Abstract
Purpose
We investigated the association between the inflammatory potential of the diet and hearing loss in the context of aging.
Methods
We studied 3435 French adults enrolled in the SU.VI.MAX 2 (2007–2009) cohort. The inflammatory potential of the diet was estimated by the Dietary Inflammatory Index (DII®) using ≥ 3 baseline 24-h dietary records. Subjective hearing loss was assessed after a mean of 12.5 ± 0.7 years by 3 individual items (ability to carry a conversation in a noisy setting, frequently asking for repetition, and need to increase the television/radio volume) and by a composite score, dichotomized for analyses. We fit sex-specific multivariable logistic regression models.
Results
Compared with males, females had higher DII scores (i.e., more pro-inflammatory diet) and less subjective hearing loss. Among males, a significant positive association between DII (continuous scale) and inability to carry a conversation in a noisy setting was found (OR = 1.10; 95% CI 1.02, 1.18), while the opposite was seen among females (OR = 0.92; 95% CI 0.87, 0.98). Regarding the need to turn up the television/radio volume, a significant positive association with DII (continuous scale) was found only among males (OR = 1.09; 95% CI 1.01, 1.18). A significant association with the subjective hearing loss composite score was found among females (ORQ3 vs Q1 = 0.74; 95% CI 0.57, 0.97).
Conclusion
The findings among males supported the hypothesis that a pro-inflammatory diet could increase risk of hearing loss, whereas the findings among females were unexpected. This study could provide impetus for future research in sensory disability and aging.
Trial registration
www.clinicaltrials.gov # NCT00272428.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Population aging is a worldwide trend that is highly correlated with the prevalence of presbycusis (or age-related sensorineural hearing loss, HL). The latter is a bilateral, irreversible condition that is manifested by a worsening ability to hear high-frequency sounds and understand speech, and that could be a precursor to dementia [1, 2]. Presbycusis results from the gradual degeneration of sensory cells (hair cells of the organ of Corti, stria vascularis, spiral ganglion neurons), accelerated by advancing age, mitochondrial DNA mutations, oxidative damage, noise exposure, ototoxic medication use, and poor diet [3,4,5,6]. Of note, the long-term adverse impact of HL on quality of life has been more salient in terms of self-perceived hearing handicap than audiometrically assessed HL [7]. Subjective HL displays a moderate, age-dependent correlation with objective HL measures [8, 9].
Aging and age-related chronic diseases have been associated with upregulation of pro-inflammatory mediators [tumor necrosis factor (TNF), interleukin-1 beta (IL-1β), interleukin-6 (IL-6), cyclooxygenase-2, cytokine-inducible nitric oxide synthase, C-reactive protein (CRP)], underlying low-grade chronic inflammation [10, 11]. Regarding presbycusis, cross-sectional results showed an age-dependent, inverse association of white blood cell count, IL-6 and CRP with hearing level [12, 13], whereas longitudinal results are less straightforward. For example, Nash et al. [14] reported an association between consistently high or increasing serum CRP and 10-year incident HL in those aged < 60 years; Lassale et al. [15] found a prospective association only between white blood cell count and HL in individuals with a median baseline age of 63 years; no significant results regarding the link between inflammation markers and HL risk emerged from the Nurses' Health Studies [16].
