Abstract
Purpose
Probiotic species of butyrate producers have been investigated for the potential in preventing and treating obesity and overweight. However, Clostridium cochlearium has not been linked with any health benefits. We hypothesized that C. cochlearium could be a promising new probiotic with health benefits in improving body weight control and insulin sensitivity.
Methods
Productions of short-chain fatty acids (SCFAs) were characterized for C. cochlearium by NMR and GC–MS analyses. Probiotic effects of C. cochlearium were evaluated through diet-induced obese (DIO) C57BL/6 mice. The influence of C. cochlearium administration on gut SCFAs was measured using GC–MS. LC–MS-based untargeted metabolomic profiling and multivariate analysis were used to assess the serum metabolic alteration, identify biomarkers and pathways in response to the C. cochlearium administration.
Results
After 17 weeks of diet intervention, body weight gain of CC group (fed with a high-fat diet supplemented with C. cochlearium) showed a 21.86% reduction from the high-fat diet (HF) control group (P < 0.001), which was specifically reflected on the significantly lowered fat mass (CC vs HF, 17.19 g vs 22.86 g, P < 0.0001) and fat percentage (CC vs HF, 41.25% vs 47.10%, P < 0.0001), and increased lean percentage (CC vs HF, 46.63% vs 43.72%, P < 0.05). C. cochlearium administration significantly reduced fasting blood glucose from week 8 (P < 0.05 or 0.01), and eventually improved insulin sensitivity (HOMA-IR, CC vs HF, 63.77 vs 143.13, P < 0.05). Overall lowered levels of SCFAs were observed in the gut content of CC group. Metabolomic analysis enabled the identification of 53 discriminatory metabolites and 24 altered pathways between CC and HF groups. In particularly, most of the pathway-matched metabolites showed positive correlations with body weight, which included glutamate, phenylalanine, ornithine, PCs, LPCs, AcCas, proline, 5,6-dihydrouracil, pyroglutamic acid, and 1-pyrroline-4-hydroxy-2-carboxylate.
Conclusions
The beneficial effects of C. cochlearium could be related to its ability to restore certain obesity-driven biomarkers and pathways, especially downregulating pathways related to specific amino acids, PCs, LPCs and AcCas. Further research is warranted to investigate related metabolites and metabolic pathways. C. cochlearium may be developed as a promising new probiotic for the prevention or alleviation of obesity and diabetes in human.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has become a major public health issue. From 1975 to 2016, the worldwide prevalence of obesity has been almost tripled to over 650 million. Together with overweight, the total number was more than 1.9 billion, accounting for 39% of adults [1]. Obesity and overweight are the primary risk factors for noncommunicable diseases including type 2 diabetes, cardiovascular diseases, non-alcoholic fatty liver and certain types of cancers [1, 2]. Lifestyle management and medical treatments are not enough to combat obesity, and it is imperative to identify new preventive and/or therapeutic strategies that are safe and sustainable [3, 4].
Probiotics has shown a potential to be developed as a safe nutritional strategy for obesity prevention and treatment [5]. The human gut is colonized by as many as 1014 bacterial cells, which tightly links the intake of nutrients to the host health and diseases [6]. As live beneficial microorganisms [7], probiotics play an important role in restoring the gut microbial dysbiosis and downstream dysregulated pathways that are highly associated with obesity and related metabolic syndrome [8]. Indeed, research has identified some potential anti-obesity probiotics, such as species of Lactobacillus, Bifidobacterium and Enterococcus [9, 10], and newly uncovered species of Pediococcus, Bacteroides, Akkermansia, Saccharomyces, Eubacterium, Parabacteroides and Clostridium [5, 11, 12]. Of which, butyrate-producing probiotics have attracted particular interest because of recent findings showing that butyrate supplement improved obesity-associated metabolic syndrome including insulin resistance and dyslipidemia [13]. Oral treatment of Eubacterium hallii, a butyrate producer, also improved insulin sensitivity in db/db mice [12].
Butyrate-producing bacteria are widely distributed in human colon, but belong to relatively fewer genera including Anaerostipes, Butyrivibrio, Coprococcus, Eubacterium, Roseburia, Ruminococcus and Clostridium [14, 15]. Clostridium is a large genus of Gram-positive and obligate anaerobic bacteria with diverse functions [16]. Although containing some human pathogens, such as difficile, botulinum and tetani, Clostridium species could be used as probiotics as well [16, 17]. C. butyricum has been found to improve not only non-antimicrobial-induced diarrhea and irritable bowel syndrome [18, 19], but also obesity and diabetes [20, 21]. Previous studies showed that C. cochlearium is an excellent butyrate producer as well [22, 23]. C. cochlearium is also a common commensal bacteria found in the gut of mammalian, such as mice, rabbits and human without associating with any harmful effects [24, 25]. Our preliminary data showed that dietary treatment of C. cochlearium reduced body weight gain in high-fat diet fed mice. Therefore, we hypothesized that C. cochlearium could be a promising new and safe probiotic with health benefits in improving body weight control and insulin sensitivity, potentially via butyrate production. In this investigation, butyrate and other short chain fatty acids (SCFAs) production of C. cochlearium was analyzed using NMR and GC–MS methods. Serum metabolites of mice were profiled by untargeted LC–MS metabolomic analysis, which led to the identification of potential biomarkers involving the mechanisms of probiotic benefits of C. cochlearium.
Methods
Preparation of C. cochlearium
C. cochlearium was purchased from ATCC (Manassas, VA, USA). It was cultured under anaerobic conditions with modified peptone yeast glucose (PYG) medium [12, 26, 27]. At the end of the exponential phase, bacterial culture was collected and centrifuged to remove media. The bacterial pellet was washed with sterile phosphate-buffered saline and mixed with 25% glycerol in medium to reach the final concentration of 1010 CFU/mL. Viability was assessed using the most probable number analysis by dilution to extinction and confirmed by microscopic analysis. Bacteria were stored at – 20 °C and used within 1 week.
