Abstract
Purpose
During the last decade more researchers have argued in favor of an increased protein intake for older adults. However, there is a lack of knowledge on the long-term effects of conforming to such a high protein intake with regards to the basal and postprandial muscle protein turnover. The purpose of this study was to compare the postprandial synthesis response in muscle proteins, and the abundance of directly incorporated food-derived amino acids following habituation to high vs. recommended level of protein intake.
Methods
In a double blinded crossover intervention 11 older male participants (66.6 ± 1.7 years of age) were habituated for 20 days to a recommended protein (RP) intake (1.1 g protein/kg lean body mass (LBM)/day) and a high protein (HP) intake (> 2.1 g protein/kg LBM/day). Following each habituation period, intrinsically labelled proteins were ingested as part of a mixed meal to determine the incorporation of meal protein-derived amino acids into myofibrillar proteins. Furthermore, the myofibrillar fractional synthesis rate (FSR) and amino acid kinetics across the leg were determined using gold standard stable isotope tracer methodologies. RT qPCR was used to assess the expression of markers related to muscle proteinsynthesis and breakdown.
Results
No impact of habituation was observed on skeletal muscle amino acid or protein kinetics. However, the shunting of amino acids directly from artery to vein was on average 2.9 \(\upmu\)mol/min higher following habituation to HP compared to RP.
Conclusions
In older males, habituation to a higher than the currently recommended protein intake did not demonstrate any adaptions in the muscle protein turnover or markers hereof when subjected to an intake of an identical mixed meal.
Clinical Trial Registry
Journal number NCT02587156, Clinicaltrials.org. Date of registration: October 27th, 2015.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Provision of protein or amino acids and resultant postprandial hyperaminoacidemia stimulates overall protein synthesis [1, 2]. The impact on skeletal muscle has been thoroughly studied [3,4,5,6,7,8] with results showing that a single serving of proteins stimulates protein synthesis in a dose dependent [9, 10], non-cumulative [11] and saturable manner [9, 10, 12] in both older and younger individuals. However, older individuals (> 65 years of age) need higher protein intakes per serving than younger counterparts (~ 22–30 years of age) to elicit a comparable response [7, 13,14,15].
These findings have led to a widespread notion that the official recommendations for optimal protein intake, which as of now is 0.8 g protein/kg body weight (BW)/day for all ages [16], should be increased for older individuals [17,18,19,20]. While these data may appear supportive of enhancing protein recommendations, an important piece is often missing; namely the study designs do not appear to consider the impact of the habituated protein intake on the dose-dependent response. In 1993 Price and colleagues habituated young and middle-aged (ages ranging from 22 to 54) participants for 2 weeks to four divergent levels of protein intake ranging from 0.36 to 2.31 g protein/kg BW/day [21]. Following the habituation period diurnal urea production, leucine oxidation and net protein balance were determined. All three parameters were significantly increased when habituated to high level of protein intakes in both the fasted and postprandial state [21]. More recently Gorissen and colleagues [22] habituated older adults to either a lower than RDA or a higher protein intake and measured basal whole body protein turnover as well as skeletal muscle protein synthesis rate before and after an intake of 25 g whey protein. With this design, the researchers could not demonstrate any impact of habituated protein intake level on protein turnover and muscle protein synthesis rates. In contrary in a similar experimental setting, we habituated older adults to WHO recommended protein intake levels and higher protein intake for 3 weeks using a cross-over design showing that whole body net protein balance in the fasted state is lower following 20 days of habituation to high protein compared to low protein [23]. Hence, when comparing higher protein intake to RDA (as suggested by other findings reviewed above) the habituated protein intake level does impact protein metabolism. As any acute effect of changing protein intake may be affected with time by adaptive mechanisms in metabolism, it is crucial to investigate a response in the habituated state, prior to suggesting permanent changes.
While former studies have investigated the impact of habituated protein intake on whole body protein metabolism, it remains unclear to our knowledge, how muscle tissue specific protein synthesis and turnover rates are affected when the target group of older individuals conform to a markedly increased daily protein intake.
The aim of the present study was to investigate muscle protein turnover in older individuals who conformed to a markedly higher protein intake in their daily diet compared to ingesting the WHO recommended protein intake. Specifically, the aims were, to investigate if the divergent protein intakes impact the protein kinetic response and how much the muscles would incorporate amino acids from identical meals. To investigate the response to an ‘every day’ postprandial condition, the meal given was a mixed meal as opposed to a protein or amino acid supplement, which expectedly will have an impact on the uptake of the amino acids from the GI tract [24] and possibly slow down breakdown due to a higher insulin secretion [25].
