Abstract
Purpose
Inflammation is a ubiquitous underlying mechanism of the links between diet and cognitive functioning. No study has yet evaluated the overall inflammatory potential of the diet, using the dietary inflammatory index (DII), in relation to cognitive functioning. In a French cohort of middle-aged adults, we evaluated the association between the DII, assessed in midlife, and cognitive performance evaluated 13 years later.
Methods
The DII is a literature-derived dietary index developed to determine the inflammatory potential of diet. The DII was estimated at baseline (1994–1996) among 3080 subjects of the SU.VI.MAX (supplementation with antioxidant vitamins and minerals) cohort. Cognitive performance was assessed in 2007–2009 via a battery of standardized neuropsychological tests. Principal component analysis was performed to extract a summary score of cognitive performance. Multivariable-adjusted linear regression analyses were performed to provide regression coefficients and 95 % confidence intervals (95 % CI).
Results
In a multivariate model, a strong inverse association was observed between a higher DII (reflecting a more inflammatory diet) and overall cognitive functioning (mean difference Q4 vs. Q1 = −1.76; 95 % CI = −2.81, −0.72, P for trend =0.002). With regard to specific cognitive domains, similar associations were observed with scores reflecting verbal memory, but not executive functioning.
Conclusion
This study suggests that a pro-inflammatory diet at midlife might be associated with subsequent lower cognitive functioning. A diet exhibiting anti-inflammatory properties may help to maintain cognitive health during aging.
Clinical trial registration
Clinicaltrials.gov (number NCT00272428).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low-grade chronic inflammation is a common underlying process for several age-related chronic diseases and conditions [1], amongst others Alzheimer’s disease [2]. At the brain level, neuro-inflammation is highly involved in neuronal damage, which may lead to cognitive decline. Besides, an elevated inflammatory status associated with activated microglia has been observed in demented patients [3–5].
Although pro-inflammatory molecules such as some cytokines are not able to cross the blood–brain barrier, it is well recognized that systemic inflammation and neuro-inflammation are closely related [6], arguing for a role of peripheral inflammation in cognitive aging.
Nevertheless, the role of systemic and neuro-inflammation in the etiology of neurodegenerative diseases is still debated [6].
Epidemiologic research focusing on the relationship between circulating pro-inflammatory markers, including C-reactive protein (CRP), interleukin (IL)-6, IL-10, tumor necrosis factor-a, and cognitive outcomes has been recently reviewed [7, 8]. Data are relatively scarce, and existing findings from longitudinal studies are contrasted, with some studies reporting a positive association [9–15] while others reporting null findings [9, 16–19]. The results also vary across the studied cognitive domains and pro-inflammatory biomarkers tested. Additionally, most studies have been carried out among the elderly, and thus, a potential for elevated inflammatory status induced by preclinical disorders cannot be excluded, thus limiting causal inference.
There is a growing body of evidence suggesting the anti-inflammatory effects of specific dietary factors including n-3 polyunsaturated fatty acids, fibers, vitamins and minerals, as well as overall dietary patterns [20, 21]. This suggests new directions for nutritional prevention via decreasing systemic inflammation by modulating cytokine production.
Recently, based upon existing mechanistic and epidemiological data from about 1943 published studies linking chronic systemic inflammation and nutritional factors, the dietary inflammatory index (DII) reflecting the overall inflammatory potential of the diet has been developed and validated [22, 23]. Unlike considering individual food or nutrients, the use of the DII may provide complementary arguments for the role of the dietary inflammatory potential in health. Furthermore, the DII has been associated with pro-inflammatory markers in some [23–27], but not all [28] epidemiological studies to date.
The purpose of the present study was to examine the long-term association between the DII evaluated in midlife using repeated 24-h records and overall and domain-specific cognitive functioning measured 13 years later, using a large cohort. Specifically, we hypothesized that long-term healthy dietary habits may help to maintain cognitive functioning during aging through their anti-inflammatory properties, expressed as lower DII scores.