Animal experiment
The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the Wayne State University. Thirty-six 6-week-old male C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA, USA). Mice were housed under 12-h light/dark cycle, controlled humidity (40% ± 10%) and constant temperature (24 ℃ ± 1 °C). After 1 week of acclimatization, mice were randomly assigned into three groups (n = 12, 6 mice per cage). The experimental group (CC) was treated with C. cochlearium (109 CFU/100 µL in sterile water) by gavage of 200 µL/mouse/day, fed ad libitum with high-fat diet. High-fat diet (HF) control and low-fat diet (LF) control groups were treated with the same dose of sterile water as the CC group. Mouse diets were purchased from Research Diets Inc. (New Brunswick, NJ, USA). The high-fat diet (D12492M) contained 5.24 kcal per gram with 60% of calories from fat and 20% of calories from carbohydrate, while the low-fat diet (D12450J) contained 3.85 kcal per gram with 10% from fat and 70% from carbohydrate. Besides, they possessed the same amounts of cellulose, minerals, and vitamins.
The experimental lasted 17 weeks, food intake and body weight were monitored weekly, the fasting blood glucose was measured at weeks 5, 8, 12, 16. At week 12, fecal pellets within 24-h were collected from each cage, and fecal calories was measured on a Bomb Calorimeter (Parr, Moline, IL). The net calorie absorption was calculated by subtracting fecal calorie defecation (cal/day/mouse) from food calorie intake (cal/day/mouse). At week 17, body composition was measured for fat and lean mass using EchoMRI-100 analyzer (Houston, TX, USA). The fasting blood glucose was measured with an Accu-check glucometer (Roche, Indianapolis, IN, USA), and the fasting insulin was measured using the ultra-sensitive mouse insulin ELISA kit (Crystal Chem, Doners Grove, IL, USA). The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as follows: [fasting glucose (mg/dL) × fasting insulin level (µU/mL)]/405 [28].
At the end, the blood samples were collected under anesthesia through cardiac puncture, followed with coagulation and centrifugation to obtain the supernatant serum. The serum samples were stored at − 80 °C until metabolomic analysis. The mice were finally euthanatized by exposing to CO2. The gut content of cecum and colon was collected and quenched immediately using liquid nitrogen, stored at − 80 °C until use.
NMR-based characterization of C. cochlearium fermentation medium
C. cochlearium and Escherichia coli (from ATCC, Manassas, VA, USA) were both cultured in PYG medium (n = 3). Specifically, 0.1 mL (109 CFU) of seeds were inoculated into 10 mL of PYG and incubated under anaerobic condition for C. cochlearium and aerobic conditions for E. coli. Then, their fermented media and a blank PYG were centrifuged to obtain clear supernatant. Samples were further prepared with phosphate buffer (pH 7.4) containing 10% D2O (Cambridge Isotope Laboratories, Inc., Andover, MA, USA), 0.5 mM sodium salt of 3-trimethylsilylpropionic acid (TSP; Sigma-Aldrich, St. Louis, MO, USA) and 1.5 mM NaN3 (Sigma-Aldrich, St. Louis, MO, USA) [29]. The proton NMR spectra were acquired on an Agilent DD2-600 MHz NMR spectrometer (Santa Clara, CA, USA), with δ in ppm related to TSP and J in Hz. The pulse sequence water ES was used to suppress water peak, and the number of scans was 64.
GC–MS measurement of SCFAs in mouse gut and bacterial media
The concentrations of short-chain fatty acids (SCFAs) in the gut content of mice and culture media of C. cochlearium and E. coli were determined through gas chromatography-mass spectrometry (GC–MS) [30, 31]. The gut contents were extracted with water containing 5 mM NaOH, in a ratio of 1 mg/50 µL. The culture media were centrifuged to obtain supernatant. Prepared samples were further derivatized by propyl chloroformate (PCF) with a mixture of propanol/pyridine (3:2, v/v), followed by extraction with hexane. The derivatized SCFAs were measured on an Agilent 6890/5973 GC–MS system (Palo Alto, CA, USA) equipped with a Thermo Scientific TG-5MS capillary column (30.0 m × 0.25 mm i.d., 0.25 µm film; Waltham, MA, USA). The injection volume was 1 μL. The carrier gas was helium with a flow rate at 2 mL/min. The temperature program was as follows: 40 °C for 2 min, increased to 130 °C at 10 °C/min, then increased to 165 °C at 5 °C/min, and increased to 300 °C at 80 °C/min, held for 2 min. The selected ion monitoring (SIM) mode was applied. The quantitative ions of acetate, propionate and butyrate were m/z 61, 75 and 71, and their retention times were 2.119, 3.309 and 4.741 min, respectively.
Untargeted LC–MS metabolomic analysis of mouse serum
Serum samples were thawed on ice, and mixed with chilled HPLC grade methanol (Merck Pvt., Mumbai, IN, USA) in the ratio of 1:3 (v/v) to precipitate protein [32]. The mixtures were vortexed for 1 min, followed by centrifugation for 5 min at 17 G. The supernatants were carefully transferred into autosampler vial and stored at 4 °C until use. A quality control (QC) sample was prepared by pooling equal volume of each serum, a blank sample was prepared using water, and both were treated following the same procedure as serum samples.
The metabolomic profiling of serum samples was performed on a Dionex UltiMate 3000 RSLC UHPLC system (Thermo Scientific, Waltham, MA, USA) coupled with a quadrupole-orbitrap mass spectrometer (Q Exactive, HF Hybrid; Thermo Scientific, Waltham, MA, USA), and equipped with an ACQUITY UPLC BEH HILIC column (130 Å, 1.7 µm, 2.1 mm × 150 mm; Waters, Milford, MA, USA). The mobile phase consisted of solvents A (10 mM ammonium formate with 0.1% formic acid, v/v) and B (acetonitrile with 0.1% formic acid, v/v). The gradient elution was as follows: 0–0.5 min, 95% of B; 0.5–9.0 min, from 95 to 40% of B; 9.0–9.1 min, from 40 back to 95% of B; 9.1–10.0 min, 95% of B. The flow rate was 0.4 mL/min, the column was kept at 30 °C, and the injection volume was 2 µL. The mass spectrometry was operated in full scan mode with a resolution of 240,000 full width at half maximum (FWHM) at 200 m/z. The data were acquired in both positive and negative ionization modes with a mass range of 70–1050 m/z.