Subjects and method
Participants
Twelve male participants aged 66.6 ± 1.7 years were recruited to this double blinded, randomized crossover-controlled trial. All included participants had their body composition determined by dual X-ray absorptiometry (DXA) scanning (Lunar iDXA; GE Medical Systems, Pewaukee, WI, USA, with enCORE v.16 software), wherefrom the LBM was used to calculate the desired dietary protein intake and the tracer amount needed. The exclusions criteria were diseases affecting; organs, muscle or connective tissue, diabetes, back pain, known atherosclerosis, alcohol intake above 21 units (1 unit is defined as 12 g of alcohol) per week as well as intake of medications (mild hypertension and cholesterol lowering drugs were allowed). Due to a mixing error of labeled proteins, one participant was excluded from all calculations, and thus, we only used 11 participants for the final analyses. Data on whole body and plasma protein turnover from this study have previously been reported [23].The participants were screened for the abovementioned criteria by oral interview and blood samples tested for the parameters shown in Table 1. Written and oral information about study design, possible side effects, risk, and discomforts were given to each participant prior to enrollment in accordance with the Helsinki declaration. All participants enrolled gave written informed consent. The study took place at Bispebjerg and Frederiksberg Hospital, Copenhagen and the study was approved by the local ethical committee of the Capital Region of Denmark, protocol number H-15005598 and the study was registered at Clinicaltrials.org as NCT02587156.
Experimental design
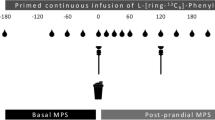
In a crossover design pictured in Fig. 1, participants went through a 20-day period of habituation to the recommended protein (RP) intake of 1.1 g protein/kg lean body mass (LBM) (corresponding to the current official recommendations of 0.8 g protein/kg BW assuming a body fat content of ~ 27%), and a 20-day period of habituation to a high level of protein (HP) intake defined as an intake > 2.1 g protein/kg LBM/day. The two periods were differentiated by four daily intakes of supplements containing 30 g of carbohydrates (20 g maltodextrin and 10 g sucrose) in the RP period and an isocaloric 20 g of protein and 10 g of carbohydrates (20 g whey hydrolysate and 10 g sucrose) in the HP period. The supplements were developed, prepared and individually but identically packed by Arla Foods Ingredients Group P/S, Viby J, Denmark. The order of the habituation periods was randomly assigned as HP/RP or RP/HP in a double blinded fashion by the investigator.
The initial 7 days of the habituation periods are categorized as a start-up period, with the only dietary alterations being the four daily intakes of the protein or carbohydrate supplements. Based on a 3-day dietary registration during the seven start-up days, participants were guided towards a diet containing 1.1 g protein/kg LBM/day, controlled by regular online dietary registrations for the remaining 13 days of the 20-day period. The supplements were spaced out dependent on the participant’s regular protein intake; thus, two supplements were given with the meal that contained the least protein, and one supplement with each of the other two main meals attempting an equal protein intake at all three main meals in the HP period. For most participants this meant 2 supplements with breakfast, 1 supplement with lunch and 1 supplement with dinner.
2 days prior to the experimental trial day the participants refrained from any sort of strenuous physical activity, and on the evening prior to the trial day all participants had a standardized meal ensuring the only difference in intake was the supplements.
After the first habituation period and experimental trial day, there was a washout period of at least 45 days before crossing over to the alternate habituation period.
Day 21: experimental trial day
The experimental trial day (i.e., day 21) was identical irrespective of the preceding habituation period, RP or HP. At 8 a.m. the participants arrived at the hospital by taxi in an overnight fasted state. Upon arrival the participants were assigned to a bed positioned in a supine position and instructed to lie still for the remainder of the trial day. A catheter was inserted in an antecubital vein, wherefrom isotopic background blood samples were taken (− 100 min). Hereafter, a primed continuous infusion of D8-phenylalalnine was started (prime: 4 mol\(\upmu\)/kg LBM, continuous infusion: 4\(\upmu\)/kg LBM/h in a fasted state, 4.8 mol\(\upmu\)/kg LBM/h in postprandial state to account for dilution in the steady state of the D8-phenylalanine isotopic enrichment by appearance of phenylalanine from the food protein). 30 min later, a primed continuous infusion of indocyanine green (ICG) was started (prime: 1 mg, continuous infusion: 7–14 mg/h into an antecubital vein). Once the primed, continuous infusions were started, a catheter was inserted in a femoral artery, femoral vein, and radial artery under local anesthesia. After placement of the catheters, the ICG infusion was moved from the antecubital vein to the femoral artery. The catheters in the radial artery and femoral vein were used for blood sampling.
Following, at least, 90 min of tracer infusion the fasting blood samples and muscle biopsy were taken, and a breakfast meal was served. The breakfast consisted of a mixed meal, with a protein content of 0.61 g/kg LBM, on average corresponding to 34.4 g protein. From the total of 0.61 g protein/kg LMB 0.54 g/kg LBM came from the intrinsically labelled milk proteins with 0.23 g/kg LBM deriving from Ca-caseinate, intrinsically labelled with 15N-phenylalanine, and 0.31 g/kg LBM deriving from whey protein, intrinsically labelled with D5-phenylalanine. The whey protein was mixed with water and served as a drink concurrent with the caseinate protein which was mixed with raspberry jam and spread with butter on bun. The total calorie intake in the meal was ~ 486 kcal, which besides the 0.61 g protein/kg LBM consisted of 1.07 g carbohydrate/kg LBM and 0.21 g fat/kg LBM.