Materials and methods
Population
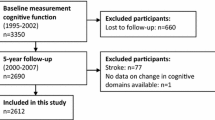
The French SU.VI.MAX «SUpplémentation en Vitamines et Minéraux AntioXydants» study (1994–2002) was a randomized, double-blind, placebo-controlled, primary prevention trial initially designed to test the potential efficacy of daily supplementation with antioxidant vitamins and minerals at nutritional doses on the incidence of cancer, ischemic heart disease, and overall mortality. It included 12,741 adult volunteers living in France (women aged 35–60 years and men aged 45–60 years). The initial follow-up was planned for 8 years [29]. Five years after the end of the trial phase, a total of 6850 individuals were included in the SU.VI.MAX 2 observational follow-up study (2007–2009). The SU.VI.MAX and SU.VI.MAX 2 studies were conducted according to the Declaration of Helsinki guidelines. All procedures involving human subjects were approved by the Ethics Committee for Studies with Human Participants of Paris-Cochin Hospital (CCPPRB No. 706 and No. 2364, respectively) and the Commission Nationale de l’Informatique et des Libertés (CNIL No. 334641 and No. 907094, respectively). Written informed consent was obtained from all subjects. The SU.VI.MAX trial was registered at www.clinicaltrials.gov under #NCT00272428.
Dietary data assessment
During the SU.VI.MAX trial, dietary data were collected through 24-h dietary records provided via computerized questionnaires using the Minitel, a small terminal widely used in France in the 1990s as an adjunct to the telephone. A maximum of six randomly distributed records was completed per year, covering weekdays and weekend days and all seasons of the year. Participants were assisted by an instruction manual that included validated photographs of >250 generic foods shown in three main portion sizes [30]. The Phenol-Explorer database [31] and a published validated composition table [32] were used to compute nutrient intakes. For the present analysis, the food and nutrient intakes were calculated as the mean reported values across all eligible 24-h records collected during the first 2 years of the SU.VI.MAX study. Next, the DII was computed using a previously published algorithm [22]. Briefly, individual intake of nutrients and food was standardized using worldwide mean and standard deviation (SD) values. To prevent skewing, z-scores were converted to centered percentiles which were then multiplied by an effect score. It was based on a literature review of 1943 studies examining the relationship between dietary constituents and inflammatory markers, to obtain a food-specific value. At the individual level, all such values were summed up to obtain the overall DII. In the present study, the DII includes data on 35 of the 45 food parameters: carbohydrates, protein, total fat, alcohol, fiber, cholesterol, saturated fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids, omega 3, omega 6, niacin, thiamin, riboflavin, vitamin B6, vitamin B12, iron, zinc, vitamin A, vitamin C, vitamin D, vitamin E, folic acid, beta carotene, anthocyanidins, flavan-3-ol, flavonols, flavonones, flavones, isoflavones, garlic, ginger, pepper, onion, and tea.
Cognitive assessment
During the SU.VI.MAX 2 study (2007–2009), all participants were invited to undergo a neuropsychological evaluation. Episodic memory was evaluated with the “rappel indicé-48” (RI-48), a validated cued recall test (list of 48 words from 12 categories). For each word correctly retrieved, one point was attributed (theoretical maximal score = 48). Lexical–semantic memory was assessed using a phonemic fluency task (citing words beginning with the letter P) and a semantic fluency task (naming as many animals as possible). For each task, the score was the number of words correctly produced during a 2-min period. Forward and backward digit span tasks (issued from the validated Wechsler adult intelligence scale-third edition [33]) were used to assess short-term and working memory. These tasks consisted in immediately repeating an increasing sequence of digits, with one point attributed for each sequence repeated correctly until the participant failed two consecutive trials of the same digit span (theoretical maximal score for each task =14). Mental flexibility was assessed with the Delis–Kaplan trail-making test (TMT) which is a number–letter switching task. The score was the time, in seconds, needed to complete task. The inverse of the TMT score was log-transformed to improve normality. We converted the initial cognitive test scores into T scores (mean = 50, SD = 10), so that a one-point difference in the test score corresponded to one-tenth of an SD difference and each score was standardized, thus allowing for comparison.
A composite cognitive score was extracted by principal component analysis (SAS Proc Factor). The factor accounted for 41 % of the initial variance. The factor loadings (i.e., correlation coefficients between the composite cognitive score and each individual neuropsychological tests) are given in Supplemental Table 1).
Covariates
Upon enrollment, information on sex, date of birth, smoking status (never-smoked, former, or current smoker), physical activity (irregular, <1 h walking/day or equivalent, ≥1 h walking/day or equivalent), formal education (primary, secondary, or post-secondary), occupational category (homemaker, farmer/manual labor, artisan/self-employed/office worker/skilled labor, managerial staff/intellectual profession), and perceived memory troubles (yes/no) was collected via self-administered questionnaires.