Due to inadequate serum amounts for 2 mice in LF group, 2 in HF group and 3 in CC group, ultimately 29 serum samples were chemically profiled in a randomized order started from the fifth injection. The QC samples were analyzed as the first three injections, then every seventh injection. The blank sample was run twice with the first one as the fourth injection, while another one as the injection 26.
Metabolomic data analysis and biomarker identification
The software Compound Discoverer 3.0 (CD; Thermo Scientific, Waltham, MA, USA) was used to align, integrate and normalize the chromatographic peaks, and produce metabolic features (m/z and retention time) based on the pooled QC sample [33, 34]. The generated feature list was filtered to remove those peaks presented in less than 50% of QC samples, had areas less than five times the background, and with relative standard deviation (RSD) greater than 30% across QC samples. To reduce the statistical influence of greatly different variance of particular metabolites, the filtered data were further normalized using Pareto scaling. Principal component analysis (PCA), and an orthogonal partial least squares-discriminant analysis (OPLS-DA) with permutation test were performed for data acquired in positive and negative ionization modes, respectively, using the SIMCA-P software (Version 14.0.1, Umetrics, Umeå, Sweden). Thereafter, discriminatory features between HF and CC groups were generated based on the criteria that values of Variable Importance in the Projection (VIP) equal to or more than two, and two tailed Student’s t test P value < 0.05.
Potential molecular formulas of metabolic features were calculated according to their accurate masses and isotope patterns. The database mzCloud, mzVault and ChemSpider were searched to identify potential candidates, and their hit accuracies were manually confirmed as well. Only metabolites with high matches to the MS/MS libraries were assigned the names of compounds. Identified differential biomarkers were further uploaded to the platform MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/MetaboAnalyst/home.xhtml) for enrichment analysis, as well as pathway searching through the Mus musculus (mouse) library of Kyoto Encyclopedia of Genes and Genomes (KEGG). Spearman’s rank correlations between 24 biomarkers hit pathways and six phenotype variables were calculated for CC and HF groups using the “psych” package in R (V 4.0.4), and the result was presented in a heat map using the “pheatmap” package.
Statistical analysis
Normal distribution was analyzed by Anderson–Darling, D’Agootino-Pearson, Shapiro–Wilk, or Kolmogorov–Smirnov in GraphPad Prism (V 7.00). The results of body weight and composition (n = 12 mice per group), fasting blood glucose (n = 12 mice per group), insulin resistance (n = 8 mice per group), and energy homeostasis (n = 2 cages per group) were presented as means ± SEM. Statistical comparisons between two groups were analyzed using Student’s t test. Significance was indicated with * for P < 0.05, ** for P < 0.01, *** for P < 0.001, and **** for P < 0.0001. SCFA concentrations of mouse gut content and bacterial fermentation were also expressed as means ± SEM (n = 3 replicates per group). Their statistical significance among three groups was compared by one-way ANOVA and Tukey’s test (P < 0.05), using the SPSS software for Windows (version 25, 2017, IBM).
Results
C. cochlearium treatment improved body composition
The initial body weights at week 1 were 22.75 g for LF group, 23.59 g for CC groups, and 25.46 g for HF group. As shown in Fig. 1, the LF control group showed the least body weight gain throughout the dietary treatment. The C. cochlearium treatment showed significantly lowered body weight gain than the HF group did from week 2 to the end of the dietary treatment (P < 0.01). At the end of the treatment, the averaged body weight gain of HF, CC and LF groups were 25.75 g, 20.12 g and 7.78 g, respectively. The HF and CC groups gained body weights 3.3 and 2.5 times the gain of LF group due to the feeding of high-fat diet. While the C. cochlearium supplementation reduced body weight gain by 5.63 g, a 21.86% reduction compared to HF group (P < 0.001, Fig. 1). Eventually, the LF group exhibited the lowest absolute body weight at week 17, while the CC group was 7.55 g lower than HF group, a 14.74% reduction (P < 0.0001, Table 1). In terms of body composition (Table 1), the three groups showed similar lean mass (19–21 g), however, the fat masses of HF and CC (22.86 g and 17.19 g) were more than three times of the LF group (5.74 g). Furthermore, C. cochlearium treatment resulted in significantly lowered fat mass (P < 0.0001) and fat percentage (P < 0.0001), while increased lean percentage (P < 0.05) than the CC group. The ratio of fat to lean mass of CC group was reduced to 0.89 from 1.08 for the HF group (P < 0.001).
Body weight (BW) gains of 17 weeks. Values are means ± SEM, n = 12 mice per group. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 indicating significance between CC and HF groups. CC the group treated with C. cochlearium and fed with high-fat diet, HF high-fat diet control group, LF low-fat diet control group
C. cochlearium treatment improved insulin sensitivity
At week 5 of the treatment, the CC group began to show a trend of lowered fasting blood glucose than the HF group (Fig. 2). The difference became significant at week 8 until the end of experiment. At week 16, the fasting blood glucose were 223.8 mg/dL for HF group, 175.1 mg/dL for CC, and 162.6 mg/dL for LF. Meanwhile, the CC group had a reduced insulin level of 140.01 µU/mL as compared to the level of 255.64 µU/mL for HF group (P < 0.05). Moreover, the CC group had a significantly lowered HOMA-IR (63.77) than the HF group did (P < 0.05), suggesting an improved insulin sensitivity by C. cochlearium treatment (Table 1).
Fasting blood glucose at weeks 5, 8, 12 and 16. Values are means ± SEM, n = 12 mice per group. *P < 0.05 and **P < 0.01 indicating significance between CC and HF groups. CC the group treated with C. cochlearium and fed with high-fat diet, HF high-fat diet control group, LF low-fat diet control group
Effects of C. cochlearium treatment on energy homeostasis
Effects of C. cochlearium treatment on food intake and fecal calories are included in Table 1. The averaged calories (cal/day/mouse) intake did not show significant difference between CC and LF, and CC and HF groups. The CC group exhibited significantly higher fecal calories than the LF group (P < 0.01), while no significant difference from the HF group. As for their net calorie absorption (calorie intake minus calorie defecation), no significant difference was observed among the three groups.