The participants were instructed to ingest the meal within 10 min, whereafter the time was set at 0 min, and the infusion rate of D8-phenylalanine was increased as stated above.
Sampling
Blood samples from the left antecubital vein was obtained prior to infusion start, and simultaneously from the femoral vein and radial artery immediately prior to eating (reported as -10, based on the average analysis of two consecutive blood samples, interspaced by 2–5 min) as well as 30, 60, 90, 120, 150, 180, and 240 min postprandial.
Muscle biopsies were taken immediately prior to eating as well as 60 and 240 min postprandial, from m. vastus lateralis using a 5-mm biopsy needle (Bergström) under local anesthesia (2–4 ml 1% lidocaine). A new incision was made for each biopsy, spaced by a minimum distance of 4 cm in a randomized order. The muscle biopsies were quickly cleaned for any visible blood and connective tissue and snap frozen in liquid nitrogen.
One or more biopsies from two of the participants were too small to conduct all planned analyses.
Production of intrinsically labeled proteins
The intrinsically labeled milk proteins were produced by infusing five Danish Holstein Friesian cows with stable isotopes, concomitantly milking the cows, and subsequently purifying the whey and caseinate protein. This has previously been described in detail by Reitelseder et al. [24]. Briefly, four cows were infused with l-[ring-D5]-phenylalanine and one cow with L-[15N]-phenylalanine. Milk was pooled from 11 milkings, pasteurized and purified, yielding L-[ring-D5]-phenylalanine labeled whey with an enrichment of 15.4 ± 0.24 mol percent excess (MPE) (mean ± SEM) and l-[15N]-phenylalanine labeled caseinate with an enrichment of 20.8 ± 0.02 MPE (mean ± SEM).
mRNA measurements
Real-time reverse transcriptase polymerase chain reaction (RT-PCR), was applied to analyze the expression of genes involved in protein breakdown (Atrogin1 and MURF), growth factors (IGF1 (IGF1Ea), MGF and Myostatin) as well as amino acid transporters (SNAT2, LAT1 and PAT1). RPLP0 was used as housekeeping gene, verified by GAPDH (Fig. 2a). Primers are shown in Table 2. As previously described [26], approximately 10 mg of muscle tissue was homogenized and RNA was extracted with TriReagent. Gel electrophoresis was performed to ensure good RNA integrity. Complementary DNA (cDNA) was synthesized from 500 ng RNA using OmniScript reverse transcriptase (Qiagen, Hilden, Germany), in the presence of a mixture of dNTP’s (nucleotides, OmniScript kit) and a poly dT primer (Invitrogen, Naerum, Denmark). cDNA was amplified in Quantitect SYBR Green master mix (Qiagen) with specific primers on a real-time PCR machine (MX3005P, Stratagene, La Jolla, Ca, USA) with the thermal profile: 10 min, 95 °C followed by 50 × [15 s, 95 °C → 30 s, 58 °C → 90 s, 63 °C (signal collection)]. Threshold cycle (Ct) values were related to a standard curve made from the cloned PCR product. The specificity of the PCR product was confirmed by a melting curve.
Expression of GAPDH (a), MuRF1 (b), Atrogin1 (c), Myostatin (d), IGF1Ea (e), MGF (f), PAT1 (g), SNAT2 (h) and LAT1 (i) in the fasted (0 h) and four hours postprandial (4 h) state following habituation to RP or HP intake. All values were normalized to the housekeeping gene RPLP0 and are related the fasting 0 h sample from the RP period. Values are shown on binary logarithmic scales as the geometric mean ± backtransformed SEM, N = 9. HP high protein period, RP Recommended protein period
Stabile isotope tracers
The stabile isotope tracers used in this study were D8-phenylalanine provided via intravenous infusion, D5-phenylalanine ingested intrinsically labelled whey protein and 15N-phenylalanine ingested via intrinsically labelled Ca-caseinate protein. These different isotopomers were differentiated during analysis based on their difference in molecular weight (mass of phenylalanine + 8, + 5 and + 1, respectively) and/or the difference in the labeled atom.
Analysis of free amino acid concentrations and tracer enrichments
Free amino acids were extracted from the muscle specimens to allow measurement of the amino acid concentrations and tracer abundances. Muscle specimens of ~ 10 mg wet weight were freeze dried and subsequently fibers were dissected free of visible blood, connective tissue and fat under a microscope in a tempered room at 18 °C and < 30% humidity. The isolated muscle fibers were weighed and homogenized in 1 ml of 6% perchloric acid with added internal standard for determination of amino acid concentrations. The samples were spun down and the supernatant containing the tissue free amino acids were extracted. The samples were then poured over cation exchange columns with resin (AG 50 W-X8 resin, Bio-Rad laboratories, Hercules, Ca, USA), which had been acidified by adding 3 × 2 ml 1 M HCl. The columns were washed five times with 3 ml of deionized water before the amino acids were eluted by adding 2 × 2 ml 4 M NH4OH collected in vials. Solvent was evaporated under a stream of N2 flow at 70 °C and derivatized using PITC derivatization agent which converted the samples into their phenylthiocabamyl (PTC) derivative. 10 µl of the 100 µl derivatized samples were loaded and analyzed on an ultra-performance liquid chromatography system coupled to a triple stage quadrupole mass spectrometer (LC–MS/MS) (Thermo Fischer Scientific, San Jose, CA, USA) as described by Bornø et al. [27].