Anthropometric and clinical measurements (including body mass index (BMI kg/m2) and blood pressure) were obtained at baseline and at the end of the follow-up, as previously described [34]. Information on hypertension (≥140/90 mmHg or medication use) and diabetes (fasting blood glucose ≥7 mmol/l, or anti-diabetic medication use) over the follow-up was also collected as previously reported [34]. During the follow-up, all reported cardiovascular events were validated by an independent expert committee. Depressive symptoms were assessed at follow-up (2007–2009) using the French version of the center for epidemiologic studies depression scale (CES-D), and the total score was used as a covariate [35].
Statistical analysis
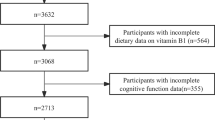
In the present analysis, we included subjects aged 45–60 years at baseline with available cognitive evaluation (N = 4447) and dietary data (i.e., ≥3 24-h records over the first 2 years of follow-up; N = 3362). Subjects with missing values for any of the covariates were excluded, leaving a final sample of 3080 participants.
We compared included and excluded subjects using Chi-square tests or Wilcoxon’s rank tests. Baseline characteristics are presented by sex-specific quartiles (Q) of the DII. Values represent mean (±SD) or percentages, and P values were calculated using linear contrast or Chi-square trend tests, as appropriate. Cross-time associations between the DII at midlife and cognitive function 13 years later were analyzed by ANCOVA. The first model was adjusted for age at the neuropsychological evaluation and sex. The second model was further adjusted for education, follow-up time between baseline and cognitive evaluation, supplementation group during the trial phase, number of 24-h dietary records, energy intake, BMI, occupational status, tobacco use status, physical activity, memory troubles, depressive symptoms concomitant with the cognitive function assessment, and history of diabetes/hypertension/cardiovascular disease.
All analyses were conducted with SAS® software (version 9.3, SAS Institute, Cary, NC, USA) with a significance level of 0.05 (two-sided tests).
Sensitivity analyses
As dietary intakes were estimated from at least three 24-h records, this might potentially lead to inability to control for intra-individual variability. In order to test the robustness of the main findings, we reanalyzed our data among participants with at least six 24-h records (N = 2695).
Results
Participant characteristics
Compared with those included, excluded participants were younger, more often female, smokers, with lower levels of physical activity and education. They also had lower energy intake, lower CES-D scores and exhibited higher (i.e., more pro-inflammatory) DII values (data not shown).
At baseline, the mean age of our study sample was 52.0 ± 4.6 years, and at the time of the cognitive evaluation, the mean age was 65.4 ± 4.6 years. The mean (SD) DII was 0.41 (1.84), and it was lower in men than in women [0.34 (1.80) and 0.97 (1.88), respectively].
Baseline characteristics across Q of the DII are presented in Table 1. Compared with those with a low DII scores, participants with a high DII scores, reflecting a more pro-inflammatory diet, were less educated, less physically active, and more often smokers.
The DII was negatively correlated with energy intake and percent of energy from carbohydrates and positively correlated with percent of energy from lipids and proteins. After adjustment for total energy, the DII was negatively correlated with folic acid, beta carotene, vitamin C, PUFA, and fiber intakes.
Association between the DII and global cognitive functioning
Table 2 presents the association between the DII modeled in Q and global cognitive functioning assessed through principal component analysis. A higher DII, reflecting a pro-inflammatory diet, was associated with lower subsequent global cognitive function [mean difference Q4–Q1 = −1.76 (−2.81; −0.72), P for trend =0.002 in the fully adjusted model].
Association between DII and individual cognitive tests
Table 3 presents the association between the DII in Q and performance on each individual cognitive test. For the RI-48 cued recall test and semantic and phonemic fluencies, a higher DII score was associated with a poorer performance.
In addition, in fully adjusted models, no association between the DII and executive functioning-related cognitive tests (the TMT, forward and backward digit span) was observed.
For comparison purposes, the associations between the DII modeled as a continuous variable and individual cognitive tests scores are shown in Fig. 1. The mean differences in cognitive performances for a 1 unit change in the DII score are shown.