Chemical characterization of C. cochlearium fermentation
The metabolites of C. cochlearium fermentation were profiled by NMR and then GC–MS methods. E. coli is not a butyrate producer [35], it was used as a negative control to distinguish the metabolites of C. cochlearium. Supplemental Fig. 1 exhibited water suppressed proton NMR for cultures of C. cochlearium and E. coli in PYG medium. Enlarged and annotated spectra are shown in Fig. 3a, b. Comparing to the PYG control, it was evident that adenosine was consumed, while adenine and acetate were generated by both bacteria. In addition, E. coli produced ethanol, lactate and succinate. It is worth noting that C. cochlearium excreted butyrate and consumed glutamate. Figure 3c shows the quantitative results of SCFAs in bacterial fermentation by GC–MS. Both bacteria significantly produced acetate (P < 0.05), while E. coli (637.27 μg/mL) more than doubled the amount C. cochlearium produced (314.05 μg/mL). There was no significant change for propionate. In particular, C. cochlearium was able to generate butyrate up to 1106.29 μg/mL, while E. coli showed no change for butyrate.
Chemical characterization for the fermentation of C. cochlearium and E. coli in PYG medium. a Enlarged proton NMR spectra with chemical shift from 5.6 to 8.7 ppm. b Proton NMR spectra with chemical shift from 0.0 to 5.4 ppm. 0.5 mM TSP (sodium salt of 3-trimethylsilylpropionic acid) was used as reference to normalize the concentrations of different samples. c GC–MS measured concentrations of acetate, propionate and butyrate. The vertical bars represent SEM (n = 3) for each data point, and different letters represent significant differences (P < 0.05). CC refers to C. cochlearium, and EC refers to E. coli
C. cochlearium treatment modified SCFAs of gut content
As for the three SCFAs in mouse gut content, HF group possessed the highest concentrations among the three groups (Fig. 4, P < 0.05). CC group had the higher level of acetate than LF, but no significant difference with LF on propionate and butyrate.
Gut acetate, propionate and butyrate in CC, HF and LF groups. The vertical bars represent SEM (n = 3 replicates per group) for each data point, and different letters represent significant differences (P < 0.05). CC the group treated with C. cochlearium and fed with high-fat diet, HF high-fat diet control group, LF low-fat diet control group
C. cochlearium treatment altered serum metabolites and pathways
LC–MS-based untargeted method was used to profile the serum metabolomic modification caused by C. cochlearium supplementation. A list of 5825 feature metabolites, including 3492 positive and 2333 negative features, was generated from the raw data processing and filtration.
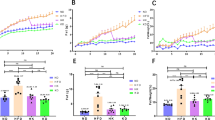
Based on the analysis on features of the positive mode, the LF group was distinctly separated from clusters of the HF and CC groups in the unsupervised PCA scores plot (Supplemental Fig. 2a), suggesting that the low-fat diet distinguished serum metabolic characterization of the LF group from the metabolites of the high-fat diet groups (the HF and CC groups). In addition, the CC group was largely separated from the HF group by component three in the PCA scores plot (Fig. 5a). In the supervised OPLS-DA plot (Fig. 5b), the HF and CC groups were significantly separated into two clusters that were far away from each other. The model presented an R2X(cum) at 0.566, a goodness-of-fit R2 at 0.956, and a goodness-of prediction Q2 at 0.781. Permutation test was further performed to validate the OPLS-DA model (Fig. 5c). As a result, all permutated blue Q2 values to the left were lower than the original point to the right (Axis Y = 0.781), and the blue regression line of Q2-points intersected the left vertical axis below zero (− 0.574), suggesting that the OPLS-DA separation was valid without overfitting [36].
Multivariate analysis of serum metabolites between CC and HF groups (N = 19). Principal component analysis (PCA) scores plots (a and d), orthogonal partial least squares-discriminant analysis (OPLS–DA) scores plots (b and e), and permutation validation of OPLS–DA models (c and f) for data collected in positive and negative ionization modes. Data were normalized by Pareto scaling. An ellipse represents the 95% confidence interval using Hotelling's T2 statistics. CC the group treated with C. cochlearium and fed with high-fat diet, HF high-fat diet control group
Features of the negative mode did not exhibit good separation between the low-fat diet group and the high-fat diet groups (Supplemental Fig. 2d). However, a trend of separation was observed between the CC and HF groups (Fig. 5d). The OPLS-DA model of the negative mode showed a significant group classification (Fig. 5e), and presented an R2X(cum) at 0.490, a goodness-of-fit R2 at 0.768, and a goodness-of prediction Q2 at 0.131. Although some permutated blue Q2-values were higher than the original point (Axis Y = 0.131), the OPLS–DA model did not overfit the data considering that the blue regression line of Q2-points intersected the vertical axis below zero (− 0.347) (Fig. 5f).
With the threshold VIP values ≥ 2 and P < 0.05, 65 discriminatory features were obtained from OPLS-DA analysis. Of which, 51 were generated from the data of the positive ionization mode and 14 from the negative mode (Supplemental Table 1). The enrichment analysis classified the 53 structurally identified metabolites into categories of amino acids and peptides (9), acylcarnitines (7), glycerophosphocholines (5), benzamides (4), hydroxy acids (2), pyrimidines (2), indoles (1), carboxylic acids (1), pyrrolines (1), organic carbonic acids (1), TCA acids (1), fatty amides (1), and others. Nine of these identified metabolites were up-regulated by the C. cochlearium treatment while the others were down-regulated. Among them, 24 identified biomarkers hit 24 pathways of mouse metabolism (Table 2). Correspondingly, most of the matched pathways were down-regulated, except for those related to arginine, LPC (18:2) and LPC (22:6).
Correlations between probiotic effects and serum biomarkers
The heat map (Fig. 6) summarized the 24 pathway-matched biomarkers into two major clusters, which differentiated the 21 down-regulated biomarkers (cluster A) from the 3 up-regulated ones (cluster B). The six phenotype variables were also separated into two primary clusters, with body lean percentage (cluster 1) as an opposite characterization to the others (cluster 2). The insulin-related variables (cluster 2.1) were further clustered from the body mass-related variables (cluster 2.2).