Analysis of muscle protein bound tracer enrichments
Before measuring enrichments in the muscle proteins, ~ 30 mg muscle specimens were homogenized in a 0.02 M Tris [pH 7.4], 0.15 M NaCl, 2 mM EDTA, 2 mM EGTA, 0.5% TritonX-100 and 0.25 M sucrose buffer. The samples were spun down and the supernatant discarded. 1.5 ml of 0.1 M Sodium Pyrophosphate/0.7 M Potassium Chloride buffer was added to the pellet followed by vigorous vortex mixing, left over night at 5 °C and spun down. This separated the collagen protein (pellet) and the myofibrillar protein (supernatant), wherefrom the myofibrillar protein was collected. The protein fractions were hydrolyzed by addition of 1 ml 1 M HCl and 1 ml resin slurry, and left overnight at 110 °C. Following hydrolysis, the samples were purified over cation exchange resin, as described for the non-protein bound samples. For measures of deuterium enrichments (D5-phenylalanine and D8-phenylalanine), samples were PITC derivatized and run on LC–MS/MS system as described above. For determining the 15-nitrogen abundances amino acids were converted to the N-acetyl-propyl (NAP) derivatives and analyzed on a gas chromatography–combustion–isotope ratio mass spectrometer (GC–C–IRMS) system as previously described by Bornø et al. [28].
Calculations
Flow was measured via Fick’s principle, by the use of the dye indocyanine green (ICG) as the indicator. The concentration of ICG was measured in plasma by assessing the absorbance by spectrophotometry at 805 nm and subtracting the sample turbidity measured at 905 nm. The concentration in each sample was then calculated by comparing the sample absorbance with the absorbance of a standard curve with known concentrations. Using Fick’s principle [29], the flow was calculated by
where CICG_V/A is the ICG concentrations in the femoral vein and artery. The average flow values from each individual trial day, was used for further calculations.
The concentration of phenylalanine was calculated as the sum of the unlabeled and the labelled phenylalanine (calculated as the tracer-to-tracee ratio (TTR) multiplied by the concentration of unlabeled). For the labelled phenylalanine both D8-phenylalanine as well as the transamination product D7-phenylalanine was measured. For all further calculations, the enrichments are MPE given by
The net balance across the leg was given by
where Ca and Cv are the total phenylalanine concentration (labeled and unlabeled) in the artery and femoral vein, respectively, and PF is the leg plasma flow (l/min).
The rate of disappearance of phenylalanine across the leg was given by
where Ea and Ev is the D8-phenylalanine enrichment in the artery and femoral vein, given in MPE.
The rate of appearance of phenylalanine across the leg was calculated as the difference between the rate of disappearance and the net balance:
Calculations of the flux from the intracellular and extracellular compartments was given by: FIn is the flux of phenylalanine in the artery feeding the muscle tissue:
where CA is the phenylalanine concentration in the artery, PF is the Plasma flow in leg.
FA→IM is the flux of phenylalanine from the artery to the intramuscular compartment:
where EIM/V/A is the D8-phenylalanine enrichment in the intramuscular compartment, vein or artery, respectively (IM/V/A).
The synthesisIM→MB: the phenylalanine uptake by the muscle-bound protein pool is the flux of phenylalanine from the intramuscular interstitial space to the muscle-bound protein pool, a measure of synthesis
The breakdownMB→IM: the phenylalanine release from the muscle-bound protein pool to the interstitial space, thus a measure of breakdown:
ShuntA→V is the flow of phenylalanine being shunted directly from the artery to the vein.
The fractional synthesis rate of amino acids into the myofibrillar protein was given by
where ΔE protein is the change in enrichment in myofibrillar protein between the fasted state and the biopsy taken 4 h postprandial. The precursor pool was given by
where \(\overline{{{\text{mean}}}}\) is the weighed mean over the 0–240 min postprandial.
Statistical analysis
The response to feeding over time after the two habituation periods was compared using two-way ANOVA with repeated measures. When there was a main interaction effect a SIDAK post hoc test was performed. When there was a main effect of time a Dunnet’s post hoc test was performed. The postprandial myofibrillar FSR measurements between habituation periods were compared using a paired t test. For subject characteristics, data is means ± SD. mRNA data is presented as geometric mean ± backtransformed SEM. All other data is presented as means ± SEM. For statistical analysis of mRNA data, Sigma Plot version 12.0 (Systat Software Inc., San Jose, CA) was applied, for all other statistical analysis GraphPad Prism 8.0 was used. With inclusion of 11 participants, we would have been able to detect a difference in the 0–4 h FSR of 0.013%/h with the expected SD of 0.01, a significance level of 5% and a power of 80%. Significant level was set to p < 0.05 and whenever 0.1 > p > 0.05 tendencies was discussed.