Given estimates that nearly half of all HL cases are preventable via cost-effective public health measures [17], dietary interventions are promising owing to diet’s role in inflammation, blood lipids, endothelial function and blood pressure [6]. CRP concentrations were shown to be 30% and 24% lower among those in the top versus the bottom quintile of the Alternate Healthy Eating Index (AHEI) and the Alternate Mediterranean Diet Index (AMED), respectively [18]; a meta-analysis of 17 trials also reported that healthy dietary patterns [Mediterranean diet, Nordic diet, Tibetan diet, Dietary Approaches to Stop Hypertension (DASH)] were associated with reduced CRP [19]. Moreover, findings from the Nurses’ Health Study II revealed that dietary patterns featuring fruit, vegetables, legumes, whole grains, nuts, poultry, and fish, with moderate alcohol intake (AMED, DASH, and AHEI-2010) were prospectively associated with lower HL risk [6]. A recent study with 734 elderly investigated the link of presbycusis with foods/beverages modeled as anti-inflammatory (fruit, vegetables, nuts, wine) or as pro-inflammatory (processed meat, sugar-rich juices, desserts, hard liquor) [20]. The researchers found that presbycusis was cross-sectionally associated with an increased intake of fruit juices and retrospectively associated with an increased intake of sugary food, high-calorie drinks, beer, and hard liquor [20]. No study has yet explored the link between the inflammatory potential of the overall diet and HL. The former is commonly estimated via a summary measure—the literature-based Dietary Inflammatory Index (DII®)—based on the evidence from 1943 peer-reviewed articles on diet and 6 of the most commonly-studied inflammatory markers [21]. This study examined the association between DII scores evaluated at midlife and subjective HL assessed later in life.
Materials and methods
We used data from the French Supplémentation en Vitamines et Minéraux Antioxydants trial (SU.VI.MAX, 1994–2002; n = 12,741; www.clinicaltrials.gov # NCT00272428) and its follow-up SU.VI.MAX 2 observational study (2007–2009; n = 6850), with males and females recruited from the general population. The former was a randomized, double-blind, placebo-controlled, primary prevention trial of the efficacy of daily antioxidant supplementation regarding risk of cancer, cardiovascular disease, and all-cause mortality. Details about the trial protocol and follow-up are published elsewhere [22]. SU.VI.MAX 2 was a cross-sectional study with 54% of the original SU.VI.MAX sample (i.e., there were no SU.VI.MAX 2 participants who had not previously been enrolled in SU.VI.MAX). All procedures were approved by the Ethics Committee for Studies with Human Participants of Paris-Cochin Hospital and the National Commission on Informatics and Liberty. Written informed consent was obtained from each participant prior to enrollment.
DII estimation
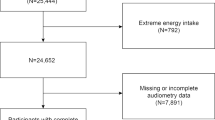
As part of the SU.VI.MAX follow-up, participants completed up to six 24-h dietary records per year with the help of a picture manual [23]. Details about the dietary data collection are available elsewhere [24]. Briefly, during the SU.VI.MAX trial (1994–2002), participants were asked to complete a 24-h dietary record every two months for a total of six such records per year. The days were assigned at random and included four weekdays and two weekend days per year. Each day of the week and all seasons had an equal chance of being covered, thus accounting not only for seasonal but also for individual variability in dietary intake. Next, nutrient intake was computed using the Phenol-Explorer database [25] and a validated food composition table [26]. For this study, mean intake of food and nutrients was calculated across all complete 24-h records (if ≥ 3 such records, in order to reduce measurement bias) collected during the first two years of follow-up (1994–1996). Hence, we excluded from the analysis 1656 individuals for whom the DII could not be calculated owing to missing dietary data or to having fewer than three 24-h dietary records. The participant inclusion flowchart is presented in Fig. 1.
DII was computed using established criteria [27]. Briefly, individual-level intake of nutrients and food was standardized to worldwide mean ± SD values, and the resulting z scores were converted to centered proportions and multiplied by a literature-derived effect score. At the individual level, all food-specific inflammatory marker values were summed to obtain the DII. Of the 45 original dietary components, the present study had data on 35: carbohydrates, fiber, protein, total/saturated/monounsaturated/polyunsaturated fatty acids, omega-3 and omega-6 fatty acids, cholesterol, niacin, thiamin, riboflavin, vitamins A, B6, B12, C, D, and E, folic acid, iron, zinc, beta-carotene, anthocyanidins, flavanols, flavonols, flavonones, flavones, isoflavones, garlic, ginger, pepper, onion, tea, and alcohol. A higher (i.e., more positive) DII corresponds to a more pro-inflammatory diet, whereas a lower (i.e., more negative) DII corresponds to a more anti-inflammatory diet [27].
