https://doi.org/10.2337/diacare.21.5.732
Vozarova B, Stefan N, Lindsay RS, Saremi A, Pratley RE, Bogardus C, Tataranni PA (2002) High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 51:1889–1895. https://doi.org/10.2337/diabetes.51.6.1889
Reyes-Gordillo K, Shah R, Muriel P (2017) Oxidative stress and inflammation in hepatic diseases: current and future therapy. Oxid Med Cell Longev 2017:3140673. https://doi.org/10.1155/2017/3140673
Sanchez-Valle V, Chavez-Tapia NC, Uribe M, Mendez-Sanchez N (2012) Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr Med Chem 19:4850–4860. https://doi.org/10.2174/092986712803341520
Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y (2015) The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci 16:26087–26124. https://doi.org/10.3390/ijms161125942
Kim SY, Lee EJ, Kim HP, Lee HS, Kim YC (2000) LCC, a cerebroside from Lycium chinense, protects primary cultured rat hepatocytes exposed to galactosamine. Phytother Res 14:448–451. https://doi.org/10.1002/1099-1573(200009)14:6%3c448::aid-ptr635%3e3.0.co;2-q
Day CR, Kempson SA (2016) Betaine chemistry, roles, and potential use in liver disease. Biochim Biophys Acta 1860:1098–1106. https://doi.org/10.1016/j.bbagen.2016.02.001
Du J, Shen L, Tan Z, Zhang P, Zhao X, Xu Y, Gan M, Yang Q, Ma J, Jiang A, Tang G, Jiang Y, Jin L, Li M, Bai L, Li X, Wang J, Zhang S, Zhu L (2018) Betaine supplementation enhances lipid metabolism and improves insulin resistance in mice fed a high-fat diet. Nutrients 10:131. https://doi.org/10.3390/nu10020131
Kitagawa E, Ota Y, Hasegawa M, Nakagawa T, Hayakawa T (2019) Accumulation of liver lipids induced by vitamin B6 deficiency was effectively ameliorated by choline and to a lesser extent, betaine. J Nutr Sci Vitaminol (Tokyo) 65:94–101. https://doi.org/10.3177/jnsv.65.94
Kwon DY, Jung YS, Kim SJ, Park HK, Park JH, Kim YC (2009) Impaired sulfur-amino acid metabolism and oxidative stress in nonalcoholic fatty liver are alleviated by betaine supplementation in rats. J Nutr 139:63–68. https://doi.org/10.3945/jn.108.094771
Xu L, Huang D, Hu Q, Wu J, Wang Y, Feng J (2015) Betaine alleviates hepatic lipid accumulation via enhancing hepatic lipid export and fatty acid oxidation in rats fed with a high-fat diet. Br J Nutr 113:1835–1843. https://doi.org/10.1017/S0007114515001130
Zhang W, Wang LW, Wang LK, Li X, Zhang H, Luo LP, Song JC, Gong ZJ (2013) Betaine protects against high-fat-diet-induced liver injury by inhibition of high-mobility group box 1 and Toll-like receptor 4 expression in rats. Dig Dis Sci 58:3198–3206. https://doi.org/10.1007/s10620-013-2775-x
Abdelmalek MF, Sanderson SO, Angulo P, Soldevila-Pico C, Liu C, Peter J, Keach J, Cave M, Chen T, McClain CJ, Lindor KD (2009) Betaine for nonalcoholic fatty liver disease: results of a randomized placebo-controlled trial. Hepatology 50:1818–1826. https://doi.org/10.1002/hep.23239
Wang Z, Yao T, Song Z (2010) Extracellular signal-regulated kinases 1/2 suppression aggravates transforming growth factor-beta1 hepatotoxicity: a potential mechanism for liver injury in methionine-choline deficient-diet-fed mice. Exp Biol Med (Maywood) 235:1347–1355. https://doi.org/10.1258/ebm.2010.010160
Liu G, Huang Y, Reis FS, Song D, Ni H (2019) Impact of nutritional and environmental factors on inflammation, oxidative stress, and the microbiome 2019. Biomed Res Int 2019:5716241. https://doi.org/10.1155/2019/5716241
Wang XL, Rainwater DL, VandeBerg JF, Mitchell BD, Mahaney MC (2001) Genetic contributions to plasma total antioxidant activity. Arterioscler Thromb Vasc Biol 21:1190–1195. https://doi.org/10.1161/hq0701.092146
Plaza-Diaz J, Solis-Urra P, Rodriguez-Rodriguez F, Olivares-Arancibia J, Navarro-Oliveros M, Abadia-Molina F, Alvarez-Mercado AI (2020) The gut barrier, intestinal microbiota, and liver disease: molecular mechanisms and strategies to manage. Int J Mol Sci 21:8351. https://doi.org/10.3390/ijms21218351
Soares JB, Pimentel-Nunes P, Roncon-Albuquerque R, Leite-Moreira A (2010) The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol Int 4:659–672. https://doi.org/10.1007/s12072-010-9219-x
Acknowledgements
This study was supported by a grant funded by the Cheonyang-gun (Chungnam, Republic of Korea). The authors would like to thank the Writing Center at Jeonbuk National University for its skilled proofreading service.
Author information
Authors and Affiliations
Contributions
MRO, SJJ, SWC, BHP, and SOL conceived the project and designed the protocol; MRO, SJJ, SWC, BHP, and SOL performed the experiments; MRO, BHP, and SOL analyzed the data and wrote the manuscript; BHP and SOL have primary responsibility for the final content of the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oh, MR., Jung, SJ., Chae, SW. et al. Lycium chinense Miller fruit extract lowers liver enzyme levels in subjects with mild hepatic dysfunction: a randomized, double-blind, placebo-controlled clinical trial. Eur J Nutr 62, 1415–1425 (2023). https://doi.org/10.1007/s00394-022-03075-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-03075-8