Results
The participant characteristics for the 11 participants, as they presented prior to the habituation periods, are shown in Table 1. The 20-day dietary composition for the two different periods is shown in Table 3. The average protein intake in the entire 20-day habituation period was 1.22 ± 0.03 g protein/LBM/day (0.82 ± 0.03 g protein/kg BW/day) in the RP period and 2.61 ± 0.06 g protein/LBM/day (1.76 ± 0.06 g/kg BW/day) in the HP period.
mRNA
mRNA expression is depicted on a binary logarithmic scale as the relative change from baseline, defined as the fasted state following habituation to RP intake (Fig. 2). GAPDH (Fig. 2a) was used to validate the housekeeping RPLP0, showing no changes in response to either time or intervention.
The relative expression of breakdown markers showed a postprandial time effect (decrease) of both MuRF1 (Fig. 2b) and Atrogin1 (Fig. 2c, p < 0.05 for both). Whereas for the relative expression of the marker for growth inhibition Myostatin (Fig. 2d) there was no effect of food intake (time) or habituated protein intake. However, a tendency for an interaction was seen (p = 0.07) likely obtained by a higher expression in the fasted state after HP compared to RP. The IGF1Ea (Fig. 2e) showed neither time effect nor interaction. However, a tendency for an effect of the habituated level of protein intake was seen (p = 0.09) with the expression tending to be higher following habituation to RP level of protein intake. For MGF (Fig. 2f) there was a significant time effect (p = 0.01) the overall time effect is likely caused by the decreased 4-h postprandial expression after the HP period. The relative expressions of amino acid transporters are shown in Fig. 2g, h, i, with no difference between fasting and postprandial (time) or between habituation states.
Flux across and within the leg
We measured the net balance as well as the flux of phenylalanine across the leg (Fig. 3). There were no changes over time or between groups, in neither the net balance (Fig. 3a), unidirectional phenylalanine uptake (Fig. 3b) nor the unidirectional phenylalanine release (Fig. 3c).
Leg plasma flow and Phenylalanine flux across the leg measured using the 2-pool model. There is no effect of either habituation or time on the net balance (a) or the unidirectional phenylalanine uptake (b). The unidirectional phe release (c), as measure of breakdown tends to increase over time (p = 0.06). The leg plasma flow is shown for all the participants, and Dunnets post hoc test reveal no changes from baseline. For the calculations of net balance and unidirectional phenylalanine release and uptake, the average flow across each trial day has been used. Values are mean ± SEM, N = 9. Phe phenylalanine
Using the intramuscular free amino acid enrichment, the flux of phenylalanine between intramuscular free amino acid pool and the intramuscular protein bound pool was assessed (Fig. 4). There were no differences between habituation periods and no difference over time for either the phenylalanine uptake by the muscle protein pool, or for the release of phenylalanine into the intramuscular free amino acid pool (Fig. 4a, b, respectively). The shunting of phenylalanine directly from the artery to the vein was on average 2.9 mol\(\upmu\)/min higher following the HP period (Fig. 4c, p < 0.05) and was significantly higher 60 min postprandial compared to fasting (Fig. 4c, p < 0.01).
Phenylalanine uptake and release by the muscle and A–V shunting measured using the 3-pool model. There is no effect of either habituation or time on the phenylalanine uptake or release by the muscle (Fig. 4a and b, respectively). The shunting of phenylalanine directly from the artery to the vein is significantly increased following habituation to higher protein and increase significantly 60 min postprandial following both habituation periods (time p = 0.01). “Dollar” denotes significant change from fasting (p < 0.05). Values are mean ± SEM, N = 9. Phe phenylalanine
Fractional synthesis rates
The fractional synthesis rates (FSR) for the myofibrillar proteins in the 4 h postprandial period are shown in Fig. 5. There was no difference between interventions for the myofibrillar protein FSR.
Nutrient protein-derived tracer abundances
The enrichment from the intrinsically labeled whey (D5-phenylalanine labeled) and Ca-caseinate (15N-phenylalanine labeled) proteins ingested in the mixed meal were detected in the myofibrillar proteins 1 and 4 h postprandial. Values are presented as mole percent excess (MPE) for D5-phenylalanine and atom percent excess (APE) for 15N-phenylalanine enrichments. The incorporation of phenylalanine deriving from both whey and caseinate was greater at 4 h compared to 1 h postprandial (p < 0.0001), Fig. 6.