Subjective hearing assessment
As part of the SU.VI.MAX 2 protocol, during the period 2007–2009, the participants underwent a comprehensive clinical examination that included an assessment of hearing level. Specifically, subjective HL was assessed with three Yes/No questions about ability to carry a conversation in a noisy setting, need to frequently ask for repetition, and need to increase the television/radio volume. A total score ranging from 0 (no problem in any area) to 3 (problems in all three areas) was calculated. Individuals with present/previous ear disease and those wearing hearing aids or other auditory devices were ineligible for the study.
Covariates
Upon enrollment in SU.VI.MAX 2, information on sex, age, education (< high school; high school diploma; Associate’s degree or equivalent; undergraduate or graduate degree), occupation (homemaker, farmer/manual labor, artisan/self-employed/office worker/skilled labor, managerial staff/intellectual profession), smoking (never, former or current smoker), and leisure–time physical activity (the equivalent of fast walking) in h/week was collected via self-report questionnaires. Objective anthropometric and clinical data also were obtained. Body weight was measured with an electronic scale following standardized procedures, with barefoot participants wearing light indoor clothing; height was measured to the nearest 0.5 cm with a wall-mounted stadiometer. Body mass index (BMI) was calculated as the weight in kg divided by the square of height in m. Blood pressure was measured following a 10-min rest with a standard mercury sphygmomanometer. If systolic blood pressure was > 160 mmHg and/or diastolic blood pressure was > 100 mmHg, the assessment was repeated following a 5-min rest [28].
Statistical analysis
The sociodemographic characteristics of included and excluded (from the present analysis) SU.VI.MAX 2 participants were compared with chi-squared tests and Student t tests. In the final sample, descriptive analyses by sex-specific DII quartiles were performed with chi-squared tests and ANOVA. The main analysis consisted of multivariable logistic regression of the cross-time association of DII [independent variable modeled on a continuous scale (Model 1) and as sex-specific quartiles (Model 2)] with subjective HL. We hypothesized that a lower DII (i.e., less diet-associated inflammation) would be protective against HL risk. As dependent variables, we individually modeled the three Yes/No items measuring perceived (subjective) HL and the summary score (range 0–3 points), which was dichotomized for the analysis (0 = no problem; ≥ 1 = some hearing problems). All models were adjusted for supplementation group during the trial, age, education, occupation, BMI, blood pressure, physical activity, number of 24-h dietary records, dietary energy intake (without alcohol), alcohol intake, and smoking status. Unlike nonsignificant interaction by age, alcohol use, smoking and supplementation group (all p > 0.10), interaction by sex was statistically significant, hence separate models were fit for males and females. Finally, a sensitivity analysis was carried out, where the DII was replaced by an energy–density DII [22], which was then modeled on a continuous scale. All tests were two-sided, and p < 0.05 was considered statistically significant. Analyses were performed using SAS® (version 9.4, SAS Institute, Inc., Cary NC, USA).
Results
Participant characteristics
The sample included 3435 participants (58% females) with a mean age at the time of HL assessment of 61.7 ± 6.1 years. The mean interval between DII estimation and subjective HL assessment was 12.5 ± 0.7 years.
When compared with SU.VI.MAX 2 participants who were not included in this analysis (n = 3415), those who were included were somewhat younger (mean age 62.4 vs. 61.7 years), more likely to have completed primary/secondary education, to be married/cohabiting (82.1% vs. 76.8%) and to have lower blood pressure (all p < 0.05, data not tabulated). There were no significant differences between included and excluded participants as regards randomization group (SU.VI.MAX trial), sex, smoking status or physical activity.
The DII was normally distributed with mean values being higher among females than among males (0.84 ± 1.88 vs. 0.12 ± 1.77; p < 0.0001). In turn, subjective HL was more frequently reported by males than by females (50.3% vs. 38.3%; p < 0.0001). The sociodemographic and health status characteristics by DII quartiles are summarized in Table 1 (females) and Table 2 (males).