Association between the dietary inflammatory index as a continuous variable and cognitive test scores. Values are adjusted mean difference (95 % confidence interval) in cognitive test scores (estimated via ANCOVA) using the first quartile of the dietary inflammatory index as reference. Adjustment is made for age, gender, education, follow-up time between baseline and cognitive evaluation, supplementation group during the trial phase and number of 24-h dietary records, energy intake, BMI, occupational status, tobacco use status, physical activity, memory troubles at baseline, depressive symptoms concomitant with the cognitive function assessment, history of diabetes/hypertension/cardiovascular disease
Sensitivity analyses
We repeated our analyses among participants with at least six 24-h records thus improving the estimation of nutritional intakes. Findings were similar in terms of direction, though the associations were strengthened (data not shown).
Discussion
In this French study, the DII estimated at midlife was inversely associated with subsequent cognitive functioning. The association was strong even after adjustment for a wide range of potential confounders, including sociodemographic, lifestyle, and health factors, in particular vascular conditions. This link was mostly driven by the associations with cognitive tests reflecting verbal memory. No association was observed with executive functioning assessed via digit span tasks and the TMT.
Inflammation and cognitive function
The few prospective cohort studies investigating the role of inflammation in later cognitive outcomes have not provided consistent findings. Methodological flaws have been advanced, such as reverse causality (due to an existing preclinical stage) and the use of a single biomarker for the assessment of inflammation, as explanations of this phenomenon [7, 8]. To the best of our knowledge, only two studies were carried out among relatively young individuals, where reverse causality was unlikely [10, 12]. In the Honolulu–Asia aging study, cognitive decline was positively associated with higher CRP concentrations in midlife, but no association was detected after removing dementia cases occurring during the follow-up [10].
In the Whitehall study [12], the authors reported a predictive role of blood concentrations of the inflammatory biomarker IL-6, but not CRP in cognitive decline (overall function and reasoning). These studies and our findings provide new insights and extend knowledge concerning the association between inflammatory status in midlife and later global cognitive health during aging. Our results support the hypothesis that a pro-inflammatory diet may impact both cognitive functioning as a whole, and regarding specific cognitive domains, by contributing to a state of systemic inflammation.
In our study, we observed a strong inverse association between a pro-inflammatory diet and cognitive performance on the fluency tests. However, further investigation with respect to the identification of cognitive domains susceptible to inflammation is needed. Different authors have reported associations with global cognitive functioning [9, 11–13], reasoning [12], motor speed [14], executive function [15], and memory function [15]. However, data are sparse and further research is needed to elucidate the differential susceptibility of specific cognitive domains to inflammation.
We identified three studies reporting significant associations between IL-6 levels and cognitive outcomes, but no association with CRP [9, 14, 36], suggesting that early inflammatory biomarkers may prospectively predict future cognitive outcomes. Moreover, these studies argue for the need to focus on several inflammatory biomarkers.
While systemic inflammation is the product of many factors including diet, obesity, and other conditions, the present study aimed to specifically focus on the part of systemic inflammation that can be attributed to nutritional factors. Although it can be assumed that dietary factors have a smaller effect on inflammation than other factors such as age and genetic factors, they are important in terms of public health since they are potentially modifiable. The scientific literature linking dietary patterns and inflammation is growing [20, 37], and the DII has been previously shown to be associated with inflammatory markers [23–27, 38]. Strong findings were obtained from the SEASONS study, a longitudinal follow-up study of 550 adult men and women in whom the DII clearly predicted interval changes in blood levels of CRP [23]. All of these studies argue for an association between DII and clinical markers of inflammation. This diet-based approach can give important new insights into the long-term preventive potential of a healthy diet, as a complement to studies focusing on single nutrients (which allows to obtain specific information on particular nutrients) or on single foods (which allows to also consider food matrix effects).
Diet quality index and cognitive function
Our findings are consistent with previous research focusing specifically on the role of vitamin D, a variety of polyphenols, beta carotene, and vitamin C and vitamin E intakes on cognitive functioning [39–41].