Incorporation of amino acids from ingested whey (D5-phenylalanine) (a) and caseinate (15 N-phenylalnine) (b) into myofibrillar proteins. The incorporation of phenylalanine from the breakfast meal into myofibrillar protein is significantly increased with time. “Dollar” denotes significant change from 1 h (p < 0.05). Values mean ± SEM, N = 9 (a, b)
Discussion
We measured basal and postprandial muscle protein turnover kinetics and related gene expression as well as how meal-protein-derived amino acids were incorporated into muscle proteins. This was measured in older men who were habituated for 20 days to daily protein intakes in accordance with WHO recommendations or markedly higher using a double-blinded, randomized cross-over design. We found that 3 weeks of habituation to a substantially higher than the standard WHO recommended daily protein intake did not change any of the measured skeletal muscle protein metabolic parameters in older males.
Previous published data [23] showed that in high turnover plasma proteins, the whey-derived amino acid tracer (D5-phenyalanine) is more abundant 4 h after meal intake when the participants are habituated to the recommended level of protein, whereas this was not the case for the caseinate-derived tracer (15N-phenylalanine). Myofibrillar protein turnover is much slower than plasma protein (approx. 1–2%/day for muscle [30] vs. for example 8–9%/day for serum albumin [31]). Thus, the amino acids from the meal are appearing much slower in the muscle proteins and are present in the muscle proteins to a far lesser degree (~ 130 fold). Moreover, in this study we found no impact of the habituated level of dietary protein intake on postprandial myofibrillar protein FSR supporting the lack of difference in dietary amino acid incorporation. This finding is in agreement with the findings by Gorissen et al. [22], who habituated older men (~ 62 years of age) to 0.7 vs. 1.5 g protein/kg/day for 14 days and measured the myofibrillar FSR in a 5 h postprandial period following ingestion of whey protein. Hence, our data supported by others, reveal no impact of habituation to a certain level of dietary protein intake on muscle protein synthesis rate when exposed to an identical protein intake be it isolated protein [22], or a mixed meal as shown in this study. The discrepancy between blood proteins’ FSRs being affected by 3 weeks of high protein habituation [23] and not the skeletal muscle may be explained by the fact that the blood proteins are mainly produced in the liver. In addition to having a higher increase in meal-derived amino acids via the portal inflow, the liver also has an enzymatic system to degrade amino acids. This multifactorial role might lead to faster adjustment of its amino acid metabolism as compared to skeletal muscle.
Estimating the protein synthesis and breakdown of the entire leg, representative for skeletal muscle, using the tracer dilution approach, has previously shown that feeding affects protein turnover via an increased synthesis and decreased breakdown [32, 33]. We show neither changes over time nor differences between habituation states for the leg phenylalanine kinetics.
However, we did observe an overall higher shunting of amino acids directly from the artery to the femoral vein (p < 0.05, Fig. 4c) in the HP compared to RP. This is despite the fact that exactly comparable protein amounts were provided on the trial days and that the circulating phenylalanine concentrations did not differ between the HP and RP period [23]. The higher shunting of amino acids indicates that uptake of available amino acids by the muscle is less effective following habituation to HP. This, however, did not translate into any changes in protein dynamics such as FSR or phenylalanine kinetics.
To evaluate the impact of habituation of protein on intramuscular protein turnover regulators real-time-qPCR analysis was applied. Neither of the selected gene expression markers for protein degradation were affected in the overnight fasted state by habituation to divergent levels of protein intake; however, both MuRF1 and Atrogin1 were decreased following feeding independent of the habituation status (Fig. 2b, c). To our knowledge, no other comparable data exists comparing a fasted and a fed state following habituation to divergent protein intakes. For the gene expression of markers commonly analyzed for protein synthesis; Myostatin, IGF1Ea and MGF (Fig. 2d, e, f), we did not find any impact of meal intake similarly to the lack of impact on muscle protein synthesis. Previously, it has been shown that exercise is required for an increased expression of these markers [34,35,36,37], and that such exercise-induced increase can be augmented when combined with feeding [3, 38, 39].
This study design does have some limitations: postprandial tracer data can be difficult to interpret due to some ubiquitous challenges. A steady state in the enrichment in the various mixing pools (e.g., veins, arteries, intramuscular pool) is an underlying assumption for the kinetic calculations. In the present study postprandial tracer fluctuations were attempted to be diminished by changing infusion rate. Despite this maneuver, the D8-phenylalanine tracer was diluted by unlabeled phenylalanine from the meal, causing a significantly decreased enrichment at 30, 60 and 90 min postprandial compared to fasting. A decreased enrichment will result in a overestimation of both synthesis and breakdown. A further limitation is the timing of the habituation period. Protein habituation studies are scarce, making it difficult to define a period needed for a proper habituation. A previous study using the nitrogen balance technique, indicates that it takes more than 2 weeks for the body to adjust to a changed protein content in the diet [40]. However, compliance to the protruded dietary guidelines is crucial, thus limiting the duration. The fact that we previously showed an impact on plasma proteins [23], which could adapt faster than muscle proteins as described above, indicating that a longer habituation period might have been warranted to find changes in muscle proteins.