Association between DII and subjective HL
The results of the sex-specific multivariable logistic regression models are presented in Table 3. Among males, a significant positive association between DII (continuous scale and quartiles) and inability to carry a conversation in a noisy setting was found (ORcont = 1.10; 95% CI 1.02–1.18; ORQ4 vs Q1 = 1.48; 95% CI 1.03–2.12), while the opposite was seen among females (ORcont = 0.92; 95% CI 0.87–0.98; ORQ4 vs Q1 = 0.69; 95% CI 0.50–0.93). Regarding the need to turn up television/radio volume, a significant positive association with DII was found only among males (ORcont = 1.09; 95% CI 1.01–1.18; ORQ4 vs Q1 = 1.53; 95% CI 1.03–2.26). No association between DII and frequently asking for repetition was found in either sex. The only significant association with the dichotomized composite score of subjective HL was seen among females (ORQ3 vs Q1 = 0.74; 95% CI 0.57–0.97). In the sensitivity analysis, where the DII was replaced by an energy–density DII, the main findings were largely replicated (Table 4).
Discussion
To our knowledge, this study was the first to investigate the association between the inflammatory potential of the overall diet and age-related HL. We prospectively studied a large sample of aging adults recruited from the general French population. The adjusted analysis provided some support for our hypothesis, especially among males in whom it was observed that less diet-related inflammation (i.e., lower DII scores) was associated in a protective fashion with subjective HL. This link was restricted to specific subjective HL measures, such as inability to carry a conversation in a noisy setting and need to turn up the television/radio volume. In turn, unexpected results were observed among females in whom DII and subjective HL were inversely associated. In our sample, males had lower mean DII (i.e., less pro-inflammatory diet) yet more subjective hearing impairment compared with females. There may also exist sex-specific predisposition to the type of presbycusis—sensory, neural, strial, and cochlear-conductive—along with the presbycusis manifestation (loss of word discrimination, alterations in physical characteristics of cochlear duct, etc.) [29]. In addition, total energy intake, which was taken into account in the main and sensitivity analyses, differs significantly between males and females [21]. Although sex-specific associations between diet quality and HL have been reported in the literature [30], further research is nonetheless warranted.
Prior studies have associated increased risk of subjective HL with lower scores on various diet quality indices (AMED, DASH, AHEI-2010, prudent dietary pattern) [6, 31]. It has been argued that dietary patterns high in fruit, vegetables, legumes, whole grains, nuts, fish, and poultry, with moderate alcohol intake and low saturated fat intake may confer protection against vascular compromise and/or cochlear blood flow reduction via reduced inflammation and blood pressure, beneficial blood lipid profiles, and endothelial function support [6]. Indeed, prudent/healthy dietary patterns have been associated with reduced CRP, E-selectin, IL-6, fasting insulin, and glucose concentrations and with increased insulin sensitivity [32, 33].
In this study, the inflammatory potential of the overall diet was estimated by the DII (and the energy–density DII in a sensitivity analysis) which has consistently shown dietary pattern differentiation similar to that obtained with other diet quality indices, such as AHEI, HEI-2010, and DASH [34]. The DII is a validated summary measure of diet-associated inflammation; it has been studied internationally with respect to its association with various physical and mental health outcomes, such as cancer, cardiovascular disease, asthma, cognitive performance, depression, sleep latency, maternal and child health, aging, and mortality [21, 35,36,37,38]. This analysis thus extends evidence about the predictive association of DII with age-related hearing impairment.