Besides, as expected, the DII was correlated with a less healthy nutritional profile [42]. Hence, our findings can be interpreted in light of previous research focusing on dietary quality using holistic approaches and the link with cognitive outcomes. Indeed, the present findings are consistent with our previous work documenting better cognitive functioning among subjects with a higher level of adherence to nutritional guidelines at midlife [43]. The findings are also consistent with those based on a priori dietary indexes, in particular the Mediterranean diet score, and a posteriori data-driven approaches such as factor analysis [44, 45]. In particular, the Mediterranean diet, which has been hypothesized to exhibit anti-inflammatory properties and beneficial vascular effects [46, 47], has been consistently associated with several cognitive outcomes in a recent meta-analysis of five longitudinal studies [48].
Strengths and limitations
Our findings should be interpreted taking into account some limitations. First, we were not able to focus on cognitive decline as cognitive performance was not measured at baseline. However, due to the relatively young age of this population heavily involved in a cohort study, it is likely that the completion of many questionnaires over a long follow-up period (13 years) argues against prevalent cognitive impairment at baseline inducing bias in any of the measures, including dietary intake. Finally, as participants of the SU.VI.MAX study were volunteers, the external validity of our findings might be limited. Concerning the use of the DII, similarly to other a priori indexes, it might be subject to some limitations, including the arbitrary selection of components and scoring methods [49]. In addition, in our study, the DII was constructed using 35 parameters, while the original index includes 45 food parameters. This may have led to some misestimation of the dietary inflammatory potential.
Our study also exhibits strengths and important original aspects, including a large cohort of community-dwelling subjects, a focus on midlife exposures, and the use of relatively accurate dietary data. Lastly, we use neuropsychological standardized tests with good sensitivity, and avoiding ceiling and floor effects.
Conclusion
In conclusion, our study supports a significant harmful role of a pro-inflammatory diet in midlife in subsequent cognitive function. This suggests that diet may act on cognitive functioning through its inflammation-inducing properties. The findings can help to refine population-level guidelines regarding the maintenance of cognitive health during aging.
Abbreviations
- BMI:
-
Body mass index
- DII:
-
Dietary inflammatory index
- CES-D:
-
Center for Epidemiologic Studies Depression Scale
- CRP:
-
C-reactive protein
- PUFA:
-
Polyunsaturated fatty acids
- Q :
-
Quartile
- TMT:
-
Trail-making test
References
Prasad S, Sung B, Aggarwal BB (2012) Age-associated chronic diseases require age-old medicine: role of chronic inflammation. Prev Med 54(Suppl):S29–S37
Rubio-Perez JM, Morillas-Ruiz JM (2012) A review: inflammatory process in Alzheimer’s disease, role of cytokines. Sci World J 2012:756357
Wyss-Coray T, Rogers J (2012) Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med 2(1):a006346
Gorelick PB (2010) Role of inflammation in cognitive impairment: results of observational epidemiological studies and clinical trials. Ann N Y Acad Sci 1207:155–162
Simen AA, Bordner KA, Martin MP, Moy LA, Barry LC (2011) Cognitive dysfunction with aging and the role of inflammation. Ther Adv Chronic Dis 2(3):175–195
Holmes C (2013) Review: systemic inflammation and Alzheimer’s disease. Neuropathol Appl Neurobiol 39(1):51–68
Bettcher BM, Kramer JH (2013) Inflammation and clinical presentation in neurodegenerative disease: a volatile relationship. Neurocase 19(2):182–200
Yang J, Fan C, Pan L, Xie M, He Q, Li D et al (2015) C-reactive protein plays a marginal role in cognitive decline: a systematic review and meta-analysis. Int J Geriatr Psychiatry 30(2):156–165
Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S et al (2003) Inflammatory markers and cognition in well-functioning African–American and white elders. Neurology 61(1):76–80
Laurin D, David CJ, Masaki KH, White LR, Launer LJ (2009) Midlife C-reactive protein and risk of cognitive decline: a 31-year follow-up. Neurobiol Aging 30(11):1724–1727
Wilson CJ, Cohen HJ, Pieper CF (2003) Cross-linked fibrin degradation products (D-dimer), plasma cytokines, and cognitive decline in community-dwelling elderly persons. J Am Geriatr Soc 51(10):1374–1381