Discussion
The primary objectives of this study were to evaluate the probiotic effects of C. cochlearium supplementation on DIO mice, and to assess their potential mechanisms of actions via chemical characterization. C. cochlearium administration significantly reduced body weight gains and body fat on mice. Body composition has been considered a more important clinical factor, because the fat mass and distribution are more associated with the metabolic disorders of obesity [37, 38]. A major one of these complications is impaired insulin sensitivity [39]. C. cochlearium intake also significantly improved insulin sensitivity and reduced blood glucose levels on mice.
Body weight change is a result of energy balance between absorption and expenditure [40]. The differentiated body weight gains and fat masses of the three groups could be attributed to the modulated energy expenditures, given that there was no difference on the net calorie absorption among them. Diet-induced energy expenditure is different among macronutrients with carbohydrate inducing 2–3 times more energy expenditure than fat does [41], which may partly explain the fact that the HF and CC groups stored more net energy than the LF group did. However, the HF and CC groups were consuming the same high-fat diet. Therefore, the 22% body weight reduction by C. cochlearium supplementation were achieved through increased energy expenditure in other two forms: resting metabolic rate (RMR) and physical activity [40]. C57BL/6 mice are susceptible to develop obesity on a high-fat diet by lowering fat oxidation [42]. Thus, C. cochlearium might stimulate fat oxidation and increase energy expenditure, leading to reduced body weight gain.
Probiotics may reduce body weight gain by modulating the composition of gut microbiota, improving the mucosal barrier function, and enhancing the host immunity [43, 44]. Nevertheless, these benefits need to be mediated by the metabolites produced by probiotics. We showed that C. cochlearium produced SCFAs, especially substantial amount of butyrate as compared to other bacteria. In bacteria, butyrate is mainly produced from pyruvate, 4-aminobutyrate, glutarate and lysine [45]. Our result suggests that C. cochlearium produces butyrate from glutamate [23].
SCFAs in the intestinal lumen are produced by the bacterial fermentation of undigested carbohydrates [46]. Gut SCFAs have been considered one of the primary contributors to the anti-obesity effect of probiotics [47, 48]. Among them, butyrate has received the most attentions due to its beneficial effects on intestinal homeostasis and cellular energy metabolism [49]. Butyrate involves in lipid and glucose metabolism by inhibiting histone deacetylase (HDAC) and subsequently activating G protein-coupled receptors (GPRs). Activation of GRPs also results in secretion of intestinal satiety hormones, such as glucagon-like peptide-1 (GLP-1) and peptide YY (PYY), leading to reduced food intake and alleviated obesity [49,50,51]. We showed that C. cochlearium was a solid butyrate producer and its dietary supplementation reduced obesity development on mice. We, therefore, hypothesized that SCFAs, particularly butyrate, play a significant role in anti-obesity activity of C. cochlearium. However, we did not find the direct evidence supporting our hypothesis since the CC group had actually lower levels of gut SCFAs. We were not able to monitor the real-time gut SCFAs as they are promptly absorbed once generated. We suspect that the C. cochlearium might also stimulate the colonic utilization of SCFAs and eventually lower their levels in the gut, resulting in an anti-obesity activity [52]. This might also explain the facts that decreased cecal and fecal SCFAs have been frequently observed in lean mice and human, rather than their obese counterparts [53,54,55].
C. cochlearium administration significantly altered the serum metabolites on DIO mice according to the multivariate analysis. We identified 53 metabolites potentially associated with the beneficial effects of C. cochlearium supplementation. The category of amino acids and peptides accounted for a major part (9 out of 53). Of which, glutamate is a significant one as it has been previously associated with obesity and related insulin resistance in human [56]. Our study showed that C. cochlearium administration decreased serum glutamate, suggesting a potential role of glutamate in mediating the anti-obesity effect of C. cochlearium. More evidence is presented in Table 2 showing that a number of glutamate-related biological pathways were also down-regulated by C. cochlearium. These pathways included aminoacyl-tRNA biosynthesis (6/48), arginine and proline metabolism (6/38), arginine biosynthesis (4/14), alanine, aspartate and glutamate metabolism (3/28), glutathione metabolism (3/28), and glyoxylate and dicarboxylate metabolism (3/32).
Phenylalanine and ornithine are two other amino acid biomarkers related to obesity or diabetes. Serum phenylalanine predicted the development of insulin resistance in a 6-year follow-up of young adults [57]. Elevated plasma ornithine concentration has been observed in both high-fat diet-induced obese C57BL/6 mice and streptozotocin (STZ) induced insulin deficient mice [58]. In our study, serum levels of phenylalanine and ornithine were positively correlated with mouse body weight, fat mass and fat/lean ratio, or HOMA-IR (P < 0.01; Fig. 6), suggesting an involvement of these two amino acids in the probiotic activity of C. cochlearium.
Our correlation test revealed that serum asparagine was positively correlated obesity and insulin resistance, while arginine had a negative association (Fig. 6). However, their roles in obesity development remain controversial. Some studies found that asparagine was elevated in obesity, while others showed an opposite relationship [59]. Serum arginine was increased in obese people, but arginine supplementation improved endothelial function and insulin sensitivity in subjects with metabolic syndrome [60]. Thus, more studies are needed to clarify their role in obesity development.
There are four major groups of lipids detected in serum: glycerophospholipids, glycerolipids, sphingolipids and non-esterified fatty acids [61]. Lipidomic characterization has established the connections between metabolic diseases and alteration of bioactive lipids, such as glycerophospholipid and sphingolipid [62]. Our metabolomic analysis showed that C. cochlearium treatment significantly altered the serum levels of two phosphatidylcholines (PCs), three lysophosphocholines (LPCs) and seven acylcarnitines (AcCas) that are supposed to be at micro- to nano-molar in blood [63].
Phosphatidylcholines (PCs) play an essential role in regulating the circulatory very low-density lipoproteins (VLDLs) and HDLs [59]. Levels of serum PCs have been positively correlated with obesity and insulin [64]. Correspondingly, our study found that C. cochlearium supplementation significantly reduced serum PC (38:3) and PC (36:4). LPC is involved in inflammatory responses and oxidative stress, primarily through the Toll-like receptors and G protein-coupled receptors G2A [65]. Plasma LPCs such as LPC (22:6) and LPC (20:3) were significantly decreased in obese subjects [66]. In our study, serum levels of LPC (22:6) and LPC (18:2) were also lower in the HF group compared with the CC group. However, serum LPC (20:3) exhibited positive correlations with body weight, fat mass, fat/lean ratio, and HOMA-IR (Fig. 6), which was inconsistent with previous reports. The inconsistency was also reported in a study showing that most plasma LPC species were reduced in obesity, while other LPCs were increased [67]. Thus, the links between LPCs and obesity could be individualized. Current lipidomic studies focus on the general profiling of LPC alteration, however, further investigation is needed to elucidate roles of specific LPCs.