Mechanistic evidence from animal models has highlighted the tendency of the mammalian inner ear to lose sensory cells with advancing age [39] as a result of chronic inflammation, oxidative stress, altered antioxidant enzyme levels, and the triggering of apoptotic pathways [40]. The mesenchymal region of the cochlea is likely a common site of inflammation [41] and a correlation has been demonstrated between pro-inflammatory cytokine concentrations and hearing thresholds after noise exposure [42]. In humans, a phenomenon termed “inflammaging” and referring to prevalent chronic inflammation in the elderly, has been studied with respect to HL; an age-dependent association has been reported between a higher white blood cell count and worse hearing level [13]. Animal models have also underscored the potential role of the diet in reducing the magnitude of age-related cochlear degeneration [43]. Next, a long-term high cholesterol and/or high-fat diet has been shown to induce HL via oxidative stress, increasing reactive oxygen species, mitochondrial damage, inner ear apoptosis and vascular compromise [44, 45]. Our models were controlled for total energy intake (along with other factors), which supports a potential causal link between long-term dietary pattern exposure and HL.
The main outcome in this study was subjective HL, which has been moderately correlated with objective HL measures [8, 9, 46]. We modeled a subjective HL summary score as well as three individual HL items, some of which could be perceived as more factual (i.e., inability to carry a conversation in a noisy setting) than others (i.e., television/radio volume which might not be perceived as loud even when turned up). Self-perceived hearing handicap has been linked to a reduced quality of life to a greater extent than audiometrically-assessed HL [7]. Prior research has shown, for example, that fewer than a quarter of those with moderate to profound HL (≥ 41 dB) perceived themselves as being hearing-handicapped [47]. A population-based study with elderly participants revealed that simply being aware of one’s hearing disability was longitudinally associated with worsening functional performance and selective attention, global cognitive deterioration, and depressive symptoms [48].
Two limitations of the present prospective study pertain to the absence of hearing level data at baseline and the fact that DII might have changed over time. At inclusion, participants were generally healthy, middle-aged volunteers (mean ages of 51.3 and 46.6 years among males and females, respectively) and we could speculate that the potential prevalence of any hearing-related conditions (and presbycusis in particular) was likely low. Another limitation pertains to the absence of information about the types of presbycusis in the study sample. Next, despite the large number of covariates included in the adjusted analysis, other potential confounders, such as tinnitus, noise exposure and genetic factors, were not included in the models. In turn, similar to other a priori indices, DII might be subject to some limitations, such as arbitrary selection of components and scoring methods [49]. In this study, DII was computed on the basis of 35 dietary parameters (versus anywhere from 27 to 30 in most other studies), yet the original index was based on 45 dietary parameters. It should also be pointed out that the lack of a dose–response relationship might be partly interpreted as possible evidence for a nonlinear association between the inflammatory potential of the diet and subjective hearing loss, to be investigated by future research. A strength of this study was the use of a large sample of middle-aged and older adults recruited from the general population. Yet, the question of external validity remains, given that the participants had engaged in a long-term nutrition-focused research and might therefore not be representative of the general French population.
As society ages, the number of individuals suffering from HL will grow. This tendency is alarming, given evidence of the association of HL with accelerated cognitive decline, poorer physical functioning, increased risk for falls, poor physician–patient communication, and poor adherence to treatment regimens [50]. The findings among males revealed that a pro-inflammatory diet could increase risk of age-related HL, providing support for the long-term preventive potential of a healthy diet. However, the findings among females were unexpected and could not be attributed to sex-specific differences in energy density; thus, they merit further investigation. Likewise, future research could replicate the models using objective HL measures. This study could inform future targeted HL prevention efforts and could serve as impetus for epidemiological research in the context of both sensory disability and aging.
Data availability
Data described in the manuscript will be made available upon request, pending application and approval.
Code availability
The SAS programs will be made available upon request, pending application and approval.