Singh-Manoux A, Dugravot A, Brunner E, Kumari M, Shipley M, Elbaz A et al (2014) Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife. Neurology 83(6):486–493
Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE (2002) Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology 59(3):371–378
Palta P, Xue QL, Deal JA, Fried LP, Walston JD, Carlson MC (2014) Interleukin-6 and C-reactive protein levels and 9-year cognitive decline in community-dwelling older women: the women’s health and aging study II. J Gerontol A Biol Sci Med Sci 70(7):873–878
Mooijaart SP, Sattar N, Trompet S, Lucke J, Stott DJ, Ford I et al (2013) Circulating interleukin-6 concentration and cognitive decline in old age: the PROSPER study. J Intern Med 274(1):77–85
Weuve J, Ridker PM, Cook NR, Buring JE, Grodstein F (2006) High-sensitivity C-reactive protein and cognitive function in older women. Epidemiology 17(2):183–189
Gimeno D, Marmot MG, Singh-Manoux A (2008) Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrinology 33(10):1322–1334
Alley DE, Crimmins EM, Karlamangla A, Hu P, Seeman TE (2008) Inflammation and rate of cognitive change in high-functioning older adults. J Gerontol A Biol Sci Med Sci 63(1):50–55
Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P (2005) Serum inflammatory proteins and cognitive decline in older persons. Neurology 64(8):1371–1377
Ahluwalia N, Andreeva VA, Kesse-Guyot E, Hercberg S (2013) Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metab 39(2):99–110
Warnberg J, Gomez-Martinez S, Romeo J, Diaz LE, Marcos A (2009) Nutrition, inflammation, and cognitive function. Ann N Y Acad Sci 1153:164–175
Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR (2014) Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 17(8):1689–1696
Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS et al (2014) A population-based dietary inflammatory index predicts levels of C-reactive protein in the seasonal variation of blood cholesterol study (SEASONS). Public Health Nutr 17(8):1825–1833
Ruiz-Canela M, Zazpe I, Shivappa N, Hebert JR, Sanchez-Tainta A, Corella D et al (2015) Dietary inflammatory index and anthropometric measures of obesity in a population sample at high cardiovascular risk from the PREDIMED (PREvencion con DIeta MEDiterranea) trial. Br J Nutr 27:1–12
Wood LG, Shivappa N, Berthon BS, Gibson PG, Hebert JR (2015) Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clin Exp Allergy 45(1):177–183
Tabung FK, Steck SE, Ma Y, Liese AD, Zhang J, Caan B et al (2015) The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: results from the women’s health initiative. Cancer Causes Control 26(3):399–408
Shivappa N, Hebert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E et al (2015) Associations between dietary inflammatory index and inflammatory markers in the Asklepios study. Br J Nutr 113(4):665–671
Alkerwi A, Vernier C, Crichton GE, Sauvageot N, Shivappa N, Hebert JR (2014) Cross-comparison of diet quality indices for predicting chronic disease risk: findings from the observation of cardiovascular risk factors in Luxembourg (ORISCAV-LUX) study. Br J Nutr 5:1–11
Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D et al (2004) The SU.VI.MAX study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med 164(21):2335–2342
Le Moullec N, Deheeger M, Preziosi P, Montero P, Valeix P, Rolland-Cachera MF et al (1996) Validation du manuel photos utilisé pour l’enquête alimentaire de l’étude SU.VI.MAX. Cah Nutr Diét 31(3):158–164
Neveu V, Perez-Jimenez J, Vos F, Crespy V, du Chaffaut L, Mennen L et al (2010) Phenol-explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford) 91:940–949
Hercberg S (coordinator) (2005) Table de composition SU.VI.MAX des aliments. Les éditions INSERM/Economica, Paris p 182
Wechsler D (1981) Wechsler adult intelligence scale-revised. Psychological Corporation, New York
Kesse-Guyot E, Andreeva VA, Jeandel C, Ferry M, Hercberg S, Galan P (2012) A healthy dietary pattern at midlife is associated with subsequent cognitive performance. J Nutr 142(5):909–915
Radloff L (1977) The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1:385–401
Rafnsson SB, Deary IJ, Smith FB, Whiteman MC, Rumley A, Lowe GD et al (2007) Cognitive decline and markers of inflammation and hemostasis: the Edinburgh Artery study. J Am Geriatr Soc 55(5):700–707
Barbaresko J, Koch M, Schulze MB, Nothlings U (2013) Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev 71(8):511–527
Wirth MD, Burch J, Shivappa N, Violanti JM, Burchfiel CM, Fekedulegn D et al (2014) Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J Occup Environ Med 56(9):986–989