During the process of fatty acid oxidation (FAO), carnitine is required to assemble and shuttle free fatty acids into the mitochondrial matrix, in the form of esterified carnitines (acylcarnitines, AcCas) [68]. Hence, circulating AcCas may reflect the level of FAO process. Increased levels of plasma AcCas were associated with higher risks of obesity and type 2 diabetes in humans [69]. We found that seven AcCa species were unanimously down-regulated in the CC group, and they were all positively associated with body weight, fat mass and fat/lean ratio (Fig. 6). The results suggest that C. cochlearium administration might stimulate the FAO process, subsequently reducing body weight gain and improving insulin sensitivity. It is worth noting that five of the seven AcCa biomarkers identified in our study were short-chain species, which was in accordance with the previous observations that short-chain AcCas were particularly elevated in diabetes subjects [68, 70].
In conclusion, our study demonstrated that dietary supplementation of C. cochlearium attenuated body weight gain and improved insulin sensitivity on high-fat diet induced obese C57BL/6 mice. We identified 53 discriminatory metabolites between CC and HF groups. Of which, 24 metabolites matched mouse metabolic pathways. Most of these metabolites showed positive correlations with body weight, which included glutamate, phenylalanine, ornithine, PCs, LPCs, AcCas, proline, 5,6-dihydrouracil, pyroglutamic acid, and 1-pyrroline-4-hydroxy-2-carboxylate. The results suggested that the beneficial effects of C. cochlearium could be related to its ability to restore certain obesity-driven biomarkers and pathways, especially downregulating pathways related to certain amino acids, PCs, LPCs and AcCas. Further research is warranted to investigate related metabolites and metabolic pathways. C. cochlearium may be developed as a promising new probiotic for the prevention or alleviation of obesity and diabetes in human.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Change history
06 April 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00394-022-02882-3
References
World Health Organization (2018) Obesity and overweight. https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 15 Sept 2021
Sanz Y, Santacruz A, Gauffin P (2010) Gut microbiota in obesity and metabolic disorders. Proc Nutr Soc 69:434–441. https://doi.org/10.1017/S0029665110001813
Muller TD, Clemmensen C, Finan B, DiMarchi RD, Tschop MH (2018) Anti-obesity therapy: from rainbow pills to polyagonists. Pharmacol Rev 70:712–746. https://doi.org/10.1124/pr.117.014803
Heymsfield SB, Wadden TA (2017) Mechanisms, pathophysiology, and management of obesity. N Engl J Med 376:254–266. https://doi.org/10.1056/NEJMra1514009
Kobyliak N, Conte C, Cammarota G, Haley AP, Styriak I, Gaspar L et al (2016) Probiotics in prevention and treatment of obesity: a critical view. Nutr Metab. https://doi.org/10.1186/s12986-016-0067-0
Sekirov I, Russell SL, Antunes LC, Finlay BB (2010) Gut microbiota in health and disease. Physiol Rev 90:859–904. https://doi.org/10.1152/physrev.00045.2009
Sanders ME (2008) Probiotics: definition, sources, selection, and uses. Clin Infect Dis 46(Suppl 2):S58-61. https://doi.org/10.1086/523341
Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G et al (2016) The gut microbiota and host health: a new clinical frontier. Gut 65:330–339. https://doi.org/10.1136/gutjnl-2015-309990
Rouxinol-Dias AL, Pinto AR, Janeiro C, Rodrigues D, Moreira M, Dias J et al (2016) Probiotics for the control of obesity—its effect on weight change. Porto Biomed J 1:12–24. https://doi.org/10.1016/j.pbj.2016.03.005
Raoult D (2009) Probiotics and obesity: a link? Nat Rev Microbiol 7:616. https://doi.org/10.1038/nrmicro2209
Chang CS, Ruan JW, Kao CY (2019) An overview of microbiome based strategies on anti-obesity. Kaohsiung J Med Sci 35:7–16. https://doi.org/10.1002/kjm2.12010
Udayappan S, Manneras-Holm L, Chaplin-Scott A, Belzer C, Herrema H, Dallinga-Thie GM et al (2016) Oral treatment with Eubacterium hallii improves insulin sensitivity in db/db mice. NPJ Biofilms Microbi. https://doi.org/10.1038/npjbiofilms.2016.9
Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M et al (2009) Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58:1509–1517. https://doi.org/10.2337/db08-1637
Chen W, Zhang S, Wu J, Ye T, Wang S, Wang P et al (2020) Butyrate-producing bacteria and the gut-heart axis in atherosclerosis. Clin Chim Acta 507:236–241. https://doi.org/10.1016/j.cca.2020.04.037
Fu X, Liu Z, Zhu C, Mou H, Kong Q (2019) Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit Rev Food Sci Nutr 59:S130–S152. https://doi.org/10.1080/10408398.2018.1542587
Yutin N, Galperin MY (2013) A genomic update on clostridial phylogeny: gram-negative spore formers and other misplaced clostridia. Environ Microbiol 15:2631–2641. https://doi.org/10.1111/1462-2920.12173
Sanchez Ramos L, Rodloff AC (2018) Identification of Clostridium species using the VITEK((R)) MS. Anaerobe 54:217–223. https://doi.org/10.1016/j.anaerobe.2018.01.007
Ling Z, Liu X, Cheng Y, Luo Y, Yuan L, Li L et al (2015) Clostridium butyricum combined with Bifidobacterium infantis probiotic mixture restores fecal microbiota and attenuates systemic inflammation in mice with antibiotic-associated diarrhea. Biomed Res Int. https://doi.org/10.1155/2015/582048
Sun YY, Li M, Li YY, Li LX, Zhai WZ, Wang P et al (2018) The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. Sci Rep. https://doi.org/10.1038/s41598-018-21241-z