References
Frisina RD, Ding B, Zhu X, Walton JP (2016) Age-related hearing loss: prevention of threshold declines, cell loss and apoptosis in spiral ganglion neurons. Aging 8:2081–2099
Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L (2011) Hearing loss and incident dementia. Arch Neurol 68:214–220
Stevens G, Flaxman S, Brunskill E, Mascarenhas M, Mathers CD, Finucane M, Global Burden of Disease Hearing Loss Expert Group (2013) Global and regional hearing impairment prevalence: an analysis of 42 studies in 29 countries. Eur J Public Health 23:146–152
Fransen E, Lemkens N, Van Laer L, Van Camp G (2003) Age-related hearing impairment (ARHI): environmental risk factors and genetic prospects. Exp Gerontol 38:353–359
Fujimoto C, Yamasoba T (2014) Oxidative stresses and mitochondrial dysfunction in age-related hearing loss. Oxid Med Cell Longev 2014:582849
Curhan SG, Wang M, Eavey RD, Stampfer MJ, Curhan GC (2018) Adherence to healthful dietary patterns is associated with lower risk of hearing loss in women. J Nutr 148:944–951
Gopinath B, Schneider J, Hickson L, McMahon CM, Burlutsky G, Leeder SR, Mitchell P (2012) Hearing handicap, rather than measured hearing impairment, predicts poorer quality of life over 10 years in older adults. Maturitas 72:146–151
Saunders GH, Frederick M, Silverman S, Arnold M, Chisolm T (2018) Community-based hearing screening: pros, cons, and lessons learned. Innov Aging 2:360
Kiely KM, Gopinath B, Mitchell P, Browning CJ, Anstey KJ (2012) Evaluating a dichotomized measure of self-reported hearing loss against gold standard audiometry: prevalence estimates and age bias in a pooled national data set. J Aging Health 24:439–458
Prasad S, Sung B, Aggarwal BB (2012) Age-associated chronic diseases require age-old medicine: role of chronic inflammation. Prev Med 54:S29–S37
Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C (2009) Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev 8:18–30
Verschuur CA, Dowell A, Syddall HE, Ntani G, Simmonds SJ, Baylis D, Gale CR, Walsh B, Cooper C, Lord JM et al (2012) Markers of inflammatory status are associated with hearing threshold in older people: findings from the Hertfordshire Ageing Study. Age Ageing 41:92–97
Verschuur C, Agyemang-Prempeh A, Newman TA (2014) Inflammation is associated with a worsening of presbycusis: evidence from the MRC national study of hearing. Int J Audiol 53:469–475
Nash SD, Cruickshanks KJ, Zhan W, Tsai MY, Klein R, Chappell R, Nieto FJ, Klein BE, Schubert CR, Dalton DS et al (2014) Long-term assessment of systemic inflammation and the cumulative incidence of age-related hearing impairment in the epidemiology of hearing loss study. J Gerontol A Biol Sci Med Sci 69:207–214
Lassale C, Vullo P, Cadar D, Batty GD, Steptoe A, Zaninotto P (2020) Association of inflammatory markers with hearing impairment: the English Longitudinal Study of Ageing. Brain Behav Immun 83:112–119
Gupta S, Curhan SG, Curhan GC (2019) Biomarkers of systemic inflammation and risk of incident hearing loss. Ear Hear 40:981–989
World Health Organization (2019) Deafness and hearing loss. WHO, Geneva
Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB (2005) Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 82:163–173
Neale EP, Batterham MJ, Tapsell LC (2016) Consumption of a healthy dietary pattern results in significant reductions in C-reactive protein levels in adults: a meta-analysis. Nutr Res 36:391–401
Sardone R, Lampignano L, Guerra V, Zupo R, Donghia R, Castellana F, Battista P, Bortone I, Procino F, Castellana M et al (2020) Relationship between inflammatory food consumption and age-related hearing loss in a prospective observational cohort: results from the Salus in Apulia study. Nutrients 2:426
Hebert JR, Shivappa N, Wirth MD, Hussey JR, Hurley TG (2019) Perspective: the Dietary Inflammatory Index (DII)—lessons learned, improvements made and future directions. Adv Nutr 10:185–195
Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, Roussel AM, Favier A, Briancon S (2004) The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med 164:2335–2342