Andreeva VA, Whegang-Youdom S, Touvier M, Assmann KE, Fezeu L, Hercberg S et al (2014) Midlife dietary vitamin D intake and subsequent performance in different cognitive domains. Ann Nutr Metab 65(1):81–89
Kesse-Guyot E, Fezeu L, Andreeva VA, Touvier M, Scalbert A, Hercberg S et al (2012) Total and specific polyphenol intakes in midlife are associated with cognitive function measured 13 years later. J Nutr 142(1):76–83
Peneau S, Galan P, Jeandel C, Ferry M, Andreeva V, Hercberg S et al (2011) Fruit and vegetable intake and cognitive function in the SU.VI.MAX 2 prospective study. Am J Clin Nutr 94:1295–1303
Turner-McGrievy GM, Wirth MD, Shivappa N, Wingard EE, Fayad R, Wilcox S et al (2015) Randomization to plant-based dietary approaches leads to larger short-term improvements in dietary inflammatory index scores and macronutrient intake compared with diets that contain meat. Nutr Res 35(2):97–106
Kesse-Guyot E, Amieva H, Castetbon K, Henegar A, Ferry M, Jeandel C et al (2011) Adherence to nutritional recommendations and subsequent cognitive performance: findings from the prospective supplementation with antioxidant vitamins and minerals 2 (SU.VI.MAX 2) study. Am J Clin Nutr 93(1):200–210
Alles B, Samieri C, Feart C, Jutand MA, Laurin D, Barberger-Gateau P (2012) Dietary patterns: a novel approach to examine the link between nutrition and cognitive function in older individuals. Nutr Res Rev 25(2):207–222
van de Rest O, Berendsen AA, Haveman-Nies A, de Groot LC (2015) Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr 6(2):154–168
Frisardi V, Panza F, Seripa D, Imbimbo BP, Vendemiale G, Pilotto A et al (2010) Nutraceutical properties of Mediterranean diet and cognitive decline: possible underlying mechanisms. J Alzheimers Dis 22(3):715–740
Gillette-Guyonnet S, Secher M, Vellas B (2013) Nutrition and neurodegeneration: epidemiological evidence and challenges for future research. Br J Clin Pharmacol 75(3):738–755
Singh B, Parsaik AK, Mielke MM, Erwin PJ, Knopman DS, Petersen RC et al (2014) Association of Mediterranean diet with mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 39(2):271–282
Waijers PM, Feskens EJ, Ocke MC (2007) A critical review of predefined diet quality scores. Br J Nutr 97(2):219–231
Acknowledgments
This research was supported by the ANR (National Research Agency, Grant No. ANR-05-PNRA-010), DGS (Ministry of Health), Médéric, Sodexo, Ipsen, MGEN, and Pierre Fabre. Mederic and MGEN are French health insurance organizations complementary to the National Health Insurance System. Ipsen and Pierre Fabre are private pharmaceutical companies; they financially supported the overall implementation of the research project. Sodexo is a food catering company that sponsored events between researchers and study participants. Sponsors were not involved in analyses or interpretation of findings. Karen E. Assmann was supported by a doctoral dissertation fellowship from University of Paris 13. Drs. Shivappa, Hebert, and Wirth were supported by grant number R44DK103377 from the United States National Institute for Diabetes and Digestive and Kidney Diseases.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. James R. Hébert owns the controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to the dietary inflammatory index (DII) from the University of South Carolina in order to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings. Dr. Nitin Shivappa is an employee of CHI. The subject matter of this paper will not have any direct bearing on that work, nor has that activity exerted any influence on this project. The other authors declare no conflict of interest.
Competing interest
EKG carried out data checking and analyses and was responsible for drafting the manuscript. She takes full responsibility for the present work. NS was involved in DII computation. KA, VAA, MT, LN, NS, JRH, MDW, SH, PG, and CJ were involved in interpreting the results and editing the manuscript. EKG, PG, and SH were responsible for developing the design and protocol of the study. All authors read and approved the final version of the manuscript. None of the authors has any competing interests, and all are independent of the funding bodies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kesse-Guyot, E., Assmann, K.E., Andreeva, V.A. et al. Long-term association between the dietary inflammatory index and cognitive functioning: findings from the SU.VI.MAX study. Eur J Nutr 56, 1647–1655 (2017). https://doi.org/10.1007/s00394-016-1211-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1211-3