Shang H, Sun J, Chen YQ (2016) Clostridium butyricum CGMCC0313.1 modulates lipid profile, insulin resistance and colon homeostasis in obese mice. PLoS ONE 11:e0154373. https://doi.org/10.1371/journal.pone.0154373
Jia L, Li D, Feng N, Shamoon M, Sun Z, Ding L et al (2017) Anti-diabetic effects of Clostridium butyricum CGMCC0313.1 through promoting the growth of gut butyrate-producingbacteria in type 2 diabetic mice. Sci Rep. https://doi.org/10.1038/s41598-017-07335-0
Buckel W, Barker H (1974) Two pathways of glutamate fermentation by anaerobic bacteria. J Bacteriol 117:1248–1260. https://doi.org/10.1128/jb.117.3.1248-1260.1974
Leutbecher U, Böcher R, Linder D, BUCKEL W (1992) Glutamate mutase from Clostridium cochlearium: purification, cobamide content and stereospecific inhibitors. Eur J Biochem 205:759–765. https://doi.org/10.1111/j.1432-1033.1992.tb16840.x
Lee W, Fujisawa T, Kawamura S, Itoh K, Mitsuoka T (1991) Isolation and identification of clostridia from the intestine of laboratory animals. Lab Anim 25:9–15. https://doi.org/10.1258/002367791780808158
Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen M-L, Bolte E et al (2002) Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis 35:S6-16. https://doi.org/10.1086/341914
Duncan SH, Louis P, Flint HJ (2004) Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol 70:5810–5817. https://doi.org/10.1128/AEM.70.10.5810-5817.2004
Louis P, Young P, Holtrop G, Flint HJ (2010) Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol 12:304–314. https://doi.org/10.1111/j.1462-2920.2009.02066.x
Ippagunta SM, Kharitonenkov A, Adams AC, Hillgartner FB (2018) Cholic acid supplementation of a high-fat obesogenic diet suppresses hepatic triacylglycerol accumulation in mice via a fbroblast growth factor 21–dependent mechanism. J Nutr 148:510–517. https://doi.org/10.1093/jn/nxy022
Beckonert O, Keun HC, Ebbels TM, Bundy J, Holmes E, Lindon JC et al (2007) Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc 2:2692–2703. https://doi.org/10.1038/nprot.2007.376
Kaspar H (2009) Amino acid analysis in biological fluids by GC-MS. PhD, University of Regensburg
Zheng X, Qiu Y, Zhong W, Baxter S, Su M, Li Q et al (2013) A targeted metabolomic protocol for short-chain fatty acids and branched-chain amino acids. Metabolomics 9:818–827. https://doi.org/10.1007/s11306-013-0500-6
Dunn WB, Broadhurst D, Begley P, Zelena E, Francis-McIntyre S, Anderson N et al (2011) Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protoc 6:1060–1083. https://doi.org/10.1038/nprot.2011.335
Lu D, Xue L, Feng C, Wu C, Xie C, Gonzalez FJ et al (2019) A systemic workflow for profiling metabolome and lipidome in tissue. J Chromatogr A 1589:105–115. https://doi.org/10.1016/j.chroma.2018.12.061
Gong J, Feng Z, Peterson AL, Carr JF, Lu X, Zhao H et al (2021) The pentose phosphate pathway mediates hyperoxia-induced lung vascular dysgenesis and alveolar simplification in neonates. JCI Insight 6:e137594. https://doi.org/10.1172/jci.insight.137594
Kataoka N, Vangnai AS, Pongtharangkul T, Yakushi T, Matsushita K (2017) Butyrate production under aerobic growth conditions by engineered Escherichia coli. J Biosci Bioeng 123:562–568. https://doi.org/10.1016/j.jbiosc.2016.12.008
Jing L, Guigonis J-M, Borchiellini D, Durand M, Pourcher T, Ambrosetti D (2019) LC-MS based metabolomic profiling for renal cell carcinoma histologic subtypes. Sci Rep 9:1–10. https://doi.org/10.1038/s41598-019-52059-y
Borga M, West J, Bell JD, Harvey NC, Romu T, Heymsfield SB et al (2018) Advanced body composition assessment: from body mass index to body composition profiling. J Investig Med 66:1–9. https://doi.org/10.1136/jim-2018-000722
Segal KR, Dunaif A, Gutin B, Albu J, Nyman A, Pi-Sunyer FX (1987) Body composition, not body weight, is related to cardiovascular disease risk factors and sex hormone levels in men. J Clin Invest 80:1050–1055. https://doi.org/10.1172/JCI113159
Hardy OT, Czech MP, Corvera S (2012) What causes the insulin resistance underlying obesity? Curr Opin Endocrinol Diabetes Obes 19:81–87. https://doi.org/10.1097/MED.0b013e3283514e13
Hill JO, Wyatt HR, Peters JC (2012) Energy balance and obesity. Circulation 126:126–132. https://doi.org/10.1161/CIRCULATIONAHA.111.087213
Jéquier E (2002) Pathways to obesity. Int J Obes 26:S12–S17. https://doi.org/10.1038/sj.ijo.0802123
Hariri N, Thibault L (2010) High-fat diet-induced obesity in animal models. Nutr Res Rev 23:270–299. https://doi.org/10.1017/S0954422410000168
Kobyliak N, Conte C, Cammarota G, Haley AP, Styriak I, Gaspar L et al (2016) Probiotics in prevention and treatment of obesity: a critical view. Nutr Metab 13:1–13. https://doi.org/10.1186/s12986-016-0067-0
Baothman OA, Zamzami MA, Taher I, Abubaker J, Abu-Farha M (2016) The role of gut microbiota in the development of obesity and diabetes. Lipids Health Dis 15:1–8. https://doi.org/10.1186/s12944-016-0278-4
Anand S, Kaur H, Mande SS (2016) Comparative in silico analysis of butyrate production pathways in gut commensals and pathogens. Front Microbiol 7:1945. https://doi.org/10.3389/fmicb.2016.01945
Cummings JH (1981) Short chain fatty acids in the human colon. Gut 22:763–779. https://doi.org/10.1136/gut.22.9.763
Butel MJ (2014) Probiotics, gut microbiota and health. Med Mal Infect 44:1–8. https://doi.org/10.1016/j.medmal.2013.10.002
Yan F, Polk DB (2010) Probiotics: progress toward novel therapies for intestinal diseases. Curr Opin Gastroenterol 26:95–101. https://doi.org/10.1097/MOG.0b013e328335239a