Le Moullec N, Deheeger M, Preziosi P, Montero P, Valeix P, Rolland-Cachera MF, Potier de Courcy G, Christides JP, Galan P, Hercberg S (1996) Validation du manuel photos utilisé pour l’enquête alimentaire de l’étude SU.VI.MAX. Cahier de Nutrition et de Diététique 31:158–164
Mennen LI, Bertrais S, Galan P, Arnault N, Potier de Couray G, Hercberg S (2002) The use of computerised 24 h dietary recalls in the French SU.VI.MAX Study: number of recalls required. Eur J Clin Nutr 56:659–665
Neveu V, Perez-Jimenez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, et al (2010) Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford) 2010:bap024
Hercberg S (2005) Table de composition SU.VI.MAX des aliments. Les Editions INSERM/Economica, Paris
Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR (2014) Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 17:1689–1696
Hercberg S, Preziosi P, Briancon S, Galan P, Triol I, Malvy D, Roussel AM, Favier A (1998) A primary prevention trial using nutritional doses of antioxidant vitamins and minerals in cardiovascular diseases and cancers in a general population: the SU.VI.MAX study-design, methods, and participant characteristics. SUpplementation en VItamines et Mineraux AntioXydants. Control Clin Trials 19:336–351
Schuknecht HF, Gacek MR (1993) Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol 102:1–16
Huang Q, Jin Y, Reed NS, Ma Y, Power MC, Talegawkar SA (2020) Diet quality and hearing loss among middle-older aged adults in the USA: findings from National Health and Nutrition Examination Survey. Public Health Nutr 23:812–820
Dawes P, Cruickshanks KJ, Marsden A, Moore DR, Munro KJ (2020) Relationship between diet, tinnitus, and hearing difficulties. Ear Hear 41:289–299
Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, Hu FB (2004) Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 80:1029–1035
Anderson AL, Harris TB, Tylavsky FA, Perry SE, Houston DK, Lee JS, Kanaya AM, Sahyoun NR (2012) Dietary patterns, insulin sensitivity and inflammation in older adults. Eur J Clin Nutr 66:18–24
Wirth MD, Hebert JR, Shivappa N, Hand GA, Hurley TG, Drenowatz C, McMahon D, Shook RP, Blair SN (2016) Anti-inflammatory dietary inflammatory index scores are associated with healthier scores on other dietary indices. Nutr Res 36:214–219
Godos J, Ferri R, Caraci F, Cosentino FII, Castellano S, Shivappa N, Hebert JR, Galvano F, Grosso G (2019) Dietary inflammatory index and sleep quality in southern Italian adults. Nutrients 1:1324
Wood LG, Shivappa N, Berthon BS, Gibson PG, Hebert JR (2015) Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clin Exp Allergy 45:177–183
Frith E, Shivappa N, Mann JR, Hebert JR, Wirth MD, Loprinzi PD (2018) Dietary inflammatory index and memory function: population-based national sample of elderly Americans. Br J Nutr 119:552–558
Kesse-Guyot E, Assmann KE, Andreeva VA, Touvier M, Neufcourt L, Shivappa N, Hebert JR, Wirth MD, Hercberg S, Galan P et al (2017) Long-term association between the dietary inflammatory index and cognitive functioning: findings from the SU.VI.MAX study. Eur J Nutr 56:1647–1655
Jiang H, Talaska AE, Schacht J, Sha SH (2007) Oxidative imbalance in the aging inner ear. Neurobiol Aging 28:1605–1612
Menardo J, Tang Y, Ladrech S, Lenoir M, Casas F, Michel C, Bourien J, Ruel J, Rebillard G, Maurice T et al (2012) Oxidative stress, inflammation, and autophagic stress as the key mechanisms of premature age-related hearing loss in SAMP8 mouse Cochlea. Antioxid Redox Signal 16:263–274
Fujioka M, Okano H, Ogawa K (2014) Inflammatory and immune responses in the cochlea: potential therapeutic targets for sensorineural hearing loss. Front Pharmacol 5:287
Arslan HH, Satar B, Serdar M, Yilmaz E (2017) Changes in proinflammatory cytokines in the cochlea in relation to hearing thresholds in noise-exposed rats. J Int Adv Otol 13:308–312
Le T, Keithley EM (2007) Effects of antioxidants on the aging inner ear. Hear Res 226:194–202