In conclusion, we found that shunting of amino acids across the leg tissue bed was higher in the postprandial condition after HP compared to RP. However, habituation to a higher protein intake than RDA neither affected surrogate measures of protein synthesis and breakdown across the leg nor did it impact basal muscle protein turnover rates or markers of improved muscle maintenance capacity. Finally, habituated protein intake above RDA did not affect mechanisms involved in the anabolic response such as myofibrillar FSR when challenged by a fixed protein intake in a standard mixed meal.
Abbreviations
- APE:
-
Atom percent excess
- BW:
-
Body weight
- DXA:
-
Dual X-ray absorptiometry
- FSR:
-
Fractional synthesis rate
- HP:
-
High protein
- ICG:
-
Indocyanine green
- LBm:
-
Lean body mass
- MPE:
-
Mole percent excess
- Phe:
-
Phenylalanine
- RP:
-
Recommended protein
References
Steffee P, Goldsmith RS, Young R et al (1976) Dietary protein intake and dynamic aspects of whole body nitrogen metabolism in adult humans. Metabolism 25:281–297
Morens C, Bos C, Pueyo ME et al (2003) Increasing habitual protein intake accentuates differences in postprandial dietary nitrogen utilization between protein sources in humans. J Nutr 133:2733–2740
Biolo G, Tipton KD, Klein S, Wolfe RR (1997) An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol 273:E122–E129
Mitchell WK, Phillips BE, Williams JP et al (2015) The impact of delivery profile of essential amino acids upon skeletal muscle protein synthesis in older men: clinical efficacy of pulse vs. bolus supply. Am J Physiol Endocrinol Metab. https://doi.org/10.1152/ajpendo.00112.2015
Reitelseder S, Agergaard J, Doessing S et al (2011) Whey and casein labeled with l-[1-13C]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion. Am J Physiol Endocrinol Metab 300:E231–E242. https://doi.org/10.1152/ajpendo.00513.2010
Groen BBL, Res PT, Pennings B et al (2012) Intragastric protein administration stimulates overnight muscle protein synthesis in elderly men. Am J Physiol Endocrinol Metab 302:E52-60. https://doi.org/10.1152/ajpendo.00321.2011
Moore DR, Churchward-Venne TA, Witard O et al (2015) Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci 70:57–62. https://doi.org/10.1093/gerona/glu103
Katsanos CS, Kobayashi H, Sheffield-Moore M et al (2005) Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 82:1065–1073
Bohé J, Low A, Wolfe RR, Rennie MJ (2003) Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol 552:315–324. https://doi.org/10.1113/jphysiol.2003.050674
Cuthbertson D, Smith K, Babraj J et al (2005) Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19:422–424. https://doi.org/10.1096/fj.04-2640fje
Areta JL, Burke LM, Ross ML et al (2013) Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol 591:2319–2331. https://doi.org/10.1113/jphysiol.2012.244897
Bohé J, Low JF, Wolfe RR, Rennie MJ (2001) Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol 532:575–579
Guillet C, Prod’homme M, Balage M et al (2004) Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J 18:1586–1587. https://doi.org/10.1096/fj.03-1341fje
Katsanos CS, Kobayashi H, Sheffield-Moore M et al (2006) A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 291:E381–E387. https://doi.org/10.1152/ajpendo.00488.2005
Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR (2000) The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab 85:4481–4490. https://doi.org/10.1210/jcem.85.12.7021
World Health Organization, Food and Agriculture Organization of the United Nations, United Nations University. Protein and amino acid requirements in human nutrition: report of a joint WHO/FAO/UNU expert consultation. World Health Organization Technical Report Series 935. Geneva: WHO; 2007
Wolfe RR (2012) The role of dietary protein in optimizing muscle mass, function and health outcomes in older individuals. Br J Nutr 108(Suppl):S88-93. https://doi.org/10.1017/S0007114512002590
Bauer J, Biolo G, Cederholm T et al (2013) Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 14:542–559. https://doi.org/10.1016/j.jamda.2013.05.021
Baum JI, Kim IY, Wolfe RR (2016) Protein consumption and the elderly: What is the optimal level of intake? Nutrients 8:1–9. https://doi.org/10.3390/nu8060359
Rafii M, Chapman K, Elango R et al (2016) Dietary protein requirement of men >65 years old determined by the indicator amino acid oxidation technique is higher than the current estimated average requirement. J Nutr 146:681–687. https://doi.org/10.3945/jn.115.225631
Price GM, Halliday D, Pacy PJ et al (1994) Nitrogen homeostasis in man: influence of protein intake on the amplitude of diurnal cycling of body nitrogen. Clin Sci (Lond) 86:91–102
Gorissen SH, Horstman AM, Franssen R et al (2016) Habituation to low or high protein intake does not modulate basal or postprandial muscle protein synthesis rates: a randomized trial. Am J Clin Nutr. https://doi.org/10.3945/ajcn.115.129924