Liu H, Wang J, He T, Becker S, Zhang G, Li D et al (2018) Butyrate: a double-edged sword for health? Adv Nutr 9:21–29
Chakraborti CK (2015) New-found link between microbiota and obesity. World J Gastrointest Pathophysiol 6:110–119. https://doi.org/10.4291/wjgp.v6.i4.110
den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM (2013) The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54:2325–2340. https://doi.org/10.1194/jlr.R036012
Fernandes J, Su W, Rahat-Rozenbloom S, Wolever T, Comelli E (2014) Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. https://doi.org/10.1038/nutd.2014.23
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444:1027–1031. https://doi.org/10.1038/nature05414
Murphy E, Cotter P, Healy S, Marques TM, O’sullivan O, Fouhy F et al (2010) Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut 59:1635–1642. https://doi.org/10.1136/gut.2010.215665
Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C et al (2010) Microbiota and SCFA in lean and overweight healthy subjects. Obesity 18:190–195. https://doi.org/10.1038/oby.2009.167
Tulipani S, Palau-Rodriguez M, Alonso AM, Cardona F, Marco-Ramell A, Zonja B et al (2016) Biomarkers of morbid obesity and prediabetes by metabolomic profiling of human discordant phenotypes. Clin Chim Acta 463:53–61. https://doi.org/10.1016/j.cca.2016.10.005
Würtz P, Soininen P, Kangas AJ, Rönnemaa T, Lehtimäki T, Kähönen M et al (2013) Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 36:648–655. https://doi.org/10.2337/dc12-0895
Sailer M, Dahlhoff C, Giesbertz P, Eidens MK, de Wit N, Rubio-Aliaga I et al (2013) Increased plasma citrulline in mice marks diet-induced obesity and may predict the development of the metabolic syndrome. PLoS ONE 8:e63950. https://doi.org/10.1371/journal.pone.0063950
Lent-Schochet D, McLaughlin M, Ramakrishnan N, Jialal I (2019) Exploratory metabolomics of metabolic syndrome: a status report. World J Diabetes 10:23. https://doi.org/10.4239/wjd.v10.i1.23
Monti LD, Casiraghi MC, Setola E, Galluccio E, Pagani MA, Quaglia L et al (2013) L-arginine enriched biscuits improve endothelial function and glucose metabolism: a pilot study in healthy subjects and a cross-over study in subjects with impaired glucose tolerance and metabolic syndrome. Metabolism 62:255–264. https://doi.org/10.1016/j.metabol.2012.08.004
Han X (2016) Lipidomics for studying metabolism. Nat Rev Endocrinol 12:668. https://doi.org/10.1038/nrendo.2016.98
Gross RW, Han X (2007) Lipidomics in diabetes and the metabolic syndrome. Meth Enzymol 433:73–90. https://doi.org/10.1016/S0076-6879(07)33004-8
Syme C, Czajkowski S, Shin J, Abrahamowicz M, Leonard G, Perron M et al (2016) Glycerophosphocholine metabolites and cardiovascular disease risk factors in adolescents: a cohort study. Circulation 134:1629–1636. https://doi.org/10.1161/CIRCULATIONAHA.116.022993
Cole LK, Vance JE, Vance DE (2012) Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochim Biophys Acta, Mol Cell Biol Lipids 1821:754–761. https://doi.org/10.1016/j.bbalip.2011.09.009
Law S-H, Chan M-L, Marathe GK, Parveen F, Chen C-H, Ke L-Y (2019) An updated review of lysophosphatidylcholine metabolism in human diseases. Int J Mol Sci 20:1149. https://doi.org/10.3390/ijms20051149
Heimerl S, Fischer M, Baessler A, Liebisch G, Sigruener A, Wallner S et al (2014) Alterations of plasma lysophosphatidylcholine species in obesity and weight loss. PLoS ONE 9:e111348. https://doi.org/10.1371/journal.pone.0111348
Barber MN, Risis S, Yang C, Meikle PJ, Staples M, Febbraio MA et al (2012) Plasma lysophosphatidylcholine levels are reduced in obesity and type 2 diabetes. PLoS ONE 7:e41456. https://doi.org/10.1371/journal.pone.0041456
Adeva-Andany MM, Calvo-Castro I, Fernández-Fernández C, Donapetry-García C, Pedre-Piñeiro AM (2017) Significance of L-carnitine for human health. IUBMB Life 69:578–594. https://doi.org/10.1002/iub.1646
Guasch-Ferré M, Ruiz-Canela M, Li J, Zheng Y, Bulló M, Wang DD et al (2019) Plasma acylcarnitines and risk of type 2 diabetes in a Mediterranean population at high cardiovascular risk. J Clin Endocrinol Metab 104:1508–1519. https://doi.org/10.1210/jc.2018-01000
Hoppel CL, Genuth SM (1982) Urinary excretion of acetylcarnitine during human diabetic and fasting ketosis. Am J Physiol Endocrinol Metab 243:E168–E172. https://doi.org/10.1152/ajpendo.1982.243.2.E168
Acknowledgements
We are grateful to the Systems Mass Spectrometry Core (SyMS-C) at the Georgia Institute of Technology for the LC-MS data acquisition.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Responsibilities were as follows: KZ and WZ designed the research, provided essential materials, and edited the manuscript. FY, WZ, PE and QA conducted the research. XJ provided essential instruments for the LC–MS data acquisition. FY analyzed the data and wrote the manuscript. KZ had primary responsibility for the final content. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they had no conflict of interest.
Ethical approval
The protocol of animal study was approved by the Institutional Animal Care and Use Committee (IACUC) at the Wayne State University, Detroit, USA.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, F., Zhu, W., Edirisuriya, P. et al. Characterization of metabolites and biomarkers for the probiotic effects of Clostridium cochlearium on high-fat diet-induced obese C57BL/6 mice. Eur J Nutr 61, 2217–2229 (2022). https://doi.org/10.1007/s00394-022-02840-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-02840-z