Du Z, Yang Y, Hu Y, Sun Y, Zhang S, Peng W, Zhong Y, Huang X, Kong W (2012) A long-term high-fat diet increases oxidative stress, mitochondrial damage and apoptosis in the inner ear of d-galactose-induced aging rats. Hear Res 287:15–24
Sikora MA, Morizono T, Ward WD, Paparella MM, Leslie K (1986) Diet-induced hyperlipidemia and auditory dysfunction. Acta Otolaryngol 102:372–381
Andreeva VA, Assmann KE, Adjibade M, Lemogne C, Hercberg S, Galan P, Kesse-Guyot E (2017) Dyslipidemia as a potential moderator of the association between hearing loss and depressive symptoms. J Nutr Health Aging 21:1291–1298
Chang HP, Ho CY, Chou P (2009) The factors associated with a self-perceived hearing handicap in elderly people with hearing impairment—results from a community-based study. Ear Hear 30:576–583
Vaccaro R, Zaccaria D, Colombo M, Abbondanza S, Guaita A (2019) Adverse effect of self-reported hearing disability in elderly Italians: results from the InveCe.Ab study. Maturitas 121:35–40
Waijers PM, Feskens EJ, Ocke MC (2007) A critical review of predefined diet quality scores. Br J Nutr 97:219–231
Weinstein B (2018) Why hearing status matters in delivery of value-based health care. Innov Aging 2:S360
Acknowledgments
The authors thank Laurent Bourhis and Nathalie Arnault for their assistance with data management.
Funding
This work was supported by the French National Research Agency (grant # ANR-05-PNRA-010), the French Ministry of Health, Médéric, Sodexo, Ipsen, MGEN and Pierre Fabre. Médéric and MGEN are French health insurance organizations complementary to the National Health Insurance System. Ipsen and Pierre Fabre are private pharmaceutical companies; they financially supported the overall implementation of the research project. Sodexo is a food-catering company that sponsored events between researchers and study participants. Drs. Shivappa, Hébert, and Wirth were supported by grant # R44DK103377 from the U.S. National Institute for Diabetes and Digestive and Kidney Diseases. The funding bodies did not have any involvement in the design or conduct of the research, in data analysis or interpretation, or in writing or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
SH, PG and EKG: SU.VI.MAX project conception, development of research plan, study oversight, data collection management; NS, JRH, and MDW: developed the DII®; SP, PG and EKG: developed the hearing data collection protocol and coordinated data collection; CJ, MT, and EKG: coordinated the calculation of DII within the SU.VI.MAX cohort; VAA: conducted research (literature review and statistical analysis) and led the writing; SP, JRH and EKG: provided analytical and theoretical guidance; all authors made major contributions to writing the manuscript, read and approved the final version; VAA: had primary responsibility for final content.
Corresponding author
Ethics declarations
Conflict of interest
The Dietary Inflammatory Index (DII®) is a registered trademark of the University of South Carolina. JR Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the DII from the University of South Carolina in order to develop computer and smartphone applications for patient counseling and dietary intervention in clinical settings. MDW and NS are employees of CHI. These activities have no direct bearing on the use of the DII as a research tool. VAA, SP, CJ, MT, SH, PG, and EKG have no conflicts of interest to report.
Ethics approval
All procedures were approved by the Ethics Committee for Studies with Human Participants of Paris-Cochin Hospital (CCPPRB #706 and #2364) and the National Commission on Informatics and Liberty (CNIL #334641 and #907094).
Consent to participate
Written informed consent was obtained from each participant prior to enrollment.
Rights and permissions
About this article
Cite this article
Andreeva, V.A., Péneau, S., Julia, C. et al. The inflammatory potential of the diet is prospectively associated with subjective hearing loss. Eur J Nutr 60, 3669–3678 (2021). https://doi.org/10.1007/s00394-021-02531-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-021-02531-1