Højfeldt G, Bülow J, Agergaard J et al (2020) Impact of habituated dietary protein intake on fasting and postprandial whole-body protein turnover and splanchnic amino acid metabolism in elderly men: a randomized, controlled, crossover trial. Am J Clin Nutr. https://doi.org/10.1093/ajcn/nqaa201
Reitelseder S, Tranberg B, Agergaard J et al (2020) Phenylalanine stable isotope tracer labeling of cow milk and meat and human experimental applications to study dietary protein-derived amino acid availability. Clin Nutr 44:1–50. https://doi.org/10.1016/j.clnu.2020.03.017
Nygren J, Nair KS (2003) Differential regulation of protein dynamics in splanchnic and skeletal muscle beds by insulin and amino acids in healthy human subjects. Diabetes 52:1377–1385
Reitelseder S, Agergaard J, Doessing S et al (2014) Positive muscle protein net balance and differential regulation of atrogene expression after resistance exercise and milk protein supplementation. Eur J Nutr 53:321–333. https://doi.org/10.1007/s00394-013-0530-x
Bornø A, Van Hall G (2014) Quantitative amino acid profiling and stable isotopically labeled amino acid tracer enrichment used for in vivo human systemic and tissue kinetics measurements. J Chromatogr B Anal Technol Biomed Life Sci 951–952:69–77. https://doi.org/10.1016/j.jchromb.2014.01.019
Bornø A, Hulston CJ, Van Hall G (2014) Determination of human muscle protein fractional synthesis rate: an evaluation of different mass spectrometry techniques and considerations for tracer choice. J Mass Spectrom 49:674–680. https://doi.org/10.1002/jms.3387
Henriksen JH, Winkler K (1987) Hepatic blood flow determination. A comparison of 99mTc-diethyl-IDA and indocyanine green as hepatic blood flow indicators in man. J Hepatol 4:66–70
Rasmussen BB, Phillips SM (2003) Contractile and nutritional regulation of human muscle growth. Exerc Sport Sci Rev 31:127–131. https://doi.org/10.1097/00003677-200307000-00005
Levitt DG, Levitt MD (2016) Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism. Int J Gen Med 9:229–255. https://doi.org/10.2147/IJGM.S102819
Smith GI, Patterson BW, Klein SJ, Mittendorfer B (2015) Effect of hyperinsulinaemia-hyperaminoacidaemia on leg muscle protein synthesis and breakdown: reassessment of the two-pool arterio-venous balance model. J Physiol 593:4245–4257. https://doi.org/10.1113/JP270774
Greenhaff PL, Karagounis LG, Peirce N et al (2008) Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab 295:E595-604. https://doi.org/10.1152/ajpendo.90411.2008
Hulmi JJ, Tannerstedt J, Selänne H et al (2009) Resistance exercise with whey protein ingestion affects mTOR signaling pathway and myostatin in men. J Appl Physiol 106:1720–1729. https://doi.org/10.1152/japplphysiol.00087.2009
Wilborn CD, Taylor LW, Greenwood M et al (2009) Effects of different intensities of resistance exercise on regulators of myogenesis. J Strength Cond Res 23:2179–2187. https://doi.org/10.1519/JSC.0b013e3181bab493
Kraemer WJ, Häkkinen K, Newton RU et al (1999) Effects of heavy-resistance training on hormonal response patterns in younger vs. older men. J Appl Physiol 87:982–992. https://doi.org/10.1152/jappl.1999.87.3.982
Willoughby DS, Stout JR, Wilborn CD (2007) Effects of resistance training and protein plus amino acid supplementation on muscle anabolism, mass, and strength. Amino Acids 32:467–477. https://doi.org/10.1007/s00726-006-0398-7
Glover EI, Phillips SM (2010) Resistance exercise and appropriate nutrition to counteract muscle wasting and promote muscle hypertrophy. Curr Opin Clin Nutr Metab Care 13:630–634. https://doi.org/10.1097/MCO.0b013e32833f1ae5
Rasmussen BB, Tipton KD, Miller SL et al (2000) An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol 88:386–392
Morse MH, Haub MD, Evans WJ, Campbell WW (2001) Protein requirement of elderly women: nitrogen balance responses to three levels of protein intake. J Gerontol A Biol Sci Med Sci 56:M724–M730. https://doi.org/10.1093/gerona/56.11.M724
Funding
The Danish Dairy Research Foundation; Arla Foods Ingredients Group P/S; The Excellence Programme 2016 University of Copenhagen—project CALM; Mejeribrugets ForskningsFond.
Author information
Authors and Affiliations
Contributions
GH, JB, GvH and LH designed the study, GH drafted the manuscript, GH, JaB, JB, LRS, GvH and LH conducted the research, GH, JB, LRS, JA, PS, GvH and LH analyzed the data, all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors report a conflict of interest related to the study.
Availability of data material
Data described in the manuscript will be made available upon request pending application and approval.
Rights and permissions
About this article
Cite this article
Højfeldt, G., Bülow, J., Agergaard, J. et al. Postprandial muscle protein synthesis rate is unaffected by 20-day habituation to a high protein intake: a randomized controlled, crossover trial. Eur J Nutr 60, 4307–4319 (2021). https://doi.org/10.1007/s00394-021-02590-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-021-02590-4